Neural and Molecular Mechanisms Involved in Controlling the Quality of Feeding Behavior: Diet Selection and Feeding Patterns
Abstract
:1. Introduction
2. Basic Concepts in the Regulation of Feeding Behavior
2.1. Information Conveyed to the Central Nervous System
2.2. Homeostatic System
2.3. Hedonic System
2.3.1. Dopamine System
2.3.2. Opioid System
2.4. Concluding Remarks on the Regulation of Feeding Behavior
3. Macronutrient-Based Diet Selection (What We Eat)
3.1. Importance of Macronutrient Selection for Controlling Weight and Health
3.2. Mechanisms That Regulate Macronutrient Preference
3.2.1. Humoral Factors that Influence Macronutrient Preference
3.2.2. Homeostatic Feeding Mechanisms that Influence Macronutrient Preference
3.2.3. The Hedonic System and Macronutrient Preference
3.2.4. Other Mechanisms that Potentially Influence Macronutrient Preference
3.3. Concluding Remarks on Macronutrient Preference
4. Feeding Pattern (When We Eat)
4.1. Importance of Feeding Patterns for Controlling Weight and Health
4.2. Molecular and Neural Bases for Circadian Regulation of Various Rhythms
4.3. Possible Mechanisms that Regulate Feeding Patterns
4.3.1. Food Anticipatory Activity (FAA) and the Food-Entrained Oscillator (FEO)
4.3.2. Are the FEO and the Ad Libitum Feeding Pattern Generator Identical
4.3.3. Neurotransmitter Systems That Show Diurnal Rhythms
4.4. HFD and Feeding Patterns
4.4.1. Impact of HFDs on Various Rhythms
4.4.2. Impact of HFD on Feeding Regulatory Mechanisms
4.4.3. HFD Disruption of Feeding Patterns
4.5. Concluding Remarks on Feeding Patterns
5. Conclusions
- (1-1)
- How is macronutrient intake sensed by the neural circuitry?
- (1-1-1)
- Could dietary components (like glucose) and intermediate metabolites directly affect neural nodes that regulated feeding behavior?
- (1-1-2)
- Are there unidentified bioactive molecules/hormones that mediate the transmission of nutrient information from the peripheral tissues to the central nervous system?
- (1-1-3)
- Does a mechanism exist that can sense the lengths of fatty acid chains (e.g., short-chain vs. long-chain), and thus, regulate preferences for specific fatty acids? For example, FGF21 assumes this role in the context of carbohydrates, and it regulates simple sugar-specific preferences (as discussed in the Section 3.2.1).
- (1-2)
- Where and how is feeding-related multimodal sensory information integrated within the brain? We may need better model organisms to study such processes, because rodent models cannnot recapitulate all the features of sensory processing observed in humans.
- (1-3)
- How do homeostatic, hedonic, and possibly multimodal integration system(s) interact with each other? The homeostatic and hedonic systems are known to interact at multiple neural nodes, but our current knowleadge is likely to be incomplete.
- (2-1)
- What is the identity of the feeding pattern generator?
- (2-1-1)
- Is it located in the brain or in the peripheral tissues?
- (2-1-2)
- Is it a single master regulator, or is it influenced by multiple oscillators?
- (2-2)
- Despite the fact that molecular clock system that generates circadian rhythms is conserved among multiple species, nocturnal and diurnal organisms eat at different times of the day. Therefore, are the mechanisms that regulate feeding patterns conserved among multiple species, particularly nocturnal and diurnal organisms?
- (2-2-1)
- If the mechanisms are conserved, do different species have different way(s) of coordinating the molecular clock with the feeding pattern generator(s)?
- (2-2-2)
- If the mechanisms are not conserved, what animal models might be best for investigating the neural mechanisms responsible for generating different feeding patterns?
- (2-3)
- To what extent does the ad libitum feeding pattern generator dictate the feeding behavior in humans?
- (3-1)
- How are the mechanisms that regulate macronutrient selection and feeding patterns regulated or altered in obesity?
- (3-2)
- If some of the mechanisms are altered, are these changes reversible upon weight loss?
- (3-3)
- If reversible, are they always reversible, or is there a point of no return?
