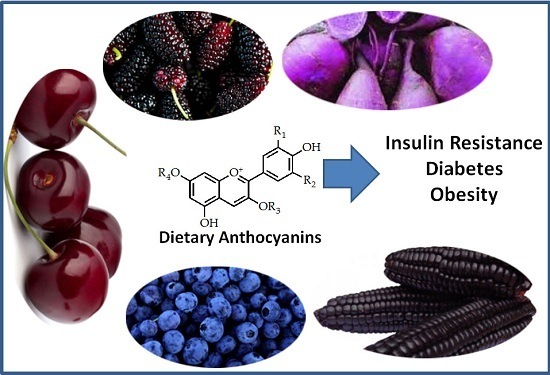
Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine
Abstract
:1. Introduction
2. Chemical Diversity of Dietary Anthocyanins
3. Natural Occurrence of Dietary Anthocyanins
4. Dietary Anthocyanins and Insulin Sensitivity/Resistance
4.1. In Vitro Protective Activity: Insulin Resistance Diabetic/Obese Condition
4.2. In Vivo Protective Activity: Insulin Resistance Diabetic/Obese Condition
5. Clinical Study
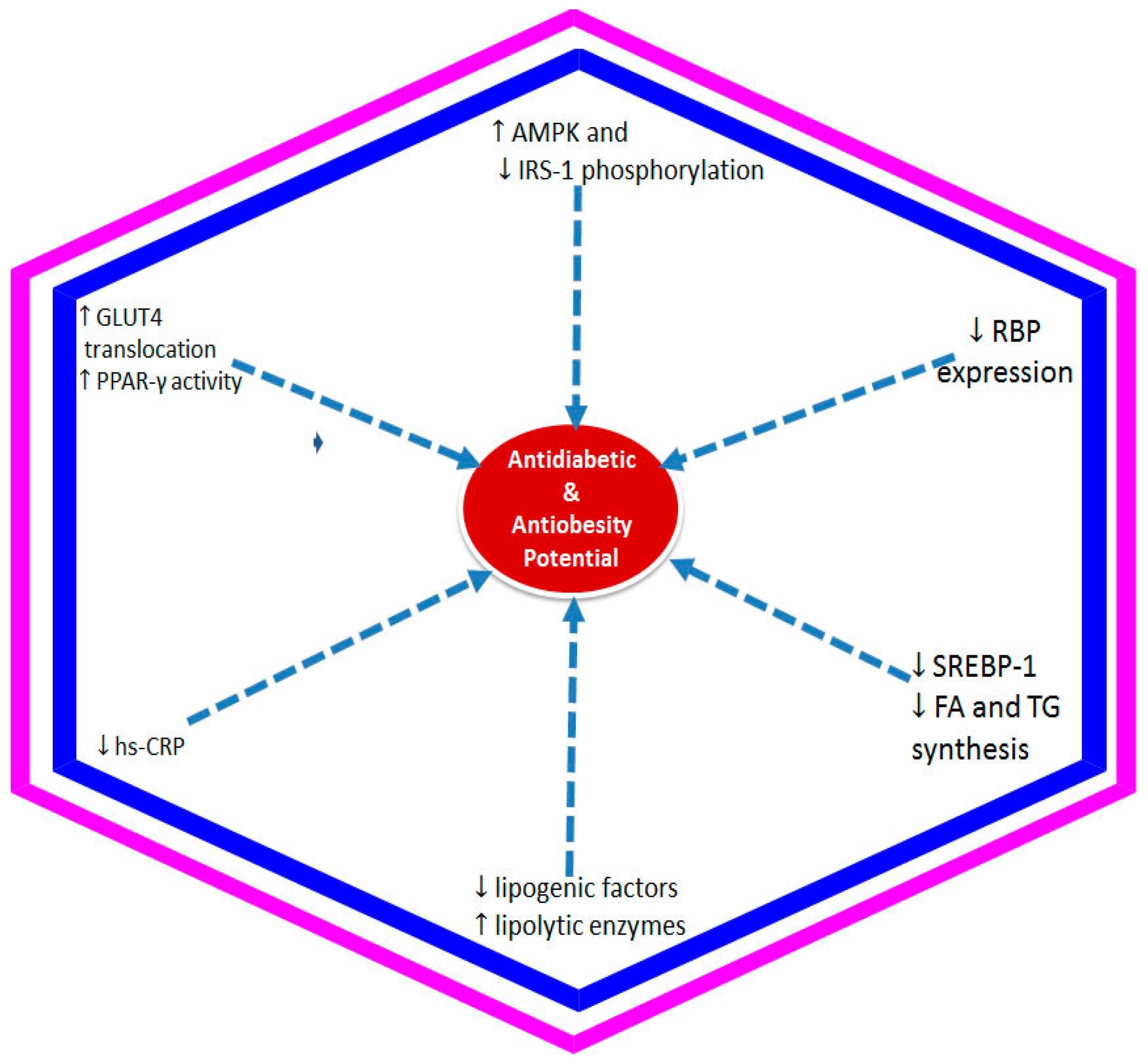
6. General Summary and Conclusions
Author Contributions
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 15 August 2017).
- World Health Organization. Diabetes. Available online: http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed on 15 August 2017).
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, X. Emerging role of JNK in insulin resistance. Curr. Diabetes Rev. 2013, 9, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Kleinridders, A.; Kahn, C. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef] [PubMed]
- Bardini, G.; Rotella, C.M.; Giannini, S. Dyslipidemia and diabetes: Reciprocal impact of impaired lipid metabolism and β-cell dysfunction on micro- and macrovascular complications. Rev. Diabet. Stud. 2012, 9, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, S.; Jayaprakash, V. Thiazolidinediones and PPAR orchestra as antidiabetic agents: From past to present. Eur. J. Med. Chem. 2016, 126, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochem. Pharmacol. 2014, 92, S73–S89. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Going back to the good old days: The merit of crude plant drug mixtures in the 21st century. Int. J. Complement. Altern. Med. 2017, 6, 1–5. [Google Scholar] [CrossRef]
- Habtemariam, S.; Varghese, G.K. Antioxidant, anti-α-glucosidase and pancreatic β-cell protective effects of methanolic extract of Ensete superbum Cheesm seeds. Asian Pac. J. Trop. Biomed. 2017, 7, 121–125. [Google Scholar] [CrossRef]
- Habtemariam, S.; Lentini, G. The therapeutic potential of rutin for diabetes: An update. Mini Rev. Med. Chem. 2015, 15, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Varghese, G.K. The antidiabetic therapeutic potential of dietary polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Smith, D.M. Correlations between anthocyanin chemistry and pollination ecology in the polemoniaceae. Biochem. Syst. Ecol. 1978, 6, 127–130. [Google Scholar] [CrossRef]
- Saito, N.; Harborne, J.B. Correlations between anthocyanin type, pollinator and flower colour in the labiatae. Phytochemistry 1992, 31, 3009–3015. [Google Scholar] [CrossRef]
- Costa, D.; Galvão, A.M.; Di Paolo, R.E.; Freitas, A.A.; Lima, J.C.; Quina, F.H.; Maçanita, A.L. Photochemistry of the hemiketal form of anthocyanins and its potential role in plant protection from UV-B radiation. Tetrahedron 2015, 71, 3157–3162. [Google Scholar] [CrossRef]
- Galaffu, N.; Bortlik, K.; Michel, M. An industry perspective on natural food colour stability. Colour Addit. Foods Beverages 2015, 91–130. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Stabilization of natural colors and nutraceuticals: Inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016, 212, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase inhibitory action of natural acylated anthocyanins. 2. α-Glucosidase inhibition by isolated acylated anthocyanins. J. Agric. Food Chem. 2001, 49, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ebuchi, S.; Fukui, K.; Matsugano, K.; Terahara, N.; Matsumoto, K. Caffeoylsophorose, a new natural alphaglucosidase inhibitor, from red vinegar by fermented purple- fleshed sweet potato. Biosci. Biotech. Biochem. 2004, 68, 2239–2246. [Google Scholar] [CrossRef]
- Iwai, K.; Kim, M.Y.; Onodera, A.; Matsue, H. α-Glucosidase inhibitory and antihyperglycemic effects of polyphenols in the fruit of Viburnum dilatatum Thunb. J. Agric. Food Chem. 2006, 54, 4588–4592. [Google Scholar] [CrossRef] [PubMed]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Chen, F.; Wang, X.; Luo, P.G.; Jiang, Y. Inhibitory effects of muscadine anthocyanins on r-glucosidase and pancreatic lipase activities. J. Agric. Food Chem. 2011, 59, 9506–9511. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Rodríguez-Werner, M.; Schlösser, A. Fractionation, enzyme inhibitory and cellular antioxidant activity of bioactives from purple sweet potato (Ipomoea batatas). Food Chem. 2017, 221, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Bento, C.; Silva, B.; Silva, L. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res. Int. 2017, 95, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Krishnasamy, G.; Muthusamy, K. In vitro evaluation of antioxidant and antidiabetic activities of Syzygium densiflorum fruits. Asian Pac. J. Trop. Dis. 2015, 5, 912–917. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of phytochemicals, antioxidant capacity, and antidiabetic activity of novel smoothies from selected Prunus fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.; Castaño-Tostado, E. Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage. Food Chem. 2015, 172, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Prathapan, A.; Krishna, M.; Nisha, V. Polyphenol rich fruit pulp of Aegle marmelos (L.) Correa exhibits nutraceutical properties to down regulate diabetic complications—An in vitro study. Food Res. Int. 2012, 48, 690–695. [Google Scholar] [CrossRef]
- Cheplick, S.; Kwon, Y.; Bhowmik, P.; Shetty, K. Phenolic-linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Biores. Technol. 2010, 101, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Kwon, Y.; Apostolidis, E.; Lajolo, F. Functionality of bioactive compounds in Brazilian strawberry (Fragaria x ananassa Duch.) cultivars: Evaluation of hyperglycemia and hypertension potential. J. Agric. Food Chem. 2008, 56, 4386–4392. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.D.S.; Kwon, Y. Evaluation of red currants (Ribes rubrum L.), black currants (Ribes nigrum L.), red and green gooseberries (Ribes uva-crispa) for potential management of type 2. J. Food Biochem. 2010, 34, 639–660. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Napierała, M.; Białas, W.; Grajek, W. Pancreatic α-amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (Aronia melanocarpa L.). Process Biochem. 2014, 49, 1457–1463. [Google Scholar] [CrossRef]
