Vitamin D and Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Literature Review Method
3. Vitamin D Function and Health
3.1. Source, Metabolism, and Storage
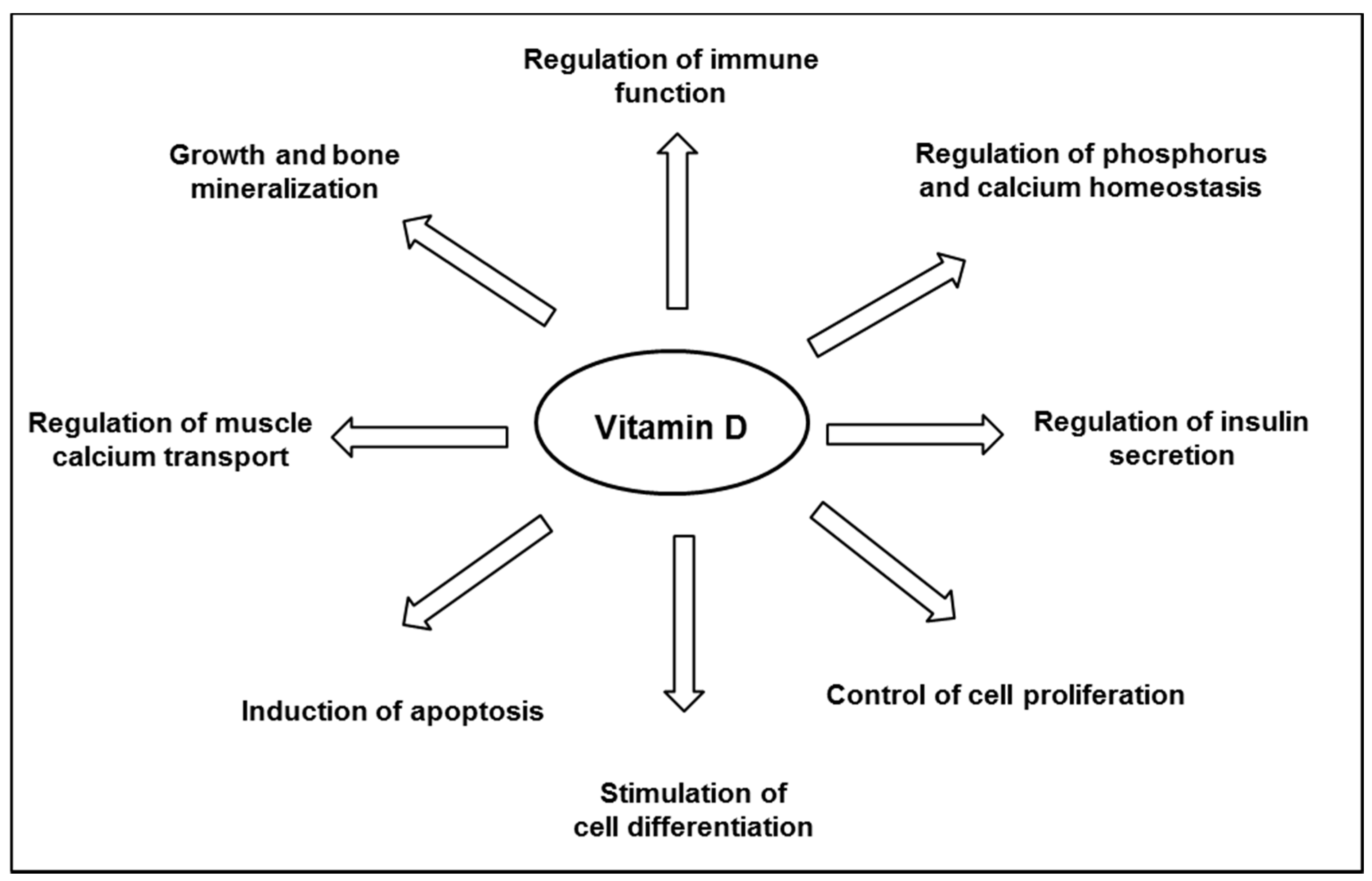
3.2. Mode of Action
3.3. Association between Vitamin D and Health Outcomes
4. The Links between Vitamin D and AMD
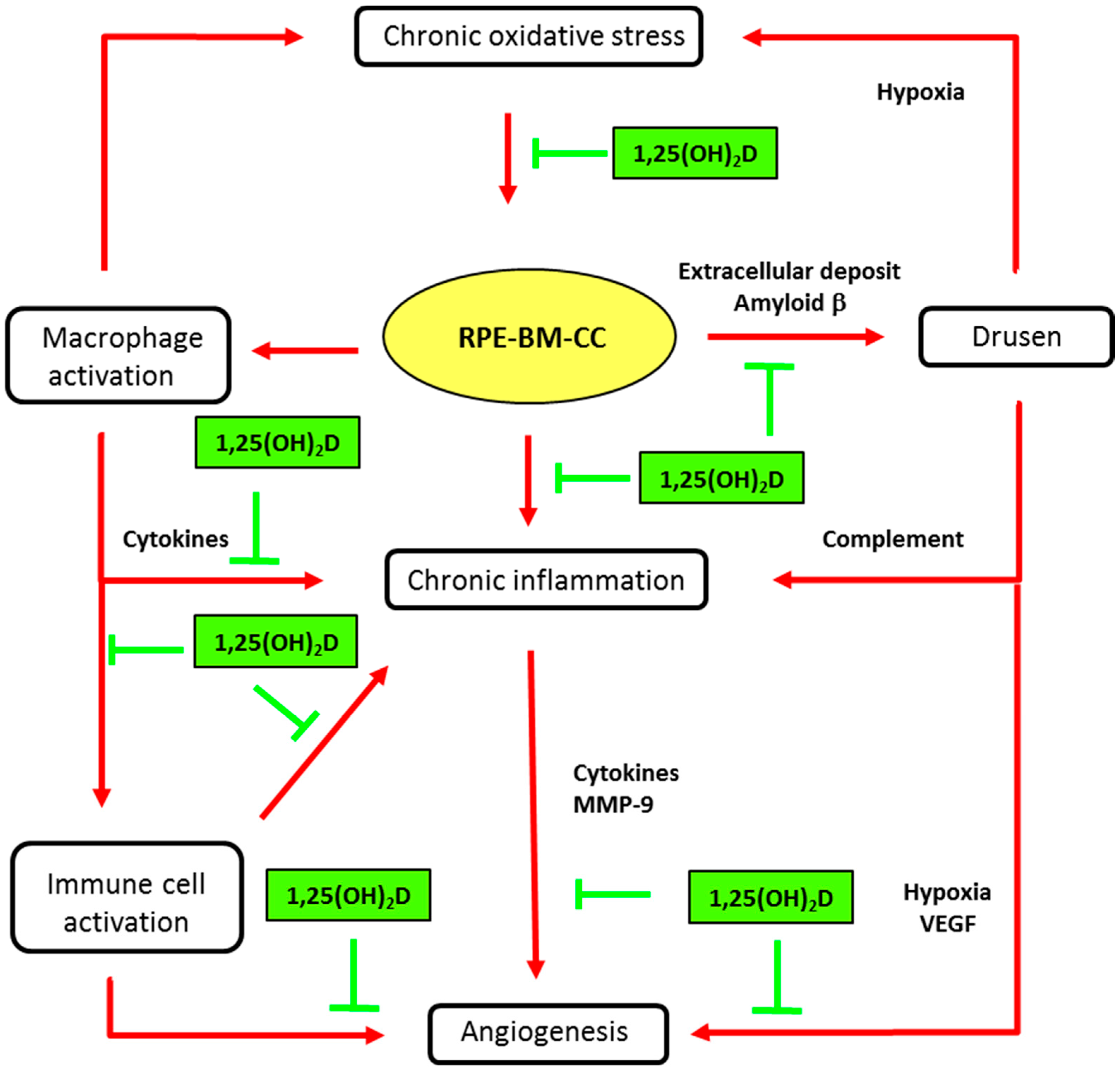
4.1. Vitamin D and AMD Pathophysiology
4.1.1. Inhibition of Chronic Oxidative Stress
4.1.2. Inhibition of Amyloid Beta Protein Deposits
4.1.3. Inhibition of Chronic Inflammation
4.1.4. Vitamin D and Angiogenesis in AMD
4.2. Vitamin D Status and AMD
4.2.1. Association between Vitamin D Plasma Levels and AMD
4.2.2. Relationship between Dietary Vitamin D and AMD
4.2.3. Genetic Link between Vitamin D and AMD
5. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Bourne, R.R.; Jonas, J.B.; Flaxman, S.R.; Keeffe, J.; Leasher, J.; Naidoo, K.; Parodi, M.B.; Pesudovs, K.; Price, H.; White, R.A.; et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010. Br. J. Ophthalmol. 2014, 98, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration-emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hartnett, M.E. Regulation of signaling events involved in the pathophysiology of neovascular AMD. Mol. Vis. 2016, 22, 189–202. [Google Scholar] [PubMed]
- Sobrin, L.; Seddon, J.M. Nature and nurture-genes and environment-predict onset and progression of macular degeneration 2014. Prog. Retin. Eye Res. 2014, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Chou, C.F.; Klein, B.E.; Zhang, X.; Meuer, S.M.; Saaddine, J.B. Prevalence of age-related macular degeneration in the US population. Arch. Ophthalmol. 2011, 129, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Ajani, U.A.; Mitchell, B.D. Familial aggregation of age-related maculopathy. Am. J. Ophthalmol. 1997, 123, 199–206. [Google Scholar] [CrossRef]