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sasaki, T. Age-associated weight gain, leptin, and sirt1: A possible role for hypothalamic sirt1 in the prevention of weight gain and aging through modulation of leptin sensitivity. Front. Endocrinol. (Lausanne) 2015, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- GBD2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar]
- McDaid, A.F.; Joshi, P.K.; Porcu, E.; Komljenovic, A.; Li, H.; Sorrentino, V.; Litovchenko, M.; Bevers, R.P.J.; Rueger, S.; Reymond, A.; et al. Bayesian association scan reveals loci associated with human lifespan and linked biomarkers. Nat. Commun. 2017, 8, 15842. [Google Scholar] [CrossRef] [PubMed]
- GBD2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (dalys) for 315 diseases and injuries and healthy life expectancy (hale), 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016, 388, 1603–1658. [Google Scholar]
- Gletsu-Miller, N.; McCrory, M.A. Modifying eating behavior: Novel approaches for reducing body weight, preventing weight regain, and reducing chronic disease risk. Adv. Nutr. 2014, 5, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Soeliman, F.A.; Azadbakht, L. Weight loss maintenance: A review on dietary related strategies. J. Res. Med. Sci. 2014, 19, 268–275. [Google Scholar] [PubMed]
- Molin Netto, B.D.; Earthman, C.P.; Farias, G.; Landi Masquio, D.C.; Grotti Clemente, A.P.; Peixoto, P.; Bettini, S.C.; von Der Heyde, M.E.; Damaso, A.R. Eating patterns and food choice as determinant of weight loss and improvement of metabolic profile after rygb. Nutrition 2017, 33, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Holden-Wiltse, J. Taste responses and food preferences in obese women: Effects of weight cycling. Int. J. Obes. Relat. Metab. Disord. 1992, 16, 639–648. [Google Scholar] [PubMed]
- Reed, D.R.; Contreras, R.J.; Maggio, C.; Greenwood, M.R.; Rodin, J. Weight cycling in female rats increases dietary fat selection and adiposity. Physiol. Behav. 1988, 42, 389–395. [Google Scholar] [CrossRef]
- Sasaki, T.; Matsui, S.; Kitamura, T. Control of appetite and food preference by nmda receptor and its co-agonist d-serine. Int. J. Mol. Sci. 2016, 17, E1018. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Morrison, C. The brain, appetite, and obesity. Annu. Rev. Psychol. 2008, 59, 55–92. [Google Scholar] [CrossRef] [PubMed]
- Basiri, M.L.; Stuber, G.D. Multimodal signal integration for feeding control. Cell 2016, 165, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Andermann, M.L.; Lowell, B.B. Toward a wiring diagram understanding of appetite control. Neuron 2017, 95, 757–778. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.J.; Spencer, N.J.; Costa, M.; Zagorodnyuk, V.P. Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 286–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Hirako, S.; Takenoya, F.; Kageyama, H.; Okabe, M.; Shioda, S. Leptin and its receptors. J. Chem. Neuroanat. 2014, 61–62, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Proenca, R.; Montez, J.M.; Carroll, K.M.; Darvishzadeh, J.G.; Lee, J.I.; Friedman, J.M. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996, 379, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Charlat, O.; Tartaglia, L.A.; Woolf, E.A.; Weng, X.; Ellis, S.J.; Lakey, N.D.; Culpepper, J.; Moore, K.J.; Breitbart, R.E.; et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996, 84, 491–495. [Google Scholar] [CrossRef]
- Havrankova, J.; Roth, J.; Brownstein, M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 1978, 272, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Vogt, M.C.; Bruning, J.C. Cns insulin signaling in the control of energy homeostasis and glucose metabolism—From embryo to old age. Trends Endocrinol. Metab. 2013, 24, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Murakami, N.; Toshinai, K.; Matsukura, S.; Niijima, A.; Matsuo, H.; Kangawa, K.; Nakazato, M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002, 123, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Neary, N.M.; Halsey, T.J.; Small, C.J.; Martinez-Isla, A.M.; Ghatei, M.A.; Theodorou, N.A.; Bloom, S.R. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J. Clin. Endocrinol. Metab. 2005, 90, 4521–4524. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Shimbara, T.; Koda, S.; Toshinai, K.; Ida, T.; Murakami, N.; Miyazato, M.; Kokame, K.; Ishizuka, Y.; Ishida, Y.; et al. Peripheral ghrelin transmits orexigenic signals through the noradrenergic pathway from the hindbrain to the hypothalamus. Cell Metab. 2006, 4, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschop, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the cns demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef]