- Zhang, A.; Rimando, A.; Fish, W.; Mentreddy, S. Serviceberry leaf extract inhibits mammalian α-glucosidase activity and suppresses postprandial glycemic. J. Ethnopharmacol. 2012, 143, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Tadera, K.; Minami, Y.; Takamatsu, K. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Carbonell-Barrachina, Á. Phenolic compounds, antioxidant and antidiabetic activity of different cultivars of Ficus carica L. fruits. J. Funct. Food 2016, 25, 421–432. [Google Scholar] [CrossRef]
- Ho, G.T.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Phenolic elderberry extracts, anthocyanins, procyanidins and metabolites influence glucose and fatty acid uptake in human skeletal muscle cells. J. Agric. Food Chem. 2017, 65, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Pogrebnyak, N.; Kuhn, P.; Poulev, A. Polyphenol-rich Rutgers Scarlet Lettuce improves glucose metabolism and liver lipid accumulation in diet-induced obese C57BL/6 mice. Nutrition 2014, 30, S52–S58. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zheng, X. Anthocyanin-rich mulberry fruit improves insulin resistance and protects hepatocytes against oxidative stress during hyperglycemia by regulating AMPK/ACC/mTOR pathway. J. Funct. Foods 2017, 30, 270–281. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Vari, R.; Filesi, C.; D’Archivio, M.; Santangelo, C.; Giovannini, C.; Iacovelli, A.; Silecchia, G.; Li Volti, G.; Galvano, F.; et al. Cyanidin-3-O-β-glucoside and protocatechuic acid exert insulin-like effects by upregulating PPAR-gamma activity in human omental adipocytes. Diabetes 2011, 60, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.H.; Yoon, H.S.; Park, H.J.; Kim, M.Y.; Shin, H.K.; Park, K.Y.; Yang, J.O.; Sohn, M.S.; Do, M.S. Anti-obesity and antioxidative effects of purple sweet potato extract in 3T3-L1 adipocytes in vitro. J. Med. Food 2011, 14, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Tanaka, M. Anthocyanidins-enriched bilberry extracts inhibit 3T3-L1 adipocyte differentiation via the insulin pathway. Nutr. Metab. 2011, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.; Martineau, L.C.; Ramassamy, C.; Matar, C.; Haddad, P.S. Fermented Canadian lowbush blueberry juice stimulates glucose uptake and AMP-activated protein kinase in insulin-sensitive cultured muscle cells and adipocytes. Can. J. Physiol. Pharmacol. 2007, 85, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.; Couture, A.; Spoor, D. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Achrekar, S.; Kaklij, G.; Pote, M. Hypoglycemic activity of Eugenia jambolana and Ficus bengalensis: Mechanism of action. In Vivo 1991, 5, 143–147. [Google Scholar] [PubMed]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin secretion by anthocyanins and anthocyanidins. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Aoki, H.; Koda, T.; Horio, F.; Takahashi, N.; Kawada, T.; Osawa, T. Anthocyanin enhances adipocytokine secretion and adipocyte-specific gene expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 2004, 316, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Ueno, Y.; Yoshikawa, T.; Kojo, H.; Osawa, T. Microarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochem. Pharmacol. 2006, 71, 1184–1197. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, S.M.; Damasceno, D.C.; Spada, A.P.; Palhares, M.; Martuchi, K.A.; Oshiiwa, M.; Sazaki, V.; da Silva, V.S. Evaluation of glycemic and lipid profile of offspring of diabetic Wistar rats treated with Malpighia emarginata juice. Exp. Diabetes Res. 2011, 2011, 173647. [Google Scholar] [CrossRef] [PubMed]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Mihova, V.; Krasnalev, I.; Borisova, P.; Belcheva, A. Antihyperlipidemic effect of Aronia melanocarpa fruit juice in rats fed a high-cholesterol diet. Plant Foods Hum. Nutr. 2007, 62, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.I.; Howard, L.R.; Prior, R.L.; Lee, S.O. Effect of Aronia melanocarpa (Black Chokeberry) supplementation on the development of obesity in mice fed a high-fat diet. J. Berry Res. 2016, 6, 203–212. [Google Scholar] [CrossRef]
- Sabahi, Z.; Khoshnood-Mansoorkhani, M.J.; Namadi, S.R.; Moein, M. Antidiabetic and Synergistic Effects of Anthocyanin Fraction from Berberis integerrima Fruit on Streptozotocin-Induced Diabetic Rats Model. Trends Phram. Sci. 2016, 2, 43–50. [Google Scholar]
- Azofeifa, G.; Quesada, S.; Navarro, L.; Hidalgo, O. Hypoglycaemic, hypolipidaemic and antioxidant effects of blackberry beverage consumption in streptozotocin-induced diabetic rats. J. Funct. Food 2016, 26, 330–337. [Google Scholar] [CrossRef]
- Guo, H.; Ling, W.; Wang, Q.; Liu, C.; Hu, Y.; Xia, M.; Feng, X.; Xia, X. Effect of anthocyanin-rich extract from black rice (Oryza sativa L. indica) on hyperlipidemia and insulin resistance in fructose-fed rats. Plant Foods Hum. Nutr. 2007, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Jin, Y.C.; Chung, J.I.; Shin, S.C.; Lee, S.J.; Seo, H.G.; Lee, J.H.; Chang, K.C.; Kim, H.J. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol. Nutr. Food Res. 2009, 53, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Ahn, I.S.; Kim, S.O.; Kong, C.S.; Chung, H.Y.; Do, M.S.; Park, K.Y. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J. Med. Food 2007, 10, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.; Benhaddou-Andaloussi, A.; Brault, A.; Harbilas, D.; Martineau, L.C.; Vallerand, D.; Ramassamy, C.; Matar, C.; Haddad, P.S. Antiobesity and antidiabetic effects of biotransformed blueberry juice in KKAy mice. Int. J. Obes. 2009, 33, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Tanone, I.I.; Urcuyo-Llanes, D.E.; Lewis, S.K.; Kirakosyan, A.; Kondoleon, M.G.; Kaufman, P.B.; Bolling, S.F. Blueberry intake alters skeletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food 2011, 14, 1511–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elks, C.M.; Terrebonne, J.D.; Ingram, D.K.; Stephens, J.M. Blueberries improve glucose tolerance without altering body composition in obese postmenopausal mice. Obesity 2015, 23, 573–580. [Google Scholar] [CrossRef] [PubMed]
