Intrauterine Zn Deficiency Favors Thyrotropin-Releasing Hormone-Increasing Effects on Thyrotropin Serum Levels and Induces Subclinical Hypothyroidism in Weaned Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. TRH Content in Median Eminence (ME)
2.3. PPII Specific Activity
2.4. Protein Determination
2.5. Serum Hormones’ Determination
2.6. Statistical Analysis
3. Results
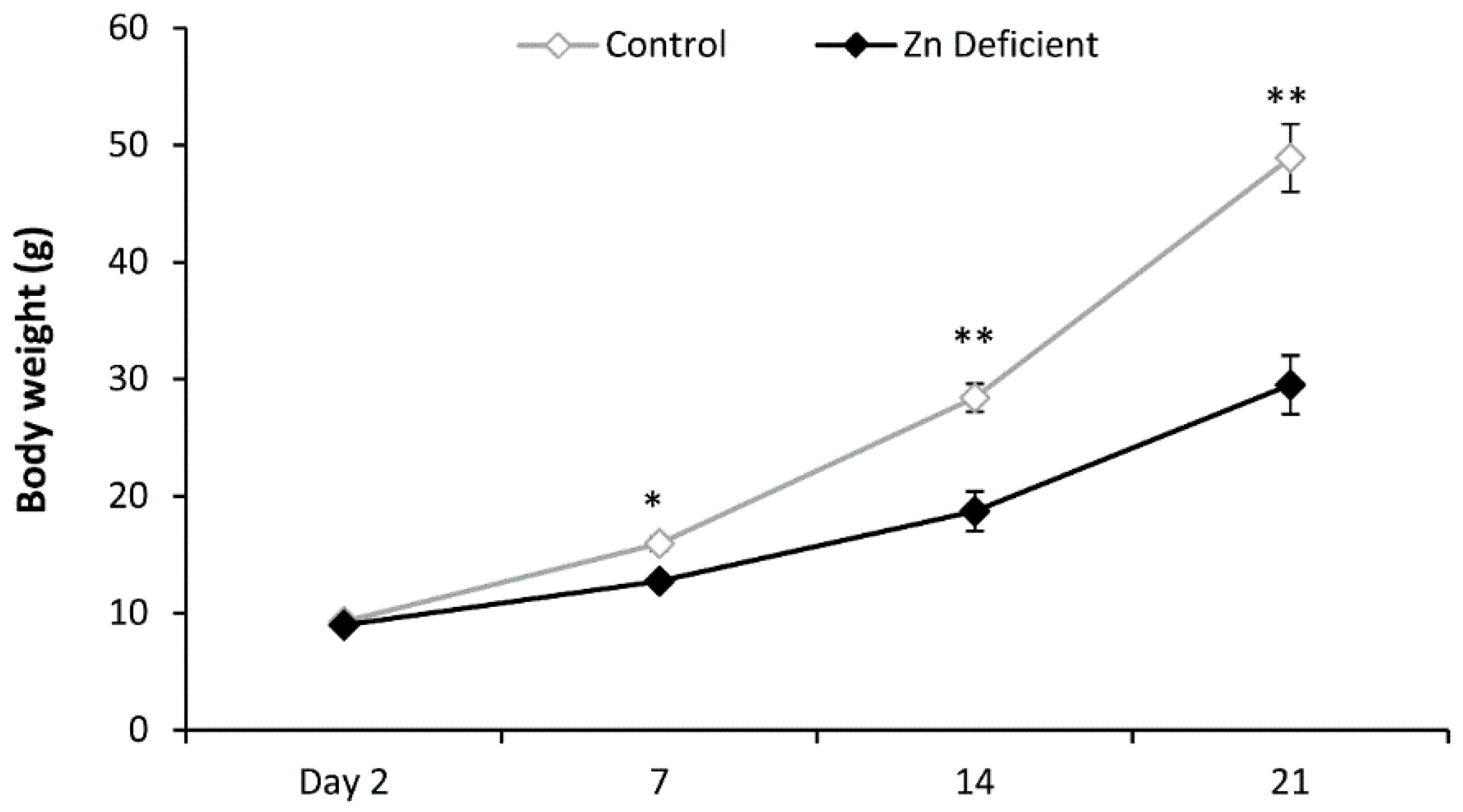
3.1. Body Weight
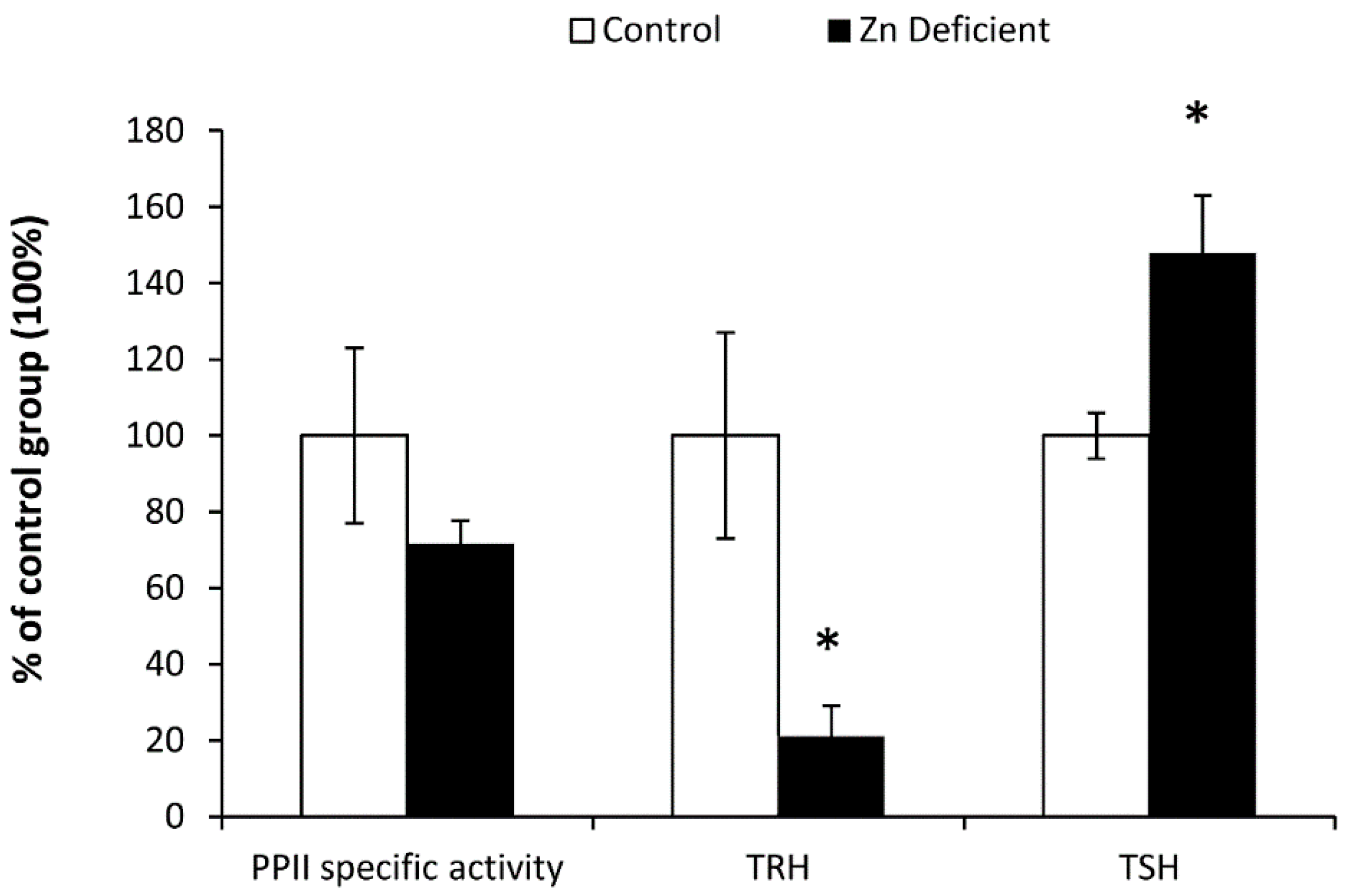
3.2. Biochemical Determinations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Duque, X.; Flores-Hernandez, S.; Flores-Huerta, S.; Mendez-Ramirez, I.; Munoz, S.; Turnbull, B.; Martinez-Andrade, G.; Ramos, R.I.; Gonzalez-Unzaga, M.; Mendoza, M.E.; et al. Prevalence of anemia and deficiency of iron, folic acid, and zinc in children younger than 2 years of age who use the health services provided by the Mexican Social Security Institute. BMC Public Health 2007, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Villalpando, S.; Garcia-Guerra, A.; Ramirez-Silva, C.I.; Mejia-Rodriguez, F.; Matute, G.; Shamah-Levy, T.; Rivera, J.A. Iron, zinc and iodide status in Mexican children under 12 years and women 12–49 years of age. A probabilistic national survey. Salud Publica Mex. 2003, 45, S520–S529. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, L.E.; Zavaleta, N.; Shankar, A.H.; Merialdi, M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am. J. Clin. Nutr. 1998, 68, 499S–508S. [Google Scholar] [PubMed]
- Shapira, N. Prenatal nutrition: A critical window of opportunity for mother and child. Womens Health 2008, 4, 639–656. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, J.R.; Supasai, S.; Kha, J.; Vaeth, B.M.; Mackenzie, G.G.; Adamo, A.M.; Oteiza, P.I. Gestational marginal zinc deficiency impaired fetal neural progenitor cell proliferation by disrupting the ERK1/2 signaling pathway. J. Nutr. Biochem. 2015, 26, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Golub, M.S.; Gershwin, M.E.; Hurley, L.S.; Saito, W.Y.; Hendrickx, A.G. Studies of marginal zinc deprivation in rhesus monkeys. IV. Growth of infants in the first year. Am. J. Clin. Nutr. 1984, 40, 1192–1202. [Google Scholar] [PubMed]