- Seddon, J.M.; Cote, J.; Page, W.F.; Aggen, S.H.; Neale, M.C. The US twin study of age-related macular degeneration: Relative roles of genetic and environmental influences. Arch. Ophthalmol. 2005, 123, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Yonekawa, Y.; Miller, J.W.; Kim, I.K. Age-related macular degeneration: advances in management and diagnosis. J. Clin. Med. 2015, 4, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W. Age-related macular degeneration revisited—Piecing the puzzle: The LXIX Edward Jackson memorial lecture. Am. J. Ophthalmol. 2013, 155, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman initiative for macular research classification committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Lawrenson, J.G. A review of the evidence for dietary interventions in preventing or slowing the progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2014, 34, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Broadhead, G.K.; Grigg, J.R.; Chang, A.A.; McCluskey, P. Dietary modification and supplementation for the treatment of age-related macular degeneration. Nutr. Rev. 2015, 73, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew, E.Y.; Clemons, T.E.; Sangiovanni, J.P.; Danis, R.P.; Ferris, F.L., 3rd; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Tohari, A.M.; Zhou, X.; Shu, X. Protection against oxidative stress by vitamin D in cone cells. Cell Biochem. Funct. 2016, 34, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Han, K.; Jee, D. Inverse relationship between high blood 25-hydroxyvitamin D and late stage of age-related macular degeneration in a representative Korean population. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4823–4831. [Google Scholar] [CrossRef] [PubMed]
- Parekh, N.; Chappell, R.J.; Millen, A.E.; Albert, D.M.; Mares, J.A. Association between vitamin D and age-related macular degeneration in the third national health and nutrition examination survey, 1988 through 1994. Arch. Ophthalmol. 2007, 125, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678S–1688S. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Pramyothin, P.; Biancuzzo, R.M.; Lu, Z.; Hess, D.T.; Apovian, C.M.; Holick, M.F. Vitamin D in adipose tissue and serum 25-hydroxyvitamin D after roux-en-Y gastric bypass. Obesity 2011, 19, 2228–2234. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Zerwekh, J.E. Blood biomarkers of vitamin D status. Am. J. Clin. Nutr. 2008, 87, 1087S–1091S. [Google Scholar] [PubMed]