- DeFuria, J.; Bennett, G.; Strissel, K.J.; Perfield, J.W., II; Milbury, P.E.; Greenberg, A.S.; Obin, M.S. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J. Nutr. 2009, 139, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Kuhn, P.; Rojo, L.E.; Lila, M.A.; Raskin, I. Blueberry polyphenol-enriched soybean flour reduces hyperglycemia, body weight gain and serum cholesterol in mice. Pharmacol. Res. 2013, 68, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tang, Q.; Gao, Z.; Yu, Z.; Song, H.; Zheng, X.; Chen, W. Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. PLoS ONE 2013, 8, e77585. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Zhao, A.; Merrow, T.; Klimis-Zacas, D. The effects of wild blueberry consumption on plasma markers and gene expression related to glucose metabolism in the obese Zucker rat. J. Med. Food 2015, 18, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Gu, L.; Hager, T.J.; Hager, A.; Howard, L.R. Whole berries versus berry anthocyanins: Interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J. Agric. Food Chem. 2008, 56, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, G.G.; Raskin, I.; Lila, M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine 2009, 16, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Overall, J.; Bonney, S.A.; Wilson, M.; Beermann, A.; Grace, M.H.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Metabolic effects of berries with structurally diverse anthocyanins. Int. J. Mol. Sci. 2017, 18, 422. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.H.; Wallig, M.; Vital, D.A.L.; de Mejia, E.G. Alcohol-free fermented blueberry-blackberry beverage phenolic extract attenuates diet-induced obesity and blood glucose in C57BL/6J mice. J. Nutr. Biochem. 2016, 31, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Jiang, Z.; Yin, J.; Long, H.; Zheng, X. Anti-obesity effects of artificial planting blueberry (Vaccinium ashei) anthocyanin in high-fat diet-treated mice. Int. J. Food Sci. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Anderson, R. An extract of chokeberry attenuates weight gain and modulates insulin, adipogenic and inflammatory signalling pathways in epididymal adipose tissue of rats fed a fructose-rich. Br. J. Nutr. 2012, 108, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.H.; Nair, M.G. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Augusti, K.T.; Daniel, R.S.; Cherian, S.; Sheela, C.G.; Nair, C.R. Effect of leucopelargonin derivative from Ficus bengalensis Linn. on diabetic dogs. Indian J. Med. Res. 1994, 99, 82–86. [Google Scholar] [PubMed]
- Cherian, S.; Kumar, R.V.; Augusti, K.T.; Kidwai, J.R. Antidiabetic effect of a glycoside of pelargonidin isolated from the bark of Ficus bengalensis Linn. Indian J. Biochem. Biophys. 1992, 29, 380–382. [Google Scholar] [PubMed]
- Iwai, K.; Onodera, A.; Matsue, H. Inhibitory effects of Viburnum dilatatum Thunb.(gamazumi) on oxidation and hyperglycemia in rats with streptozotocin-induced diabetes. J. Agric. Food Chem. 2004, 52, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Lenquiste, S.A.; Batista, Â.G.; da Silva Marineli, R.; Dragano, N.R.V.; Maróstica, M.R., Jr. Freeze-dried jaboticaba peel added to high-fat diet increases HDL-cholesterol and improves insulin resistance in obese rats. Food Res. Int. 2012, 49, 153–160. [Google Scholar] [CrossRef]
- Sarikaphuti, A.; Nararatwanchai, T. Preventive effects of Morus alba L. anthocyanins on diabetes in Zucker diabetic fatty rats. Exp. Ther. Med. 2013, 6, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Liu, L.; Chuang, C.; Chyau, C. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Argic. Food Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Singab, A.; El-Beshbishy, H.; Yonekawa, M. Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Yamakawa, T.; Kamei, J.; Kadonosono, K.; Tanaka, S. Anti-hyperglycemic effects of plum in a rat model of obesity and type 2 diabetes, Wistar fatty rat. Biomed. Res. 2005, 26, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, Y.; Huang, T.; Yamahara, J. Pomegranate flower: A unique traditional antidiabetic medicine with dual PPAR-α/-γ activator properties. Diabetes Obes. Metab. 2008, 10, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, S.; Kota, B.; Peng, G.; Li, G. Punica granatum flower extract, a potent α-glucosidase inhibitor, improves postprandial hyperglycemia in Zucker diabetic fatty rats. J. Ethnopharmacol. 2005, 99, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Horio, F.; Uchida, K.; Aoki, H.; Osawa, T. Dietary cyanidin 3-O-beta-d-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 2003, 133, 2125–2130. [Google Scholar] [PubMed]
- Sasaki, R.; Nishimura, N.; Hoshino, H.; Isa, H.; Kadowaki, M.; Ichi, T.; Tanaka, A.; Nishiumi, S.; Fukuda, I.; Ashida, H.; et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem. Pharmacol. 2007, 74, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ebuchi, S.; Kobayashi, M.; Fukui, K. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the α-glucosidase inhibitory action. J. Agric. Food. Chem. 2002, 50, 7244–7248. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Amresh, G.; Sahu, P.; Mishra, N. Antihyperlipedemic activity, haematological effects and histopathological analysis of Sapindus mukorossi Gaerten fruits in streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2012, 5, 518–522. [Google Scholar] [CrossRef]
- Wu, T.; Tang, Q.; Yu, Z.; Gao, Z.; Hu, H.; Chen, W.; Zheng, X.; Yu, T. Inhibitory effects of sweet cherry anthocyanins on the obesity development in C57BL/6 mice. Int. J. Food Sci. Nutr. 2014, 65, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Titta, L.; Trinei, M.; Stendardo, M.; Berniakovich, I.; Petroni, K.; Tonelli, C.; Riso, P.; Porrini, M.; Minucci, S.; Pelicci, P.G.; et al. Blood orange juice inhibits fat accumulation in mice. Int. J. Obese (Lond.) 2010, 34, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.; Singer, A.; Kirakosyan, A. Altered hyperlipidemia, hepatic steatosis, and hepatic peroxisome proliferator-activated receptors in rats with intake of tart cherry. J. Med. Food. 2008, 11, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Welch, A.A.; Spector, T.; Macgregor, A.; Cassidy, A. Intake of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J. Nutr. 2014, 144, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Esmaillzadeh, A.; Tahbaz, F.; Gaieni, I. Cholesterol-lowering effect of concentrated pomegranate juice consumption in type II diabetic patients with hyperlipidemia. See comment in PubMed Commons below. Int. J. Vitam. Nutr. Res. 2006, 76, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.A.; Jafari-Menshadi, F.; Zinsaz, A.; Sadafi, Z. Effect of concentrated pomegranate juice consumption on glucose and lipid profile concentrations in Type 2 diabetic patients. Zahedan J. Res. Med. Sci. 2013, 15, 40–42. [Google Scholar]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Udani, J.; Singh, B.; Singh, V. Effects of Acai (Euterpe oleracea Mart.) berry preparation on metabolic parameters in a healthy overweight population: A pilot study. Nutr. J. 2011, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Sheikh, N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell. Immunol. 