- Kralik, A.; Eder, K.; Kirchgessner, M. Influence of zinc and selenium deficiency on parameters relating to thyroid hormone metabolism. Horm. Metab. Res. 1996, 28, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Amani, R.; Saeidi, S.; Nazari, Z.; Nematpour, S. Correlation between dietary zinc intakes and its serum levels with depression scales in young female students. Biol. Trace Elem. Res. 2010, 137, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.J. Decreased zinc and increased copper in individuals with anxiety. Nutr. Metab. Insights 2011, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hagmeyer, S.; Haderspeck, J.C.; Grabrucker, A.M. Behavioral impairments in animal models for zinc deficiency. Front. Behav. Neurosci. 2014, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Seven, M.; Basaran, S.Y.; Cengiz, M.; Unal, S.; Yuksel, A. Deficiency of selenium and zinc as a causative factor for idiopathic intractable epilepsy. Epilepsy Res. 2013, 104, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Palka, G.; Lio, S.; Bucci, I.; De, R.P.; Stuppia, L.; Monaco, F. Is zinc deficiency a cause of subclinical hypothyroidism in Down syndrome? Ann. Genet. 1990, 33, 9–15. [Google Scholar] [PubMed]
- Bucci, I.; Napolitano, G.; Giuliani, C.; Lio, S.; Minnucci, A.; Di, G.F.; Calabrese, G.; Sabatino, G.; Palka, G.; Monaco, F. Zinc sulfate supplementation improves thyroid function in hypozincemic Down children. Biol. Trace Elem. Res. 1999, 67, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Mogulkoc, R.; Bediz, C.S.; Kul, A.; Ugur, A. Pinealectomy and zinc deficiency have opposite effects on thyroid hormones in rats. Endocr. Res. 2003, 29, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.Y.; Sun, J.Y.; Wang, J.F. The effect of peripheral administration of zinc on food intake in rats fed Zn-adequate or Zn-deficient diets. Biol. Trace Elem. Res. 2008, 124, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Salas, E.; Alcantara-Alonso, V.; Matamoros-Trejo, G.; Vargas, M.A.; Morales-Mulia, M.; de Gortari, P. Mediobasal hypothalamic and adenohypophyseal TRH-degrading enzyme (PPII) is down-regulated by zinc deficiency. Int. J. Dev. Neurosci. 2015, 46, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Czekay, G.; Bauer, K. Identification of the thyrotropin-releasing-hormone-degrading ectoenzyme as a metallopeptidase. Biochem. J. 1993, 290, 921–926. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, B.; O’Cuinn, G. Localization of a narrow-specificity thyroliberin hydrolyzing pyroglutamate aminopeptidase in synaptosomal membranes of guinea-pig brain. Eur. J. Biochem. 1984, 144, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Garat, B.; Miranda, J.; Charli, J.L.; Joseph-Bravo, P. Presence of a membrane bound pyroglutamyl amino peptidase degrading thyrotropin releasing hormone in rat brain. Neuropeptides 1985, 6, 27–40. [Google Scholar] [CrossRef]