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3–36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after rygb. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Trumbauer, M.E.; Chen, A.S.; Weingarth, D.T.; Adams, J.R.; Frazier, E.G.; Shen, Z.; Marsh, D.J.; Feighner, S.D.; Guan, X.M.; et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide y and agouti-related protein. Endocrinology 2004, 145, 2607–2612. [Google Scholar] [CrossRef] [PubMed]
- Mullier, A.; Bouret, S.G.; Prevot, V.; Dehouck, B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J. Comp. Neurol. 2010, 518, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Krashes, M.J.; Lowell, B.B.; Garfield, A.S. Melanocortin-4 receptor-regulated energy homeostasis. Nat. Neurosci. 2016, 19, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kitamura, T. Roles of foxo1 and sirt1 in the central regulation of food intake. Endocr. J. 2010, 57, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Girardet, C.; Butler, A.A. Neural melanocortin receptors in obesity and related metabolic disorders. Biochim. Biophys. Acta 2014, 1842, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Mercer, R.E.; Chee, M.J.; Colmers, W.F. The role of npy in hypothalamic mediated food intake. Front. Neuroendocrinol. 2011, 32, 398–415. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Robinson, T.E. Parsing reward. Trends Neurosci. 2003, 26, 507–513. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E.; Aldridge, J.W. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr. Opin. Pharmacol. 2009, 9, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Correa, M. The mysterious motivational functions of mesolimbic dopamine. Neuron 2012, 76, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007, 30, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.J.; Hollon, N.G.; Phillips, P.E. Pavlovian valuation systems in learning and decision making. Curr. Opin. Neurobiol. 2012, 22, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Norgren, R.; Hajnal, A.; Mungarndee, S.S. Gustatory reward and the nucleus accumbens. Physiol. Behav. 2006, 89, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Globa, A.K.; Mills, F.; Naef, L.; Qiao, M.; Bamji, S.X.; Borgland, S.L. Consumption of palatable food primes food approach behavior by rapidly increasing synaptic density in the vta. Proc. Natl. Acad. Sci. USA 2016, 113, 2520–2525. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Jones-Gotman, M.; Dagher, A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 2003, 19, 1709–1715. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Telang, F. Overlapping neuronal circuits in addiction and obesity: Evidence of systems pathology. Philos. Trans. R Soc. Lond. B Biol. Sci. 2008, 363, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Steinbusch, L.; Labouebe, G.; Thorens, B. Brain glucose sensing in homeostatic and hedonic regulation. Trends Endocrinol. Metab. 2015, 26, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Figlewicz, D.P.; Evans, S.B.; Murphy, J.; Hoen, M.; Baskin, D.G. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (vta/sn) of the rat. Brain Res. 2003, 964, 107–115. [Google Scholar] [CrossRef]
- Leshan, R.L.; Opland, D.M.; Louis, G.W.; Leinninger, G.M.; Patterson, C.M.; Rhodes, C.J.; Munzberg, H.; Myers, M.G., Jr. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J. Neurosci. 2010, 30, 5713–5723. [Google Scholar] [CrossRef] [PubMed]
- Alhadeff, A.L.; Rupprecht, L.E.; Hayes, M.R. Glp-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 2012, 153, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Abizaid, A.; Liu, Z.W.; Andrews, Z.B.; Shanabrough, M.; Borok, E.; Elsworth, J.D.; Roth, R.H.; Sleeman, M.W.; Picciotto, M.R.; Tschop, M.H.; et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Investig. 2006, 116, 3229–3239. [Google Scholar] [CrossRef] [PubMed]
- Jerlhag, E.; Egecioglu, E.; Dickson, S.L.; Douhan, A.; Svensson, L.; Engel, J.A. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict. Biol. 2007, 12, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Fadel, J.; Deutch, A.Y. Anatomical substrates of orexin-dopamine interactions: Lateral hypothalamic projections to the ventral tegmental area. Neuroscience 2002, 111, 379–387. [Google Scholar] [CrossRef]