- Adams, J.S.; Hewison, M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch. Biochem. Biophys. 2012, 523, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C. Rapid, membrane-initiated actions of 1,25 dihydroxyvitamin D: What are they and what do they mean? J. Nutr. 2004, 134, 3215–3218. [Google Scholar] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.C.; Xu, H.; El-Tanani, M.; Crowe, P.; Bingham, V. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: Operational networks and tissue-specific growth control. Biochem. Pharmacol. 2010, 79, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goltzman, D.; Hendy, G.N.; White, J.H. Vitamin D and its receptor during late development. Biochim. Biophys. Acta 2015, 1849, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Adamopoulos, C.; Basdra, E.K.; Papavassiliou, A.G. Role of vitamin D in atherosclerosis. Circulation 2013, 128, 2517–2531. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Grande, J.P.; Roche, P.C.; Campbell, R.J.; Kumar, R. Immuno-localization of the calcitriol receptor, calbindin-D28k and the plasma membrane calcium pump in the human eye. Curr. Eye Res. 1995, 14, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Spira, A.; Holick, M.F. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: A randomized double-blind clinical trial. PLoS ONE 2013, 8, e58725. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; van Schoor, N.M. The effect of vitamin D on bone and osteoporosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Body, J.J.; Bergmann, P.; Boonen, S.; Devogelaer, J.P.; Gielen, E.; Goemaere, S.; Kaufman, J.M.; Rozenberg, S.; Reginster, J.Y. Extraskeletal benefits and risks of calcium, vitamin D and anti-osteoporosis medications. Osteoporos. Int. 2012, 23 (Suppl. 1), S1–S23. [Google Scholar] [CrossRef] [PubMed]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Scientific Advisory Committee on Nutrition. Vitamin D and Health. Avaliable online: https://www.gov.uk/government/publications/sacn-vitamin-d-and-health-report (accessed on 26 September 2017).