- Friedman, T.C.; Wilk, S. Delineation of a particulate thyrotropin-releasing hormone-degrading enzyme in rat brain by the use of specific inhibitors of prolyl endopeptidase and pyroglutamyl peptide hydrolase. J. Neurochem. 1986, 46, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Ponce, G.; Charli, J.L.; Pasten, J.A.; Aceves, C.; Joseph-Bravo, P. Tissue-specific regulation of pyroglutamate aminopeptidase II activity by thyroid hormones. Neuroendocrinology 1988, 48, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- de Gortari, P.; Fernandez-Guardiola, A.; Martinez, A.; Cisneros, M.; Joseph-Bravo, P. Changes in TRH and its degrading enzyme pyroglutamyl peptidase II, during the development of amygdaloid kindling. Brain Res. 1995, 679, 144–150. [Google Scholar] [CrossRef]
- Joseph-Bravo, P.; Charli, J.L.; Palacios, J.M.; Kordon, C. Effect of neurotransmitters on the in vitro release of immunoreactive thyrotropin-releasing hormone from rat mediobasal hypothalamus. Endocrinology 1979, 104, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosebrough, N.; Farr, A.; Randall, R. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Coppola, A.; Meli, R.; Diano, S. Inverse shift in circulating corticosterone and leptin levels elevates hypothalamic deiodinase type 2 in fasted rats. Endocrinology 2005, 146, 2827–2833. [Google Scholar] [CrossRef] [PubMed]
- Pekary, A.E.; Sattin, A.; Blood, J. Rapid modulation of TRH and TRH-like peptide release in rat brain and peripheral tissues by leptin. Brain Res. 2010, 1345, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Ohta, K.; Haraguchi, K.; Onaya, T. Cloning and functional expression of a thyrotropin receptor cDNA from rat fat cells. J. Biol. Chem. 1995, 270, 10833–10837. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kobayashi, T. Expression of functional TSH receptor in white adipose tissues of hyt/hyt mice induces lipolysis in vivo. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1569–E1575. [Google Scholar] [CrossRef] [PubMed]
- Denereaz, N.; Lemarchand-Beraud, T. Severe but not mild alterations of thyroid function modulate the density of thyroid-stimulating hormone receptors in the rat thyroid gland. Endocrinology 1995, 136, 1694–1700. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; McDonald, D.; Hope, T.J.; Prabhakar, B.S. Upon thyrotropin binding the thyrotropin receptor is internalized and localized to endosome. Endocrinology 2004, 145, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Donangelo, C.M.; King, J.C. Maternal zinc intakes and homeostatic adjustments during pregnancy and lactation. Nutrients 2012, 4, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Masters, D.G.; Keen, C.L.; Lonnerdal, B.; Hurley, L.S. Release of zinc from maternal tissues during zinc deficiency or simultaneous zinc and calcium deficiency in the pregnant rat. J. Nutr. 1986, 116, 2148–2154. [Google Scholar] [PubMed]