- Hommel, J.D.; Trinko, R.; Sears, R.M.; Georgescu, D.; Liu, Z.W.; Gao, X.B.; Thurmon, J.J.; Marinelli, M.; DiLeone, R.J. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006, 51, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Labouebe, G.; Liu, S.; Dias, C.; Zou, H.; Wong, J.C.; Karunakaran, S.; Clee, S.M.; Phillips, A.G.; Boutrel, B.; Borgland, S.L. Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids. Nat. Neurosci. 2013, 16, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Mebel, D.M.; Wong, J.C.; Dong, Y.J.; Borgland, S.L. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur. J. Neurosci. 2012, 36, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Skibicka, K.P.; Hansson, C.; Alvarez-Crespo, M.; Friberg, P.A.; Dickson, S.L. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 2011, 180, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Borgland, S.L.; Chang, S.J.; Bowers, M.S.; Thompson, J.L.; Vittoz, N.; Floresco, S.B.; Chou, J.; Chen, B.T.; Bonci, A. Orexin a/hypocretin-1 selectively promotes motivation for positive reinforcers. J. Neurosci. 2009, 29, 11215–11225. [Google Scholar] [CrossRef] [PubMed]
- Patyal, R.; Woo, E.Y.; Borgland, S.L. Local hypocretin-1 modulates terminal dopamine concentration in the nucleus accumbens shell. Front. Behav. Neurosci. 2012, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, G.; Wegener, G.; Hasselstrom, J.; Hansen, T.V.; Wortwein, G.; Fink-Jensen, A.; Woldbye, D.P. Neuropeptide y infusion into the shell region of the rat nucleus accumbens increases extracellular levels of dopamine. Neuroreport 2009, 20, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.J.; Wetzel, A.; Sinno, M.H.; Skunde, M.; Bendszus, M.; Preissl, H.; Enck, P.; Herzog, W.; Friederich, H.C. Integration of homeostatic signaling and food reward processing in the human brain. JCI Insight 2017, 2, e92970. [Google Scholar] [CrossRef] [PubMed]
- Toll, L.; Bruchas, M.R.; Calo, G.; Cox, B.M.; Zaveri, N.T. Nociceptin/orphanin fq receptor structure, signaling, ligands, functions, and interactions with opioid systems. Pharmacol. Rev. 2016, 68, 419–457. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Romero-Pico, A.; Vazquez, M.J.; Novelle, M.G.; Lopez, M.; Dieguez, C. The opioid system and food intake: Homeostatic and hedonic mechanisms. Obes. Facts 2012, 5, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Akil, H.; Watson, S.J.; Young, E.; Lewis, M.E.; Khachaturian, H.; Walker, J.M. Endogenous opioids: Biology and function. Annu. Rev. Neurosci. 1984, 7, 223–255. [Google Scholar] [CrossRef] [PubMed]
- Appleyard, S.M.; Hayward, M.; Young, J.I.; Butler, A.A.; Cone, R.D.; Rubinstein, M.; Low, M.J. A role for the endogenous opioid beta-endorphin in energy homeostasis. Endocrinology 2003, 144, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Le Merrer, J.; Becker, J.A.; Befort, K.; Kieffer, B.L. Reward processing by the opioid system in the brain. Physiol. Rev. 2009, 89, 1379–1412. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.J. Effects of opiate agonists and antagonists on fluid intake and saccharin choice in the rat. Neuropharmacology 1983, 22, 323–328. [Google Scholar] [CrossRef]
- Cooper, S.J.; Turkish, S. Effects of naltrexone on food preference and concurrent behavioral responses in food-deprived rats. Pharmacol. Biochem. Behav. 1989, 33, 17–20. [Google Scholar] [CrossRef]
- Evans, K.R.; Vaccarino, F.J. Amphetamine- and morphine-induced feeding: Evidence for involvement of reward mechanisms. Neurosci. Biobehav. Rev. 1990, 14, 9–22. [Google Scholar] [CrossRef]
- Lynch, W.C. Opiate blockade inhibits saccharin intake and blocks normal preference acquisition. Pharmacol. Biochem. Behav. 1986, 24, 833–836. [Google Scholar] [CrossRef]
- Drewnowski, A.; Krahn, D.D.; Demitrack, M.A.; Nairn, K.; Gosnell, B.A. Taste responses and preferences for sweet high-fat foods: Evidence for opioid involvement. Physiol. Behav. 1992, 51, 371–379. [Google Scholar] [CrossRef]
- Fantino, M.; Hosotte, J.; Apfelbaum, M. An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am. J. Physiol. 1986, 251, R91–R96. [Google Scholar] [PubMed]
- Yeomans, M.R.; Gray, R.W. Selective effects of naltrexone on food pleasantness and intake. Physiol. Behav. 1996, 60, 439–446. [Google Scholar] [CrossRef]
- Zhang, M.; Kelley, A.E. Enhanced intake of high-fat food following striatal mu-opioid stimulation: Microinjection mapping and fos expression. Neuroscience 2000, 99, 267–277. [Google Scholar] [CrossRef]