2017, 315, 18–26. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Olefsky, J.M. Inflammation and insulin resistance. FEBS Lett. 2007, 582, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Maezono, K.; Osman, A.; Pendergrass, M.; Patti, M.E.; Pratipanawatr, T.; DeFronzo, R.A.; Kahn, C.R.; Mandarino, L.J. Insulin resistance differentially affects the PI 3-kinase and MAP kinase mediated signaling in human muscle. J. Clin. Investig. 2000, 105, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Arora, S.; Goswami, B.; Mallika, V. Metabolic syndrome: A review of emerging markers and management. Diabetes Met. Syndrome Clin. Res. Rev. 2009, 3, 240–254. [Google Scholar] [CrossRef]
- Wang, D.; Zou, T.; Yang, Y.; Yan, X.; Ling, W. Cyanidin-3-O-β-glucoside with the aid of its metabolite protocatechuic acid, reduces monocyte infiltration in apolipoprotein E-deficient mice. Biochem. Pharmacol. 2011, 82, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Serraino, I.; Dugo, P.; Di Paola, R.; Mondello, L.; Genovese, T.; Morabito, D.; Dugo, G.; Sautebin, L.; Caputi, A.P.; et al. Protective effects of anthocyanins from blackberry in a rat model of acute lung inflammation. Free Radic. Res. 2003, 37, 891–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Saleem, M.; Krueger, C.G.; Reed, J.D.; Mukhtar, H. Anthocyanin and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-κB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer 2005, 113, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yi, L.; Jin, X.; Fu, Y.J.; Zhu, J.D.; Mi, M.T.; Zhang, Q.Y.; Ling, W.H.; Yu, B. Inhibitory effect of delphinidin on monocyte-endothelial cell adhesion induced by oxidized low-density lipoprotein via ROS/p38MAPK/NF-kB pathway. Cell Biochem. Biophys. 2011, 61, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Dai, G.; Zheng, X. Mulberry anthocyanin extract ameliorates insulin resistance by regulating PI3K/AKT pathway in HepG2 cells and db/db mice. J. Nutr. Biochem. 2016, 36, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Rudich, A.; Kozlovsky, N.; Potashnik, R.; Bashan, N. Oxidant stress reduces insulin responsiveness in 3T3-L1 adipocytes. Am. J. Physiol. 1997, 272, E935–E940. [Google Scholar] [PubMed]
- Kameji, H.; Mochizuki, K.; Miyoshi, N.; Goda, T. β-Carotene accumulation in 3T3-L1 adipocytes inhibits the elevation of reactive oxygen species and the suppression of genes related to insulin sensitivity induced by tumor necrosis factor-α. Nutrition 2010, 26, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Han, G.D. Ameliorating effects of fermented rice bran extract on oxidative stress induced by high glucose and hydrogen peroxide in 3T3-L1 adipocytes. Plant Foods Hum. Nutr. 2011, 66, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhao, Y.; Suo, S.; Liu, Y.; Zhao, B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radic. Biol. Med. 2012, 52, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Varghese, G.K. A novel diterpene skeleton: Identification of a highly aromatic, cytotoxic and antioxidant 5-methyl-10-demethyl-abietane-type diterpene from Premna serratifolia. Phyther. Res. 2015, 29, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Investigation into the antioxidant and antidiabetic potential of Moringa stenopetala: Identification of the active principles. Nat. Prod. Commun. 2015, 10, 475–478. [Google Scholar] [PubMed]
- Habtemariam, S.; Varghese, G.K. Extractability of rutin in herbal tea preparations of Moringa stenopetala leaves. Beverages 2015, 1, 169–182. [Google Scholar] [CrossRef]
- Roselli, M.; Lentini, G.; Habtemariam, S. Phytochemical, antioxidant and anti-α-glucosidase activity evaluations of Bergenia cordifolia. Phyther. Res. 2012, 26, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S.; Cowley, R.A. Antioxidant and anti-α-glucosidase ccompounds from the rhizome of Peltiphyllum peltatum (Torr.) Engl. Phytother. Res. 2012, 26, 1656–1660. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Methyl-3-O-methyl gallate and gallic acid from the leaves of Peltiphyllum peltatum: Isolation and comparative antioxidant, prooxidant, and cytotoxic effects in neuronal cells. J. Med. Food 2011, 14, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Juan-Badaturuge, M.; Habtemariam, S.; Thomas, M.J.K. Antioxidant compounds from a South Asian beverage and medicinal plant, Cassia auriculata. Food Chem. 2011, 125, 221–225. [Google Scholar] [CrossRef]
- Juan-Badaturugea, M.; Habtemariam, S.; Jackson, C.; Thomas, M.J.K. Antioxidant principles of Tanacetum vulgare L. aerial part. Nat. Prod. Commun. 2009, 4, 1561–1564. [Google Scholar]
- Habtemariam, S.; Dagne, E. Comparative antioxidant, prooxidant and cytotoxic activity of sigmoidin A and eriodictyol. Planta Med. 2010, 76, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Activity-guided isolation and identification of free Radical-scavenging components from ethanolic extract of Boneset (Leaves of Eupatorium perfoliatum). Nat. Prod. Commun. 2008, 3, 1317–1320. [Google Scholar]
- Habtemariam, S.; Jackson, C. Antioxidant and cytoprotective activity of leaves of Peltiphyllum peltatum (Torr.) Engl. Food Chem. 2007, 105, 498–503. [Google Scholar] [CrossRef]
- Habtemariam, S. Flavonoids as inhibitors or enhancers of the cytotoxicity of tumor necrosis factor-alpha in L-929 tumor cells. J. Nat. Prod. 1997, 60, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Modulation of tumour necrosis factor-α-induced cytotoxicity by polyphenols. Phyther. Res. 1997, 11, 277–280. [Google Scholar] [CrossRef]