- Levenson, C.W. Zinc regulation of food intake: New insights on the role of neuropeptide Y. Nutr. Rev. 2003, 61, 247–249. [Google Scholar] [PubMed]
- Chowanadisai, W.; Kelleher, S.L.; Lonnerdal, B. Maternal zinc deficiency raises plasma prolactin levels in lactating rats. J. Nutr. 2004, 134, 1314–1319. [Google Scholar] [PubMed]
- Blake, N.G.; Eckland, D.J.; Foster, O.J.; Lightman, S.L. Inhibition of hypothalamic thyrotropin-releasing hormone messenger ribonucleic acid during food deprivation. Endocrinology 1991, 129, 2714–2718. [Google Scholar] [CrossRef] [PubMed]
- Van Haasteren, G.A.; Linkels, E.; van, T.H.; Klootwijk, W.; Kaptein, E.; de Jong, F.H.; Reymond, M.J.; Visser, T.J.; de Greef, W.J. Effects of long-term food reduction on the hypothalamus-pituitary-thyroid axis in male and female rats. J. Endocrinol. 1996, 150, 169–178. [Google Scholar] [CrossRef] [PubMed]
- de Gortari, P.; Gonzalez-Alzati, M.E.; Cisneros, M.; Joseph-Bravo, P. Effect of fasting on the content of thyrotropin-releasing hormone and its RNAm in the central nervous system and pyroglutamyl peptidase II activity in the anterior pituitary of post-weaned and adult rats. Nutr. Neurosci. 2000, 3, 255–265. [Google Scholar] [CrossRef]
- Geras, E.J.; Gershengorn, M.C. Evidence that TRH stimulates secretion of TSH by two calcium-mediated mechanisms. Am. J. Physiol. 1982, 242, E109–E114. [Google Scholar] [PubMed]
- Nillni, E.A. Regulation of the hypothalamic thyrotropin releasing hormone (TRH) neuron by neuronal and peripheral inputs. Front. Neuroendocrinol. 2010, 31, 134–156. [Google Scholar] [CrossRef] [PubMed]
- Dyess, E.M.; Segerson, T.P.; Liposits, Z.; Paull, W.K.; Kaplan, M.M.; Wu, P.; Jackson, I.M.; Lechan, R.M. Triiodothyronine exerts direct cell-specific regulation of thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus. Endocrinology 1988, 123, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Mogulkoc, R. Leptin, NPY, Melatonin and Zinc Levels in Experimental Hypothyroidism and Hyperthyroidism: The Relation to Zinc. Biochem. Genet. 2017, 55, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.; Joseph-Bravo, P.; Cisneros, M.; Vargas, M.A.; Charli, J.L. Regional distribution of in vitro release of thyrotropin releasing hormone in rat brain. Peptides 1987, 8, 291–298. [Google Scholar] [CrossRef]