- Katsuura, Y.; Taha, S.A. Modulation of feeding and locomotion through mu and delta opioid receptor signaling in the nucleus accumbens. Neuropeptides 2010, 44, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Katsuura, Y.; Heckmann, J.A.; Taha, S.A. Mu-opioid receptor stimulation in the nucleus accumbens elevates fatty tastant intake by increasing palatability and suppressing satiety signals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R244–R254. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.C.; Berridge, K.C. Opioid hedonic hotspot in nucleus accumbens shell: Mu, delta, and kappa maps for enhancement of sweetness “Liking” And “Wanting”. J. Neurosci. 2014, 34, 4239–4250. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.E.; Bless, E.P.; Swanson, C.J. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J. Pharmacol. Exp. Ther. 1996, 278, 1499–1507. [Google Scholar] [PubMed]
- Yeomans, M.R.; Blundell, J.E.; Leshem, M. Palatability: Response to nutritional need or need-free stimulation of appetite? Br. J. Nutr. 2004, 92, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Hayward, M.D.; Schaich-Borg, A.; Pintar, J.E.; Low, M.J. Differential involvement of endogenous opioids in sucrose consumption and food reinforcement. Pharmacol. Biochem. Behav. 2006, 85, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Mahler, S.V.; Berridge, K.C. Which cue to “Want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J. Neurosci. 2009, 29, 6500–6513. [Google Scholar] [CrossRef] [PubMed]
- Mendez, I.A.; Ostlund, S.B.; Maidment, N.T.; Murphy, N.P. Involvement of endogenous enkephalins and beta-endorphin in feeding and diet-induced obesity. Neuropsychopharmacology 2015, 40, 2103–2112. [Google Scholar] [CrossRef] [PubMed]
- Berridge, K.C.; Kringelbach, M.L. Pleasure systems in the brain. Neuron 2015, 86, 646–664. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Henderson, S.A.; Levine, A.; Hann, C. Taste and food preferences as predictors of dietary practices in young women. Public Health Nutr. 1999, 2, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Hayes, J.E.; Sullivan, B.S.; Faghri, P. Surveying food and beverage liking: A tool for epidemiological studies to connect chemosensation with health outcomes. Ann. N. Y. Acad. Sci. 2009, 1170, 558–568. [Google Scholar] [CrossRef] [PubMed]
- The 2015 Food & Health Survey: Consumer Attitudes toward Food Safety, Nutrition & Health; International Food Information Council Foundation: Washington, DC, USA, 2015.
- Ventura, A.K.; Worobey, J. Early influences on the development of food preferences. Curr. Biol. 2013, 23, R401–R408. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, R.; Cavelaars, A.; Geelen, A.; Nikolic, M.; Altaba, I.I.; Vinas, B.R.; Ngo, J.; Golsorkhi, M.; Medina, M.W.; Brzozowska, A.; et al. Socio-economic determinants of micronutrient intake and status in europe: A systematic review. Public Health Nutr. 2014, 17, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Pirastu, N.; Robino, A.; Lanzara, C.; Athanasakis, E.; Esposito, L.; Tepper, B.J.; Gasparini, P. Genetics of food preferences: A first view from silk road populations. J. Food Sci. 2012, 77, S413–S418. [Google Scholar] [CrossRef] [PubMed]
- Feeney, E.; O’Brien, S.; Scannell, A.; Markey, A.; Gibney, E.R. Genetic variation in taste perception: Does it have a role in healthy eating? Proc. Nutr. Soc. 2011, 70, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Pallister, T.; Sharafi, M.; Lachance, G.; Pirastu, N.; Mohney, R.P.; MacGregor, A.; Feskens, E.J.; Duffy, V.; Spector, T.D.; Menni, C. Food preference patterns in a uk twin cohort. Twin Res. Hum. Genet. 2015, 18, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Groschl, M.; Knerr, I.; Topf, H.G.; Schmid, P.; Rascher, W.; Rauh, M. Endocrine responses to the oral ingestion of a physiological dose of essential amino acids in humans. J. Endocrinol. 2003, 179, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Knerr, I.; Groschl, M.; Rascher, W.; Rauh, M. Endocrine effects of food intake: Insulin, ghrelin, and leptin responses to a single bolus of essential amino acids in humans. Ann. Nutr. Metab. 2003, 47, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Markus, R.; Panhuysen, G.; Tuiten, A.; Koppeschaar, H. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol. Behav. 2000, 70, 333–342. [Google Scholar] [CrossRef]
- Lehnert, H.; Wurtman, R.J. Amino acid control of neurotransmitter synthesis and release: Physiological and clinical implications. Psychother. Psychosom. 1993, 60, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J.; Fernstrom, J.D. Control of brain neurotransmitter synthesis by precursor availability and nutritional state. Biochem. Pharmacol. 1976, 25, 1691–1696. [Google Scholar] [CrossRef]