- Habtemariam, S. Catechols and quercetin reduce MTT through iron ions: A possible artefact in cell viability assay. Phyther. Res. 1995, 9, 603–605. [Google Scholar] [CrossRef]
- Acquaviva, R.; Russo, A.; Galvano, F.; Galvano, G.; Barcellona, M.L.; Li-Volti, G.; Vanella, A. cyanidin and cyanidin 3-O-beta-d-glucoside as DNA cleavage protectors and antioxidants. Cell Biol. Toxicol. 2003, 19, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Lazze, M.C.; Pizzala, R.; Savio, M.; Stivala, L.A.; Prosperi, E.; Bianchi, L. Anthocyanins protect against DNA damage induced by tert-butyl-hydroperoxide in rat smooth muscle and hepatoma cells. Mutat. Res. 2003, 535, 103–115. [Google Scholar] [CrossRef]
- Matsufuji, H.; Shibamoto, T. Inhibition of malonaldehyde formation in oxidized calf thymus DNA with synthetic and natural antioxidants. J. Agric. Food. Chem. 2004, 52, 5759–5763. [Google Scholar] [CrossRef] [PubMed]
- Serraino, I.; Dugo, L.; Dugo, P. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003, 73, 1097–1114. [Google Scholar] [CrossRef]
- Toufektsian, M.; Lorgeril, M.D.; Nagy, N. Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J. Nutr. 2008, 138, 747–752. [Google Scholar] [PubMed]
- Bendia, E.; Benedetti, A.; Baroni, G.S.; Candelaresi, C.; Macarri, G.; Trozzi, L.; Di Sario, A. Effect of cyanidin 3-O-β-glucopyranoside on hepatic stellate cell proliferation and collagen synthesis induced by oxidative stress. Dig. Liver Dis. 2005, 37, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Tahara, S.; Baba, N. Inhibition of linoleic acid hydroperoxide-induced toxicity in cultured human fibroblasts by anthocyanidins. Biosci. Biotechnol. Biochem. 2003, 67, 1391–1393. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, E.; Igarashi, K.; Kubo, K.; Molyneux, J.; Kubomura, K. Protective effects of boysenberry anthocyanins on oxidative stress in diabetic rats. Food Sci. Technol. Res. 2003, 9, 345–349. [Google Scholar] [CrossRef]
- Feshani, A.M.; Kouhsari, S.M.; Mohammadi, S. Vaccinium arctostaphylos, a common herbal medicine in Iran: Molecular and biochemical study of its antidiabetic effects on alloxan-diabetic Wistar rats. J. Ethnopharmacol. 2011, 133, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, F.; Ji, B.; Wang, R.; Yang, J.; Liu, H.; Zhou, F. Anthocyanins-rich extract of wild Chinese blueberry protects glucolipotoxicity-induced INS832/13 beta-cell against dysfunction and death. J. Food Sci. Technol. 2015, 52, 3022–3029. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMPK: A target for drugs and natural products with effects on both diabetes and cancer. Diabetes 2013, 62, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.H.; Lee, H.A.; Park, M.H.; Han, J.S. Mulberry (Morus alba L.) fruit extract containing anthocyanins improves glycemic control and insulin sensitivity via activation of AMP-activated protein kinase in diabetic C57BL/Ksj-db/db mice. J. Med. Food 2016, 19, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Talagavadi, V.; Rapisarda, P.; Galvano, F.; Pelicci, P.; Giorgio, M. Cyanidin-3-O-β-glucoside and protocatechuic acid activate AMPK/mTOR/S6K pathway and improve glucose homeostasis in mice. J. Funct. Foods 2016, 21, 338–348. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Z.; Park, J.H.; Ryu, O.H.; Choi, M.K.; Lee, J.Y. Anti-diabetic effect of purple corn extract on C57BL/KsJ db/db mice. Nutr. Res. Pract. 2015, 9, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jia, Q.; Wang, Y.; Zhang, Y.; Xia, M. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP-PKA-dependent signaling pathway. Free Rad. Biol. Med. 2012, 52, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, H-K.; Kim, J.N.; Han, S.N.; Nam, J-H.; Na, H-N.; Ha, T.J. Black soybean anthocyanins inhibit adipocyte differentiation in 3T3-L1 cells. Nutr. Res. 2012, 32, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Guo, J.; Jiang, X.; Li, Z.; Ling, W. Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: Involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem. Toxicol. 2012, 50, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Craig, R.L.; Chu, W.S.; Elbein, S.C. Retinol binding protein 4 as a candidate gene for type 2 diabetes and predi abetic intermediate traits. Mol. Genet. Metab. 2007, 90, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.M.; Youn, B.S.; Lee, H.; Lee, N.; Min, S.S.; Kwak, S.H.; Lee, H.K.; Park, K.S. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care 2006, 29, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.E.; Yang, Q.; Bluher, M.; Hammarstedt, A.; Ciaraldi, T.P.; Henry, R.R.; Wason, C.J.; Oberbach, A.; Jansson, P.A.; Smith, U.; et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 2006, 354, 2552–2563. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; de Freitas, V.; Reis, C.; Mateus, N. A new approach on the gastric absorption of anthocyanins. Food Funct. 2012, 3, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wallace, T.C.; Keatley, K.E.; Failla, M.L.; Giusti, M.M. Stability of blackraspberry anthocyanins in the digestive tract lumen and transport efficiency into gastric and small intestinal tissues in the rat. J. Agric. Food Chem. 2009, 7, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Fernandes, I.; Brás, N.F.; Faria, A.; De Freitas, V.; Calhau, C.; Mateus, N. Experimental and theoretical data on the mechanism by which red wineanthocyanins are transported through a human MKN-28 gastric cell model. J. Agric. Food Chem. 2015, 63, 7685–7692. [Google Scholar] [CrossRef] [PubMed]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Talavera, S.; Gonthier, M.P.; Texier, O.; Scalbert, A.; Lamaison, J.L.; Rémésy, C. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. J. Nutr. 2003, 133, 1296–1301. [Google Scholar] [PubMed]
- Kay, C.D.; Mazza, G.J.; Holub, B.J. Anthocyanins exist in the circulation primarily as metabolites in adult men. J. Nutr. 2005, 135, 2582–2588. [Google Scholar] [PubMed]
- Kay, C.D.; Mazza, G.; Holub, B.J.; Wang, J. Anthocyanin metabolites in human urine and serum. Br. J. Nutr. 2004, 91, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.-A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Hidalgo, M.; Sanchez-Moreno, C.; Pelaez, C.; Requena, T.; dePascual-Teresa, S. Bioconversion of anthocyanin glycosides by Bifidobacteria and Lactobacillus. Food Res. Int. 2009, 42, 1453–1461. [Google Scholar] [CrossRef]