- Diano, S.; Naftolin, F.; Goglia, F.; Horvath, T.L. Fasting-induced increase in type II iodothyronine deiodinase activity and messenger ribonucleic acid levels is not reversed by thyroxine in the rat hypothalamus. Endocrinology 1998, 139, 2879–2884. [Google Scholar] [CrossRef] [PubMed]
- Uribe, R.M.; Redondo, J.L.; Charli, J.L.; Joseph-Bravo, P. Suckling and cold stress rapidly and transiently increase TRH mRNA in the paraventricular nucleus. Neuroendocrinology 1993, 58, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T.; Rudeen, P.K. Ethanol blocks the cold-induced increase in thyrotropin-releasing hormone mRNA in paraventricular nuclei but not the cold-induced increase in thyrotropin. Brain Res. Mol. Brain Res. 1992, 13, 321–330. [Google Scholar] [CrossRef]
- Jaimes-Hoy, L.; Joseph-Bravo, P.; de Gortari, P. Differential response of TRHergic neurons of the hypothalamic paraventricular nucleus (PVN) in female animals submitted to food-restriction or dehydration-induced anorexia and cold exposure. Horm. Behav. 2008, 53, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Koh, J.; Choi, D.W. Zinc selectively blocks the action of N-methyl-d-aspartate on cortical neurons. Science 1987, 236, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.L.; Vyklicky, L., Jr. The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. J. Physiol. 1989, 415, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Amico-Ruvio, S.A.; Murthy, S.E.; Smith, T.P.; Popescu, G.K. Zinc effects on NMDA receptor gating kinetics. Biophys. J. 2011, 100, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, G.; Lechan, R.M.; Liposits, Z.; Fekete, C. Glutamatergic innervation of corticotropin-releasing hormone- and thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. Brain Res. 2005, 1039, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Itoh, H.; Yamada, K.; Tamano, H.; Oku, N. Enhancement of hippocampal mossy fiber activity in zinc deficiency and its influence on behavior. Biometals 2008, 21, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Doboszewska, U.; Szewczyk, B.; Sowa-Kucma, M.; Noworyta-Sokolowska, K.; Misztak, P.; Golebiowska, J.; Mlyniec, K.; Ostachowicz, B.; Krosniak, M.; Wojtanowska-Krosniak, A.; et al. Alterations of Bio-elements, Oxidative, and Inflammatory Status in the Zinc Deficiency Model in Rats. Neurotox. Res. 2016, 29, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Someya, Y.; Sato, S.; Shirato, K.; Jinde, M.; Ishida, S.; Akimoto, S.; Kobayashi, K.; Sakakibara, Y.; Suzuki, Y.; et