- Acworth, I.N.; During, M.J.; Wurtman, R.J. Tyrosine: Effects on catecholamine release. Brain Res. Bull. 1988, 21, 473–477. [Google Scholar] [CrossRef]
- Lehnert, H.; Reinstein, D.K.; Strowbridge, B.W.; Wurtman, R.J. Neurochemical and behavioral consequences of acute, uncontrollable stress: Effects of dietary tyrosine. Brain Res. 1984, 303, 215–223. [Google Scholar] [CrossRef]
- Kuwata, H.; Iwasaki, M.; Shimizu, S.; Minami, K.; Maeda, H.; Seino, S.; Nakada, K.; Nosaka, C.; Murotani, K.; Kurose, T.; et al. Meal sequence and glucose excursion, gastric emptying and incretin secretion in type 2 diabetes: A randomised, controlled crossover, exploratory trial. Diabetologia 2016, 59, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Bemis, T.; Brychta, R.; Chen, K.Y.; Courville, A.; Crayner, E.J.; Goodwin, S.; Guo, J.; Howard, L.; Knuth, N.D.; et al. Calorie for calorie, dietary fat restriction results in more body fat loss than carbohydrate restriction in people with obesity. Cell Metab. 2015, 22, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Guo, J. Obesity energetics: Body weight regulation and the effects of diet composition. Gastroenterology 2017, 152, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Micha, R.; Wu, J.H.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: A systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (pure): A prospective cohort study. Lancet 2017. [Google Scholar] [CrossRef]
- Van Horn, L.; Carson, J.A.; Appel, L.J.; Burke, L.E.; Economos, C.; Karmally, W.; Lancaster, K.; Lichtenstein, A.H.; Johnson, R.K.; Thomas, R.J.; et al. Recommended dietary pattern to achieve adherence to the american heart association/american college of cardiology (aha/acc) guidelines: A scientific statement from the american heart association. Circulation 2016, 134, e505–e529. [Google Scholar] [CrossRef] [PubMed]
- Von Holstein-Rathlou, S.; BonDurant, L.D.; Peltekian, L.; Naber, M.C.; Yin, T.C.; Claflin, K.E.; Urizar, A.I.; Madsen, A.N.; Ratner, C.; Holst, B.; et al. Fgf21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 2016, 23, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Owen, B.M.; Song, P.; Hernandez, G.; Zhang, Y.; Zhou, Y.; Scott, W.T.; Paratala, B.; Turner, T.; Smith, A.; et al. Fgf21 regulates sweet and alcohol preference. Cell Metab. 2016, 23, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Maratos-Flier, E. Understanding the physiology of fgf21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Soberg, S.; Sandholt, C.H.; Jespersen, N.Z.; Toft, U.; Madsen, A.L.; von Holstein-Rathlou, S.; Grevengoed, T.J.; Christensen, K.B.; Bredie, W.L.P.; Potthoff, M.J.; et al. Fgf21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab. 2017, 25, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.M.; Fritzen, A.M.; Sjoberg, K.A.; Myrmel, L.S.; Madsen, L.; Wojtaszewski, J.F.; Richter, E.A.; Kiens, B. Circulating fgf21 in humans is potently induced by short term overfeeding of carbohydrates. Mol. Metab. 2017, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Shimbara, T.; Mondal, M.S.; Kawagoe, T.; Toshinai, K.; Koda, S.; Yamaguchi, H.; Date, Y.; Nakazato, M. Central administration of ghrelin preferentially enhances fat ingestion. Neurosci. Lett. 2004, 369, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Schele, E.; Bake, T.; Rabasa, C.; Dickson, S.L. Centrally administered ghrelin acutely influences food choice in rodents. PLoS ONE 2016, 11, e0149456. [Google Scholar] [CrossRef] [PubMed]
- Perello, M.; Sakata, I.; Birnbaum, S.; Chuang, J.C.; Osborne-Lawrence, S.; Rovinsky, S.A.; Woloszyn, J.; Yanagisawa, M.; Lutter, M.; Zigman, J.M. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol. Psychiatry 2010, 67, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Skibicka, K.P.; Hansson, C.; Egecioglu, E.; Dickson, S.L. Role of ghrelin in food reward: Impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict. Biol. 2012, 17, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Van der Klaauw, A.A.; Keogh, J.M.; Henning, E.; Stephenson, C.; Kelway, S.; Trowse, V.M.; Subramanian, N.; O’Rahilly, S.; Fletcher, P.C.; Farooqi, I.S. Divergent effects of central melanocortin signalling on fat and sucrose preference in humans. Nat. Commun. 2016, 7, 13055. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Rimmington, D.; O’Rahilly, S.; Coll, A.P. Pro-opiomelanocortin modulates the thermogenic and physical activity responses to high-fat feeding and markedly influences dietary fat preference. Endocrinology 2007, 148, 5331–5338. [Google Scholar] [CrossRef] [PubMed]