- Forester, S.C.; Waterhouse, A.L. Identification of Cabernet sauvignon anthocyanin gut microflora metabolites. J. Agric. Food Chem. 2008, 56, 9299–9304. [Google Scholar] [CrossRef] [PubMed]
- Keppler, K.; Humpf, H.-U. Metabolism of anthocyanis and their phenolic degradation products by the intestinal microflora. Bioorg. Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef] [PubMed]

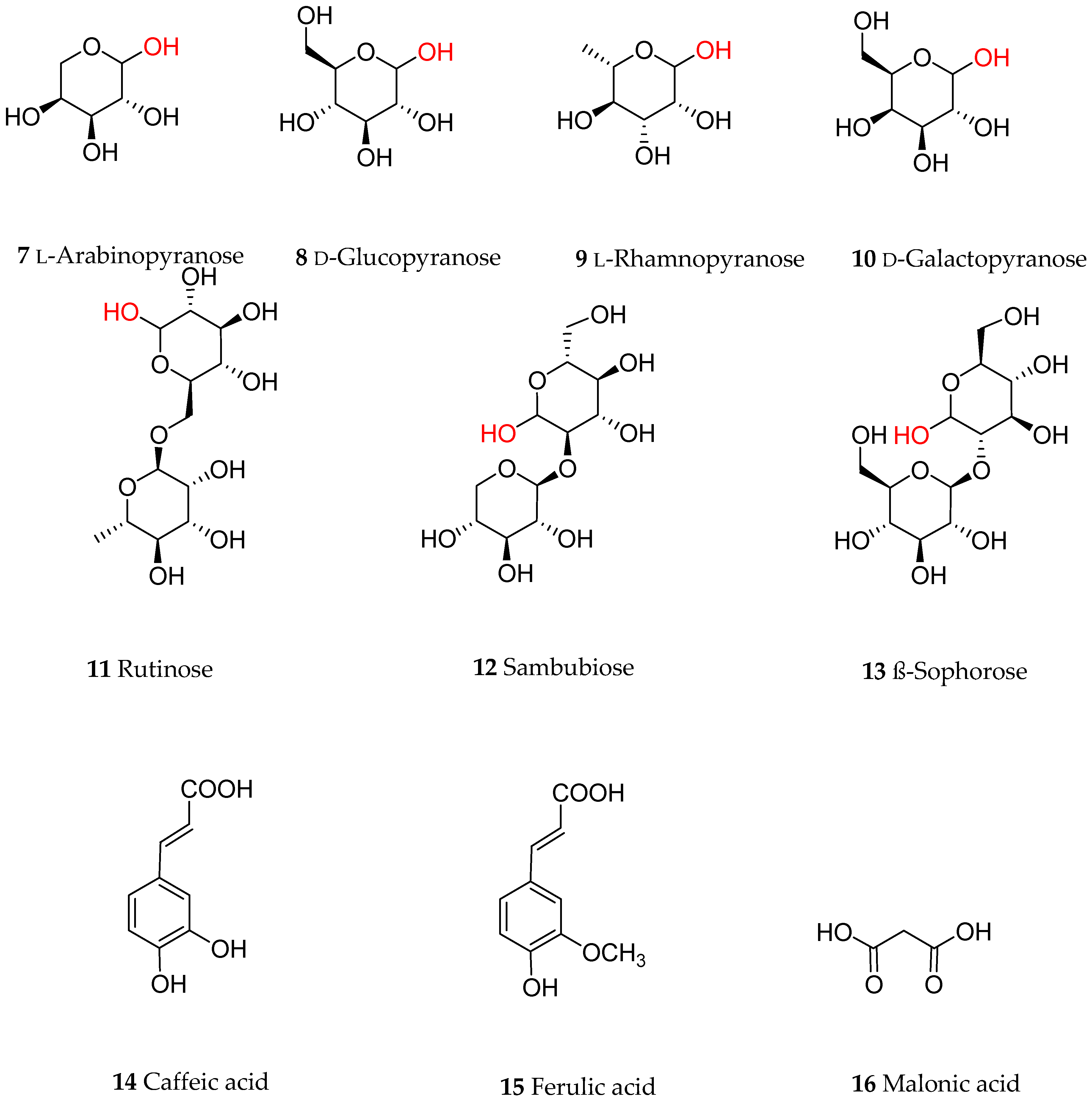
| Compound | Cyanidin Glycosides | Compound | Delphinidin, Pelargonidin and Peonidin Glycosides |
|---|---|---|---|
| 17 | Cyanidin-3-O-arabinoside | 26 | Delphinidin-3-O-galactoside |
| 18 | Cyanidin-3-O-galactoside | 27 | Delphinidin-3-O-glucoside |
| 19 | Cyanidin-3-O-glucoside | 28 | Delphinidin-3-O-sambubioside-5-O-glucoside |
| 20 | Cyanidin-3-O-glucosyl-rutinoside | 29 | Pelargonidin-3-O-galactoside |
| 21 | Cyanidin-3-O-rutinoside | 30 | Pelargonidin-3-O-glucoside3 |
| 22 | Cyanidin-3-O-sambubioside | 31 | Pelargonidin-3-O-rutinoside |
| 23 | Cyanidin-3-O-sophoroside | 32 | Pelargonidin-3-O-(2-O-(6-O-(E-3-O-(β-d-glucopyranosyl)caffeyl)-β-d-glucopyranosyl)-6-O-E-caffeoyl-β-d-glucopyranoside)-5-O-β-d-glucopyranoside |
| 24 | Cyanidin-3,5-O-diglucoside | 33 | Peonidin-3-O-rutinoside |
| 25 | Cyanidin-3-O-malonylglucoside | 34 | Peonidin-3-O-(2-O-(6-O-E-feruloyl-β-d-glucopyranosyl)-6-O-E-caffeoyl-β-d-glucopyranoside)-5-O-β-d-glucopyranoside |
| Anthocyanins | Plant Name and Part Used | In Vitro Model/Activity | References |
|---|---|---|---|
| Purified acylated anthocyanins: e.g., 32 | Morning glory (red flower) | α-Glucosidase/Enzyme inhibition | [20] |
| 6-O-Caffeoylsophorose of the diacylated anthocyanin, 34 | Fermented purple-fleshed sweet potato | α-Glucosidase/Enzyme inhibition | [21] |
| Purified acylated anthocyanin: 22 but not 19 or cyanidin (3) | Viburnum dilatatum fruits | α-Glucosidase/Enzyme inhibition | [22] |
| Anthocyanins 18, 19 and 24 | Synthetic source | α-Glucosidase and pancreatic α-amylase/Enzyme inhibition | [23] |
| Methanolic extracts and purified anthocyanins: 24 as well as cyanidin (3) | Noble Muscadine grape - whole fruit and skin | α-Glucosidase and pancreatic lipase/Enzyme inhibition | [24] |
| Anthocyanins enriched water extract | Ipomoea batatas (purple sweet potato) | α-Amylase and α-glucosidase/Enzyme inhibition | [25] |
| Aqueous ethanol (70%) extract containing 19, 21, 31 and 33 | Pulp of Sweet cherry cultivars | α-Glucosidase/Enzyme inhibition | [26] |
| Ethanol extract | Syzygium dessiflorum fruits | α-Amylase/Enzyme inhibition | [27] |
| Smoothies containing anthocyanins 19–21, 23 and 33 | Fruits of sour cherry (Prunus cerasus, Prunus persica, Prunus armeniaca and plum) | α-Amylase and α-glucosidase/Enzyme inhibition | [28] |
| Aqueous extract | Roselle (Hibiscus sabdarilfa) flowers | α-Amylase and α-glucosidase/Enzyme inhibition | [29] |
| Methanol extract | Aegle marmelos fruit pulp | α-Amylase and α-glucosidase/Enzyme inhibition; L6 rat skeletal muscle cells/Increase glucose uptake | [30] |
| Aqueous and methanol extracts | Strawberries fruits | α-Amylase and α-glucosidase/Enzyme inhibition | [31] |
| Aqueous extract | Brazilian strawberry cultivar (Fragaria x ananassa) | α-Amylase and α-glucosidase/Enzyme inhibition | [32] |
| Aqueous extracts | Red current, black current, red and green goose berries | α-Amylase and α-glucosidase/Enzyme inhibition | [33] |
| Aqueous, methanolic, and acetic acid extracts; Major component are 17–19 | black chokeberry (Aronia melanocarpa) berries | α-Amylase and lipase/Enzyme inhibition | [34] |
| Aqueous and ethyl acetate extracts | Serviceberry plant samples (leaves, twig and berries) | α-Glucosidase/Enzyme inhibition | [35] |
| Purified flavonoids including anthocyanins | Standard chemicals | α-Glucosidase/Enzyme inhibition | [36] |
| Aqueous methanol extracts containing anthocyanins (21, 24 and 31) | Fig (Ficus carica) fruits | α-Amylase and α-glucosidase/Enzyme inhibition | [37] |
| Ethanol and methanol extracts and purified anthocyanins: 19 and 22 | Elderberries (Sambucus nigra) | α-Glucosidase and α-amylase/Enzyme inhibition; skeletal muscle cells/Stimulate glucose uptake | [38] |
| Anthocyanin-rich formulation and purified anthocyanin: 28 | Maqui berry (Aristotelia chilensis) | H4IIE rat liver cells and L6 mycotubes/Decrease glucose production, increase glucose uptake and enhanced insulin stimulated downregulation of gluconeogenic enzyme, glucose-6-phosphatase | [39] |
| Aqueos extract containing 19 and 25 | Rutgers Scarlet Lettuce | H4IIE rat hepatoma cells/Inhibition of glucose production | [40] |
| Acidified ethanol extract and purified anthocyanins: 19, 21 and 30 | Mulberry (Morus alba) | HepG2 cells/Increase glucose uptake | [41] |
| Purified anthocyanin: 19 | Standard reference | Human omental adipocytes and 3T3-L1 cells/Increase glucose transport, GLUT4 membrane translocation and insulin sensitivity (insulin like activity); | [42] |
| Aqueous extract containing high concentration of acylated cyanidin and peonidin | Purple sweet potato | 3T3-L1 adipocytes/Suppress leptin secretion and expression of lipogenic and inflammatory factors; promoted lipolytic action | [43] |
| Standardized extract containing 25% anthocyanins | Bilberry | 3T3-L1 cell line/Decrease adipocyte differentiation via insulin signaling pathway | [44] |
| Fermented Juice | Lowbush blueberry fruits | Insulin sensitive cultured muscle cells and adipocytes/Stimulate glucose uptake; increase insulin sensitivity | [45] |
| Ethanol extract | Canadian lowbush blueberry (Vaccinium angustifolium) fruits | Replicating βTC-tet cells/Increase proliferation | [46] |
| Aqueous extract | Eugenia jambolana seeds and pulp | Cultured Islets of Langerhans cells of normal and diabetic rats/Stimulate insulin release | [47] |
| Purified anthocyanins: 18, 19, 27 and 29 | Cornus fruits (C. officinalis and C. mas) | Rodent pancreatic β-cells (INS-1832/13)/Increased insulin secretion and prevent insulin resistance | [48] |
| Anthocyanins * | Plant Name and Part Used | Animal Model | Anti-diabetic and/or Anti-obesity Activity | References |
|---|---|---|---|---|