al. Dietary zinc-deficiency and its recovery responses in rat liver cytosolic alcohol dehydrogenase activities. J. Toxicol. Sci. 2011, 36, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Lomeda, R.A.; Ryu, S.H.; Sohn, H.Y.; Shin, H.I.; Beattie, J.H.; Kwun, I.S. Zinc deficiency negatively affects alkaline phosphatase and the concentration of Ca, Mg and P in rats. Nutr. Res. Pract. 2007, 1, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Dahlheim, H.; White, C.L.; Rothemund, J.; von Lutterotti, N.; Jacob, I.C.; Rosenthal, J. Effect of zinc depletion on angiotensin I-converting enzyme in arterial walls and plasma of the rat. Miner. Electrolyte Metab. 1989, 15, 125–129. [Google Scholar] [PubMed]
- White, C.L.; Pschorr, J.; Jacob, I.C.; von Lutterotti, N.; Dahlheim, H. The effect of zinc in vivo and in vitro on the activities of angiotensin converting enzyme and kininase-I in the plasma of rats. Biochem. Pharmacol. 1986, 35, 2489–2493. [Google Scholar] [CrossRef]
- Reeves, P.G.; O’Dell, B.L. An experimental study of the effect of zinc on the activity of angiotensin converting enzyme in serum. Clin. Chem. 1985, 31, 581–584. [Google Scholar] [PubMed]
- Vargas, M.A.; Herrera, J.; Uribe, R.M.; Charli, J.L.; Joseph-Bravo, P. Ontogenesis of pyroglutamyl peptidase II activity in rat brain, adenohypophysis and pancreas. Dev. Brain Res. 1992, 66, 251–256. [Google Scholar] [CrossRef]
- Takeda, A. Zinc homeostasis and functions of zinc in the brain. Biometals 2001, 14, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Licastro, F.; Mocchegiani, E.; Zannotti, M.; Arena, G.; Masi, M.; Fabris, N. Zinc affects the metabolism of thyroid hormones in children with Down’s syndrome: Normalization of thyroid stimulating hormone and of reversal triiodothyronine plasmic levels by dietary zinc supplementation. Int. J. Neurosci. 1992, 65, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Franklyn, J.A. The thyroid—Too much and too little across the ages. The consequences of subclinical thyroid dysfunction. Clin. Endocrinol. 2013, 78, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Parle, J.V.; Franklyn, J.A.; Cross, K.W.; Jones, S.C.; Sheppard, M.C. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin. Endocrinol. 1991, 34, 77–83. [Google Scholar]