- Koegler, F.H.; Schaffhauser, A.O.; Mynatt, R.L.; York, D.A.; Bray, G.A. Macronutrient diet intake of the lethal yellow agouti (ay/a) mouse. Physiol. Behav. 1999, 67, 809–812. [Google Scholar] [CrossRef]
- Tempel, D.L.; Leibowitz, K.J.; Leibowitz, S.F. Effects of pvn galanin on macronutrient selection. Peptides 1988, 9, 309–314. [Google Scholar] [CrossRef]
- Adams, A.C.; Clapham, J.C.; Wynick, D.; Speakman, J.R. Feeding behaviour in galanin knockout mice supports a role of galanin in fat intake and preference. J. Neuroendocrinol. 2008, 20, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Karatayev, O.; Baylan, J.; Leibowitz, S.F. Increased intake of ethanol and dietary fat in galanin overexpressing mice. Alcohol 2009, 43, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Barson, J.R.; Morganstern, I.; Leibowitz, S.F. Galanin and consummatory behavior: Special relationship with dietary fat, alcohol and circulating lipids. EXS 2010, 102, 87–111. [Google Scholar] [PubMed]
- Leibowitz, S.F.; Akabayashi, A.; Wang, J. Obesity on a high-fat diet: Role of hypothalamic galanin in neurons of the anterior paraventricular nucleus projecting to the median eminence. J. Neurosci. 1998, 18, 2709–2719. [Google Scholar] [PubMed]
- Wang, J.; Dourmashkin, J.T.; Yun, R.; Leibowitz, S.F. Rapid changes in hypothalamic neuropeptide y produced by carbohydrate-rich meals that enhance corticosterone and glucose levels. Brain Res. 1999, 848, 124–136. [Google Scholar] [CrossRef]
- McCue, D.L.; Kasper, J.M.; Hommel, J.D. Regulation of motivation for food by neuromedin u in the paraventricular nucleus and the dorsal raphe nucleus. Int. J. Obes. (Lond.) 2017, 41, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Benzon, C.R.; Johnson, S.B.; McCue, D.L.; Li, D.; Green, T.A.; Hommel, J.D. Neuromedin u receptor 2 knockdown in the paraventricular nucleus modifies behavioral responses to obesogenic high-fat food and leads to increased body weight. Neuroscience 2014, 258, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Stanley, B.G.; Daniel, D.R.; Chin, A.S.; Leibowitz, S.F. Paraventricular nucleus injections of peptide yy and neuropeptide y preferentially enhance carbohydrate ingestion. Peptides 1985, 6, 1205–1211. [Google Scholar] [CrossRef]
- Slawecki, C.J.; Betancourt, M.; Walpole, T.; Ehlers, C.L. Increases in sucrose consumption, but not ethanol consumption, following icv npy administration. Pharmacol. Biochem. Behav. 2000, 66, 591–594. [Google Scholar] [CrossRef]
- Elbers, C.C.; de Kovel, C.G.; van der Schouw, Y.T.; Meijboom, J.R.; Bauer, F.; Grobbee, D.E.; Trynka, G.; van Vliet-Ostaptchouk, J.V.; Wijmenga, C.; Onland-Moret, N.C. Variants in neuropeptide y receptor 1 and 5 are associated with nutrient-specific food intake and are under recent selection in europeans. PLoS ONE 2009, 4, e7070. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, J.K.; Furman, K.; Gumbs, M.C.; Eggels, L.; Opland, D.M.; Land, B.B.; Kolk, S.M.; Narayanan, N.S.; Fliers, E.; Kalsbeek, A.; et al. Neuropeptide y activity in the nucleus accumbens modulates feeding behavior and neuronal activity. Biol. Psychiatry 2015, 77, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Grinevich, V.; Desarmenien, M.G.; Chini, B.; Tauber, M.; Muscatelli, F. Ontogenesis of oxytocin pathways in the mammalian brain: Late maturation and psychosocial disorders. Front. Neuroanat. 2015, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Klockars, A.; Levine, A.S.; Olszewski, P.K. Central oxytocin and food intake: Focus on macronutrient-driven reward. Front. Endocrinol. (Lausanne) 2015, 6, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amico, J.A.; Vollmer, R.R.; Cai, H.M.; Miedlar, J.A.; Rinaman, L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1798–R1806. [Google Scholar] [CrossRef] [PubMed]
- Miedlar, J.A.; Rinaman, L.; Vollmer, R.R.; Amico, J.A. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1063–R1068. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Rinaman, L.; Vollmer, R.R.; Amico, J.A. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1828–R1833. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, P.K.; Klockars, A.; Olszewska, A.M.; Fredriksson, R.; Schioth, H.B.; Levine, A.S. Molecular, immunohistochemical, and pharmacological evidence of oxytocin’s role as inhibitor of carbohydrate but not fat intake. Endocrinology 2010, 151, 4736–4744. [Google Scholar] [CrossRef] [PubMed]