| Juice | Acerola (Malpighia emarginata DC.) fruits | Diabetic Wister rats | Reduction in blood glucose level, cholesterol and triglyceride | [51] |
| Juice | Aronia melanocarpa Fruits | Diabetic rat | Lower glucose and lipid level | [52] |
| Juice | Aronia melanocarpa (black chokeberry) | Obese mice under high fat diet | Lower lipid level | [53] |
| Athocyanin fractyion of ethanol extract | Berberis integerrima fruits | STZ-induced diabetic rats | Reduce glucose level and increase glycogen | [54] |
| Juice containing 19 and 25 | Blackberries (Rubus adenotrichos Schltdl. Cv. “vino”) | STZ-induced diabetic rats | Decreases the levels of glucose, triacylglycerols and cholesterol | [55] |
| Anthocyanin rich fractions | Black rice (Oryza sativa) | Rats fed high fructose rich diet for 4 weeks | Increase plasma insulin level and insulin sensitivity; prevent insulin resistance; hypolipidemic effect | [56] |
| Anthocyanin rich preparation | Black soybean seed coat | STZ induced diabetic rats | Protect pancreatic tissue from apoptosis, regulation of glucose transport; prevent insulin resistance; hypolipidemic effect | [57] |
| Anthocyanin rich preparation | Black soybean (Glycine max L.) | Rats fed with high fat diet | Decrease body weight gain; suppress weight gain in liver, epidymal and perirenal fat pads; improve lipid profile, serum triglyceride and cholesterol level | [58] |
| Juice | Blueberries | Obese rodent fed with high fat diet | Improve insulin resistance and glucose tolerance | [59,60,61] |
| Powder diet | Blueberries | Obese and insulin resistance mice fed with 60% high fat diet and 4% blueberries | Lower plasma glucose; increase insulin sensitivity; reduce adipocyte cell death | [62] |
| Juice/Powder | Blueberries | Obese rodent | Decrease body weight gain and lipid accumulation; increase insulin sensitivity | [59,63,64] |
| Powder | Blueberries | Obese rat | Anti-obesity effect; increase glucose absorption | [65] |
| Powder | Blueberry | Obese mice fed with high fat diet | Decrease body weight and body fat accumulation | [66] |
| Powder | Blueberry | Obesity prone rat (Zucher fatty and Zucker lean) | Reduces triglycerides, fasting insulin; improve insulin sensitivity | [60] |
| Extract (unknown) | Blueberries (Vaccinium myrtillus) or Bilberries (Vaccinium cyanococcus) | Type-2 diabetic male KK-Aγ mice | Amloriate insulin sensitivity; improve diabetic condition; suppress glucose production and lipid content in the liver | [67] |
| Methanol extract and anthocyanin fraction | Blueberry (Vaccinium angustifolium) | Diabetic mice | Hypoglycemic activity | [68] |
| Powdered formulation or juice | Blueberry, Black current, Concord grape, Black raspberry and Maqui berry | Obese mice with high fat diet | Improve insulin sensitivity | [69] |
| Fermented beverage | Blueberry and blackberry | Obese mice fed with high fat diet | Reduce fasting blood glucose level; prevent obesity | [70] |
| Juice | Blueberry and Mulberry | Obese mice fed with high fat diet | Decrease body weight gain and serum cholesterol level; reduce insulin resistance, lipid accumulation and leptin secretion | [64] |
| Powder | Blueberry (Vaccinium ashei) | Obese male mice under high fat diet | Decrease serum glucose; improve lipid profile | [71] |
| Powder | Blueberry | Female mice fed with high fat diet | Supplement prevent glucose and insulin tolerance in obese post-menopausal mice | [61] |
| Spraydried (CellBerry®) | Chokeberry | Rats fed with high fructose-rich diet | Reduce weight gain; modulate insulin, adipogenic and inflammatory signaling pathways | [72] |
| Purified anthocyanins: 18, 29 and 26 | Cornelian cherry (Cornus mas) | Obese and insulin resistance mice fed with high fat diet | Decrease body weight and accumulation of lipids and triglyceride in the liver; increase insulin level; preserve islet architecture | [73] |
| Ethanol extract and purified anthocyanin: derivatives of pelargonidin glycoside | Bark of Ficus benghalensis Linn. | Alloxan-induced diabetic dogs and rats | Hypoglycemic effect; stimulate insulin secretion | [74,75] |
| Freeze-dried powder and crude extract preparations | Gamazumi (Viburnum dilatatum) | STZ-induced hyperglycemic rats | Decrease plasma glucose level; antioxidant activity | [76] |
| Freeze dried Jabuticaba peel containing 19 and 27 | Jabuticaba (Myrciaria spp.) peel | Rats under high fat diet | Increase glucose and insulin tolerance; reduce serum insulin resistance; increase HDL level | [77] |
| Anthocyanin fraction from aqueous methanol (70%) extract 28 | Maqui berry (Aristotelia chilensis) | Hyperglycemic obese mice fed high fat diet | Improve fasting blood glucose level and glucose tolerance | [39] |
| Aqueous ethanol (50%) extract containing 1, 19 and 21 | Morus alba fruits | Zucker diabetic fatty rats | Decrease glucose level; maintain insulin level and β cell histology | [78] |
| Juice predominantly containg 19 and 21 | Morus australis fruits | Obese and insulin resistance mice fed with high fat diet | Suppress weight gain and insulin resistance; attenuate lipid accumulation; lower the size of adipocytes | [64] |
| Aqueous ethanol (70%) and the ethyl acetate fraction | Mulberry (Morus alba L.) fruit | STZ induced diabetic mice | Hypoglycemic effect | [79] |
| Aqueous extract | Mulberry (Morus alba L.) fruits | Male Syrian golden hamster | Prevent obesity; reduce hepatic lipogenesis, body weight gain and fat accumulation | [80] |
| Acidified ethanol extract containing 19 and 20 | Mulberry (Morus alba L.) fruit | db/db diabetic mice | -cell protection; reverse insulin resistance | [41] |
| Aqueous ethanol (70%) extract | Mulberry root bark | STZ induced diabetic rats | Reduces serum glucose and lipid peroxides; increased insulin level | [81] |
| Concentrated juice | Plums | Wistar fatty rats | Reduce blood glucose; increase insulin sensitivity | [82] |
| Methanol extract | Pomegranate (Punica granatum Linn.) flowers | Zucker diabetic fatty rats | Decreases plasma glucose level | [83,84] |
| Powder rich in 19 | Purple corn | High fat diet induced insulin resistance mice | Reverse insulin resistance; suppress weight gain and hypertrophy of adipocytes | [85] |
| Purified anthocyanin: 19 | Purple corn | Diabetic KKA-γ mice | Reduces blood glucose level; enhance insulin sensitivity | [86] |