- Mueller, A.; Schofl, C.; Dittrich, R.; Cupisti, S.; Oppelt, P.G.; Schild, R.L.; Beckmann, M.W.; Haberle, L. Thyroid-stimulating hormone is associated with insulin resistance independently of body mass index and age in women with polycystic ovary syndrome. Hum. Reprod. 2009, 24, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, J.B. Subclinical Hypothyroidism in PCOS: Impact on Presentation, Insulin Resistance, and Cardiovascular Risk. Biomed. Res. Int. 2016, 2016, 2067087. [Google Scholar] [CrossRef] [PubMed]
- Unal, E.; Akin, A.; Yildirim, R.; Demir, V.; Yildiz, I.; Haspolat, Y.K. Association of Subclinical Hypothyroidism with Dyslipidemia and Increased Carotid Intima-Media Thickness in Children. J. Clin. Res. Pediatr. Endocrinol. 2017, 9, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Decandia, F. Risk factors for cardiovascular disease in subclinical hypothyroidism. Ir. J. Med. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.B.; Li, H.B.; Zhu, X.R.; Song, H.L.; Zhao, Y.Y.; Yang, J.K. Subclinical hypothyroidism and the risk of chronic kidney disease in T2D subjects: A case-control and dose-response analysis. Medicine 2017, 96, e6519. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.R.; Kalantarhormozi, M.; Izadi, F.; Arkia, E.; Rashidi, M.; Pourbehi, F.; Daneshifard, F.; Rahbar, A. Relationship between Body Mass Index, Waist-to-Hip Ratio, and Serum Lipid Concentrations and Thyroid-Stimulating Hormone in the Euthyroid Adult Population. Iran. J. Med. Sci. 2017, 42, 301–305. [Google Scholar] [PubMed]
| Group | T3 (ng/dL) | T4 (μg/dL) | Leptin (ng/mL) | Corticosterone (ng/mL) |
|---|---|---|---|---|
| Control | 8.17 ± 0.15 | 8.5 ± 1 | 4.3 ± 0.8 | 149 ± 19 |
| Zn-deficient | 7.35 ± 0.49 | 8.86 ± 1.1 | 1.86 ± 0.2 * | 99 ± 17 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcántara-Alonso, V.; Alvarez-Salas, E.; Matamoros-Trejo, G.; De Gortari, P. Intrauterine Zn Deficiency Favors Thyrotropin-Releasing Hormone-Increasing Effects on Thyrotropin Serum Levels and Induces Subclinical Hypothyroidism in Weaned Rats. Nutrients 2017, 9, 1139. https://doi.org/10.3390/nu9101139
Alcántara-Alonso V, Alvarez-Salas E, Matamoros-Trejo G, De Gortari P. Intrauterine Zn Deficiency Favors Thyrotropin-Releasing Hormone-Increasing Effects on Thyrotropin Serum Levels and Induces Subclinical Hypothyroidism in Weaned Rats. Nutrients. 2017; 9(10):1139. https://doi.org/10.3390/nu9101139
Chicago/Turabian StyleAlcántara-Alonso, Viridiana, Elena Alvarez-Salas, Gilberto Matamoros-Trejo, and Patricia De Gortari. 2017. "Intrauterine Zn Deficiency Favors Thyrotropin-Releasing Hormone-Increasing Effects on Thyrotropin Serum Levels and Induces Subclinical Hypothyroidism in Weaned Rats" Nutrients 9, no. 10: 1139. https://doi.org/10.3390/nu9101139