- Morrison, M.A.; Silveira, A.C.; Huynh, N.; Jun, G.; Smith, S.E.; Zacharaki, F.; Sato, H.; Loomis, S.; Andreoli, M.T.; Adams, S.M.; et al. Systems biology-based analysis implicates a novel role for vitamin D metabolism in the pathogenesis of age-related macular degeneration. Hum. Genom. 2011, 5, 538–568. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.; Appukuttan, B.; Binek, S.J.; Planck, S.R.; Stout, J.T.; Rosenbaum, J.T.; Smith, J.R. Prediction of cis-regulatory elements controlling genes differentially expressed by retinal and choroidal vascular endothelial cells. J. Ocul. Biol. Dis. Inform. 2008, 1, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Alsalem, J.A.; Patel, D.; Susarla, R.; Coca-Prados, M.; Bland, R.; Walker, E.A.; Rauz, S.; Wallace, G.R. Characterization of vitamin D production by human ocular barrier cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2140–2147. [Google Scholar] [CrossRef] [PubMed]
- Parmeggiani, F.; Romano, M.R.; Costagliola, C.; Semeraro, F.; Incorvaia, C.; D’Angelo, S.; Perri, P.; De Palma, P.; De Nadai, K.; Sebastiani, A. Mechanism of inflammation in age-related macular degeneration. Mediat. Inflamm. 2012, 2012, 546786. [Google Scholar] [CrossRef] [PubMed]
- Kinnunen, K.; Petrovski, G.; Moe, M.C.; Berta, A.; Kaarniranta, K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012, 90, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Vaishnav, A.; Murillo, G.; Alimirah, F.; Torres, K.E.; Mehta, R.G. Protection against cellular stress by 25-hydroxyvitamin D3 in breast epithelial cells. J. Cell Biochem. 2010, 110, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Diker-Cohen, T.; Koren, R.; Ravid, A. Programmed cell death of stressed keratinocytes and its inhibition by vitamin D: The role of death and survival signaling pathways. Apoptosis 2006, 11, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ohno-Matsui, K.; Ichinose, S.; Sato, T.; Iwata, N.; Saido, T.C.; Hisatomi, T.; Mochizuki, M.; Morita, I. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J. Clin. Investig. 2005, 115, 2793–2800. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ohno-Matsui, K.; Morita, I. Elevated amyloid β production in senescent retinal pigment epithelium, a possible mechanism of subretinal deposition of amyloid β in age-related macular degeneration. Biochem. Biophys. Res. Commun. 2012, 423, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Talaga, K.; Rivest, A.; Barron, E.; Hageman, G.; Johnson, L. Characterization of beta amyloid assemblies in drusen: The deposits associated with aging and age-related macular degeneration. Exp. Eye Res. 2004, 78, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer's disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Ding, J.D.; Lin, J.; Mace, B.E.; Herrmann, R.; Sullivan, P.; Bowes Rickman, C. Targeting age-related macular degeneration with Alzheimer’s disease based immunotherapies: Anti-Amyloid-Beta antibody attenuates pathologies in an age-related macular degeneration mouse model. Vis. Res. 2008, 48, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Rekhi, E.; Hoh Kam, J.; Jeffery, G. Vitamin D rejuvenates aging eyes by reducing inflammation, clearing amyloid beta and improving visual function. Neurobiol. Aging 2012, 33, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.; Mihai, D.M.; Washington, I. Morphological and physiological retinal degeneration induced by intravenous delivery of vitamin A dimers in rabbits. Dis. Model. Mech. 2015, 8, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Khandhadia, S.; Cipriani, V.; Yates, J.R.; Lotery, A.J. Age-related macular degeneration and the complement system. Immunobiology 2012, 217, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Muckersie, E.; Forrester, J.V.; Xu, H. Immune activation in retinal aging: A gene expression study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5888–5896. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Helming, L.; Böse, J.; Ehrchen, J.; Schiebe, S.; Frahm, T.; Geffers, R.; Probst-Kepper, M.; Balling, R.; Lengeling, A. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood 2005, 106, 4351–4358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Ohno-Matsui, K.; Yoshida, T.; Shimada, N.; Ichinose, S.; Sato, T.; Mochizuki, M.; Morita, I. Amyloid-beta up-regulates complement factor B in retinal pigment epithelial cells through cytokines released from recruited macrophages/microglia: Another mechanism of complement activation in age-related macular degeneration. J. Cell Physiol. 2009, 220, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, G.; Niven, J.; Forrester, J.V.; Crane, I.J. Retinal pigment epithelial cell apoptosis is influenced by a combination of macrophages and soluble mediators present in age-related macular degeneration. Curr. Eye Res. 2016, 41, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Camelo, S. Potential sources and roles of adaptive immunity in age-related macular degeneration: Shall we rename AMD into autoimmune macular disease? Autoimmune Dis. 2014, 2014, 532487. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wei, L.; Meyerle, C.; Tuo, J.; Sen, H.N.; Li, Z.; Chakrabarty, S.; Agron, E.; Chan, C.C.; Klein, M.L.; et al. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. J. Transl. Med. 2011, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Pantalena, L.C.; Liu, X.K.; Gaffen, S.L.; Liu, H.; Rohowsky-Kochan, C.; Ichiyama, K.; Yoshimura, A.; Steinman, L.; Christakos, S.; et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.; Weinreb, R.N. Ophthalmic drug discovery: Novel targets and mechanisms for retinal diseases and glaucoma. Nat. Rev. Drug Discov. 2012, 11, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, E.; Geirsdottir, A.; Sigurdsson, H. Metabolic physiology in age related macular degeneration. Prog. Retin. Eye Res. 2011, 30, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Majewski, S.; Skopinska, M.; Marczak, M.; Szmurlo, A.; Bollag, W.; Jablonska, S. Vitamin D3 is a potent inhibitor of tumor cell-induced angiogenesis. J. Investig. Dermatol. Symp. Proc. 1996, 1, 97–101. [Google Scholar] [PubMed]
- Ben-Shoshan, M.; Amir, S.; Dang, D.T.; Dang, L.H.; Weisman, Y.; Mabjeesh, N.J. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol. Cancer Ther. 2007, 6, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Arjamaa, O.; Nikinmaa, M.; Salminen, A.; Kaarniranta, K. Regulatory role of HIF-1alpha in the pathogenesis of age-related macular degeneration (AMD). Ageing Res. Rev. 2009, 8, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Mantell, D.J.; Owens, P.E.; Bundred, N.J.; Mawer, E.B.; Canfield, A.E. 1alpha,25-dihydroxyvitamin D(3) inhibits angiogenesis in vitro and in vivo. Circ. Res. 2000, 87, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.M.; Scheef, E.A.; Wang, S.; Mehraein, F.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Calcitriol is a potent inhibitor of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Bahar-Shany, K.; Ravid, A.; Koren, R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J. Cell Physiol. 2010, 222, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Steen, B.; Sejersen, S.; Berglin, L.; Seregard, S.; Kvanta, A. Matrix metalloproteinases and metalloproteinase inhibitors in choroidal neovascular membranes. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2194–2200. [Google Scholar]
- Nita, M.; Strzalka-Mozik, B.; Grzybowski, A.; Mazurek, U.; Romaniuk, W. Age-related macular degeneration and changes in the extracellular matrix. Med. Sci. Monit. 2014, 20, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Drouet, M.; Duval, G.T.; Paré, P.Y.; Leruez, S.; Dinomais, M.; Milea, D. Circulating vitamin D concentration and age-related macular degeneration: Systematic review and meta-analysis. Maturitas 2016, 88, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Weng, Y.; Guo, X.; Feng, L.; Xia, H.; Jiang, Z.; Lou, J. The association between serum vitamin D levels and age-related macular degeneration: A systematic meta-analytic review. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2168–2177. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Voland, R.; Sondel, S.A.; Parekh, N.; Horst, R.L.; Wallace, R.B.; Hageman, G.S.; Chappell, R.; Blodi, B.A.; Klein, M.L.; et al. Vitamin D status and early age-related macular degeneration in postmenopausal women. Arch. Ophthalmol. 2011, 129, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Itty, S.; Day, S.; Lyles, K.W.; Stinnett, S.S.; Vajzovic, L.M.; Mruthyunjaya, P. Vitamin D deficiency in neovascular versus nonneovascular age-related macular degeneration. Retina 2014, 34, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Golan, S.; Shalev, V.; Treister, G.; Chodick, G.; Loewenstein, A. Reconsidering the connection between vitamin D levels and age-related macular degeneration. Eye 2011, 25, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Day, S.; Acquah, K.; Platt, A.; Lee, P.P.; Mruthyunjaya, P.; Sloan, F.A. Association of vitamin D deficiency and age-related macular degeneration in medicare beneficiaries. Arch. Ophthalmol. 2012, 130, 1070–1071. [Google Scholar] [CrossRef] [PubMed]
- Cougnard-Grégoire, A.; Merle, B.M.; Korobelnik, J.F.; Rougier, M.B.; Delyfer, M.N.; Féart, C.; Le Goff, M.; Dartigues, J.F.; Barberger-Gateau, P.; Delcourt, C. Vitamin D deficiency in community-dwelling elderly is not associated with age-related macular degeneration. J. Nutr. 2015, 145, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight, ultraviolet radiation, vitamin D and skin cancer: How much sunlight do we need? Adv. Exp. Med. Biol. 2014, 810, 1–16. [Google Scholar] [PubMed]
- Seddon, J.M.; Reynolds, R.; Shah, H.R.; Rosner, B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: epigenetic implications. Ophthalmology 2011, 118, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Aoki, A.; Inoue, M.; Nguyen, E.; Obata, R.; Kadonosono, K.; Shinkai, S.; Hashimoto, H.; Sasaki, S.; Yanagi, Y. Dietary n-3 fatty acid, α-Tocopherol, Zinc, vitamin D, vitamin C, and β-carotene are associated with age-related macular degeneration in Japan. Sci. Rep. 2016, 6, 20723. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bedell, M.; Zhang, K. Age-related macular degeneration: Genetic and environmental factors of disease. Mol. Interv. 2010, 10, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Meyers, K.J.; Liu, Z.; Engelman, C.D.; Wallace, R.B.; LeBlanc, E.S.; Tinker, L.F.; Iyengar, S.K.; Robinson, J.G.; Sarto, G.E.; et al. Association between vitamin D status and age-related macular degeneration by genetic risk. JAMA Ophthalmol. 2015, 133, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Camp, N.J.; Sun, H.; Tong, Z.; Gibbs, D.; Cameron, D.J.; Chen, H.; Zhao, Y.; Pearson, E.; Li, X.; et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science 2006, 314, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Pahl, L.; Schubert, S.; Skawran, B.; Sandbothe, M.; Schmidtke, J.; Stuhrmann, M. 1,25-Dihydroxyvitamin D decreases HTRA1 promoter activity in the rhesus monkey—A plausible explanation for the influence of vitamin D on age-related macular degeneration? Exp. Eye Res. 2013, 116, 234–239. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Layana, A.G.; Minnella, A.M.; Garhöfer, G.; Aslam, T.; Holz, F.G.; Leys, A.; Silva, R.; Delcourt, C.; Souied, E.; Seddon, J.M. Vitamin D and Age-Related Macular Degeneration. Nutrients 2017, 9, 1120. https://doi.org/10.3390/nu9101120
Layana AG, Minnella AM, Garhöfer G, Aslam T, Holz FG, Leys A, Silva R, Delcourt C, Souied E, Seddon JM. Vitamin D and Age-Related Macular Degeneration. Nutrients. 2017; 9(10):1120. https://doi.org/10.3390/nu9101120
Chicago/Turabian StyleLayana, Alfredo Garcia, Angelo Maria Minnella, Gerhard Garhöfer, Tariq Aslam, Frank G. Holz, Anita Leys, Rufino Silva, Cécile Delcourt, Eric Souied, and Johanna M. Seddon. 2017. "Vitamin D and Age-Related Macular Degeneration" Nutrients 9, no. 10: 1120. https://doi.org/10.3390/nu9101120