| Purified anthocyanin: 34 | Purple sweet potato | Male Sprague Dawley rats | Increase plasma insulin sensitivity; decrease α-glucosidase activity | [87] |
| Aqueous alcohol extracts containing 25 | Rutgers Scarlet Lettuce | High fat diet induced obese mice | Improve glucose metabolism; decrease total liver lipid | [40] |
| Aqueos alcohol extract | Sapindus mukorossi fruits | STZ induced diabetic rats | Decrease blood glucose level and lipid level | [88] |
| Aqueous and ethyl acetate extracts | Serviceberry plant samples (leaves, twig and berries) | Diet induced obese and hyperglycemic mice | Lower blood glucose; delay absorption of carbohydrate; inhibit intestinal α-glucosidase activity | [35] |
| Purified anthocyanin: 20, 21 and 31 | Sweet cherry (Prunus avium) | Male mice fed with high fat diet | Prevent body weight gain; reduces size of adipocytes; decrease leptin secretion, serum glucose triglyceride, total cholesterol and low density lipoprotein | [89] |
| Anthocyanin-enriched juices and purified 19 | Sweet orange (Citrus sinensis), Moro (a blood orange) | Obese mice fed with high fat diet | Improve glucose tolerance, insulin sensitivity; reduce hepatic accumulation of lipid | [90] |
| Freeze-dried | Tart cherries | Male rats | Decrease fasting glucose level; increase plasma insulin level | [91] |
| Clinical Trial Identifier No. | Objective | Voluntary and Dose | Status |
|---|---|---|---|
| NCT01245270 | Single supplement of standardized bilberry extract (36% w/w anthocyanins) modifies glycemic response in persons with type-2 diabetes controlled by diet and lifestyle | 8 male patients of age between 40 and 70 years with type-2 diabetes given a single oral capsule of 0.47 g standardized blueberry extract followed by a polysaccharide drink in a double blind cross over intervention | Completed |
| NCT01005420 | The effect of anthocyanins in the form of blueberry powder on enhancing insulin sensitivity in insulin resistant and obese human | 37 male and female of age 20 years and older taking 45 g of blueberry powder per day as a smoothie | Completed |
| NCT02689765 | Effect of purified anthocyanins from bilberries and black currant on insulin resistance, glucose and lipid metabolism disorders | 160 humans both male and female with type-2 diabetes of age 40–75 years taking two 80 mg anthocyanin capsule twice a day for 24 weeks | Completed |
| NCT 01883401 | Investigate the effect of low and high doses of freeze-dried strawberries in cardiovascular risk factor in subjects with abnormal adiposity and dyslipidemia | 60 male and female patients of age between 19 and 72 years were given 25 and 50 g of dried strawberries | Completed |
| NCT 02340039 | The acute effect of black currant and apple extract on postprandial glycemia | 34 male and female patients of age between 20 and 60 years were given 600 mg of black current anthocyanins and 600 mg of apple polyphenols | Completed |
| NCT 02650726 | Effect of purified anthocyanins on high density lipoprotein and endothelial function in subjects with type-2 diabetes | 80 male and female patients of age between 40 and 60 years were given daily dose of 320 mg anthocyanin for 24 weeks in a randomized double blinded placebo-controlled trial | Completed |
| NCT 01053793 | A trial to measure the glycemic index and polyphenol bioavailability of four different varieties of potato | 10 male and female patients of age between 18 and 50 years were given 50 g of cooked purple, red, yellow and white potatoes | Completed |
| NCT 02317211 | Effect of purified anthocyanins on oxidative stress and glycemic control in subjects with type-2 diabetes | 70 patients of age between 25 and 65 years were given 320 mg anthocyanin daily for 12 weeks in a randomized double blinded placebo-controlled trial | Completed |
| NCT 01720511 | Pilot study of the effect of purple rice on glucose tolerance, serum lipid and inflammation | 10 male and female patients of age 18 years and above were given one cup of rice into their dishes and consumed at lunch and dinner each day for 4 weeks (equivalent to 4 ounces of uncooked rice/day) | Completed |
| NCT O2035592 | Dose dependent impact of blueberry powder intake on insulin sensitivity and resistance | 144 male and female of age 50–74 years | Active |
| NCT 02291250 | Effect of black currant containing anthocyanins on glucose metabolism | 16 obese male female of 21–70 age | Recruiting |
| NCT02972996 | Effect of blueberry consumption on cardiometabolic prevention in type-2 diabetes patients | 48 male patients of age between 45 and 75 years were given 22 g of blueberry powder in a randomized experiment | Recruiting |
| NCT 03213288 | The effect of bilberry fruit and black rice derived anthocyanins on lipid status in adults | 50 male and female patients of age 45 years and older were given 320 mg of anthocyanins for 28 days | Recruiting |
| NCT 02940080 | Effect of anthocyanins extracted from purple potatoes on healthy study subjects Postprandial glycaemia and insulinemia | 20 male patients of age between 18 and 45 years were given 168 mg of anthocyanins extracted from purple-flashed potatoes added to steam-cooked mashed potatoes in water | Recruiting |
| NCT 02291250 | Effect of soft fruit on postprandial blood glucose | 16 overweight male and female patients of age between 21 and 70 years were given black current (200 g) and green currant (200 g) | Recruiting |
| NCT01180712 | Study of oral anthocyanins on insulin resistance | 60 obese type-2 diabetic males of age 40–70 years taking 1.4 g of concentrate blueberry extract in a hard gelatin capsule administered thrice a day for 21 days | Recruiting |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belwal, T.; Nabavi, S.F.; Nabavi, S.M.; Habtemariam, S. Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine. Nutrients 2017, 9, 1111. https://doi.org/10.3390/nu9101111
Belwal T, Nabavi SF, Nabavi SM, Habtemariam S. Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine. Nutrients. 2017; 9(10):1111. https://doi.org/10.3390/nu9101111
Chicago/Turabian StyleBelwal, Tarun, Seyed Fazel Nabavi, Seyed Mohammad Nabavi, and Solomon Habtemariam. 2017. "Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine" Nutrients 9, no. 10: 1111. https://doi.org/10.3390/nu9101111