- Herisson, F.M.; Brooks, L.L.; Waas, J.R.; Levine, A.S.; Olszewski, P.K. Functional relationship between oxytocin and appetite for carbohydrates versus saccharin. Neuroreport 2014, 25, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.D.; Haynes, P.J.; Anderson, A.B.; Turnbull, A.C. Plasma oxytocin concentrations during the menstrual cycle. Eur. J. Obstet. Gynecol. Reprod. Biol. 1981, 12, 195–200. [Google Scholar] [CrossRef]
- Salonia, A.; Nappi, R.E.; Pontillo, M.; Daverio, R.; Smeraldi, A.; Briganti, A.; Fabbri, F.; Zanni, G.; Rigatti, P.; Montorsi, F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm. Behav. 2005, 47, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.K.; Pei, H.; Burnett, K.H.; Myers, M.G., Jr.; Rhodes, C.J.; Olson, D.P. Control of food intake and energy expenditure by nos1 neurons of the paraventricular hypothalamus. J. Neurosci. 2014, 34, 15306–15318. [Google Scholar] [CrossRef] [PubMed]
- van der Klaauw, A.A.; Ziauddeen, H.; Keogh, J.M.; Henning, E.; Dachi, S.; Fletcher, P.C.; Farooqi, I.S. Oxytocin administration suppresses hypothalamic activation in response to visual food cues. Sci. Rep. 2017, 7, 4266. [Google Scholar] [CrossRef] [PubMed]
- Mullis, K.; Kay, K.; Williams, D.L. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res. 2013, 1513, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Herisson, F.M.; Waas, J.R.; Fredriksson, R.; Schioth, H.B.; Levine, A.S.; Olszewski, P.K. Oxytocin acting in the nucleus accumbens core decreases food intake. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.S.; Perea-Martinez, I.; Dvoryanchikov, G.; Yoshida, M.; Nishimori, K.; Roper, S.D.; Chaudhari, N. Oxytocin signaling in mouse taste buds. PLoS ONE 2010, 5, e11980. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, M.S.; Perea-Martinez, I.; Abouyared, M.; St John, S.J.; Chaudhari, N. Oxytocin decreases sweet taste sensitivity in mice. Physiol. Behav. 2015, 141, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, R.; Uemoto, M.; Inui, A.; Asakawa, A.; Ueno, N.; Ishibashi, C.; Hirono, S.; Yukioka, H.; Kato, A.; Shinfuku, N.; et al. Melanin-concentrating hormone enhances sucrose intake. Int. J. Mol. Med. 2005, 15, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Domingos, A.I.; Sordillo, A.; Dietrich, M.O.; Liu, Z.W.; Tellez, L.A.; Vaynshteyn, J.; Ferreira, J.G.; Ekstrand, M.I.; Horvath, T.L.; de Araujo, I.E.; et al. Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. eLife 2013, 2, e01462. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Adamantidis, A.; Ackroff, K. Mch receptor deletion does not impair glucose-conditioned flavor preferences in mice. Physiol. Behav. 2016, 163, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, S.C.; Britton, K.T.; Koob, G.F. Both conditioned taste preference and aversion induced by corticotropin-releasing factor. Pharmacol. Biochem. Behav. 1991, 40, 717–721. [Google Scholar] [CrossRef]
- Kumar, B.A.; Papamichael, M.; Leibowitz, S.F. Feeding and macronutrient selection patterns in rats: Adrenalectomy and chronic corticosterone replacement. Physiol. Behav. 1988, 42, 581–589. [Google Scholar] [CrossRef]
- Kumar, B.A.; Leibowitz, S.F. Impact of acute corticosterone administration on feeding and macronutrient self-selection patterns. Am. J. Physiol. 1988, 254, R222–R228. [Google Scholar] [PubMed]
- Bligh, M.E.; Douglass, L.W.; Castonguay, T.W. Corticosterone modulation of dietary selection patterns. Physiol. Behav. 1993, 53, 975–982. [Google Scholar] [CrossRef]
- Teegarden, S.L.; Bale, T.L. Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Physiol. Behav. 2008, 93, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Yan, X.B.; Hofman, M.A.; Swaab, D.F.; Zhou, J.N. Increased expression level of corticotropin-releasing hormone in the amygdala and in the hypothalamus in rats exposed to chronic unpredictable mild stress. Neurosci. Bull. 2010, 26, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, S.C.; Koob, G.F. Corticotropin-releasing factor modulates dietary preference in nutritionally and physically stressed rats. Psychopharmacology (Berl) 1992, 109, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Hajnal, A.; Smith, G.P.; Norgren, R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R31–R37. [Google Scholar] [CrossRef] [PubMed]
- Carleton, A.; Accolla, R.; Simon, S.A. Coding in the mammalian gustatory system. Trends Neurosci. 2010, 33, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Chung, S.; Lu, D.P.; Cho, Y.K. Descending projections from the nucleus accumbens shell suppress activity of taste-responsive neurons in the hamster parabrachial nuclei. J. Neurophysiol. 2012, 108, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ferreira, J.G.; Zhou, L.; Shammah-Lagnado, S.J.; Yeckel, C.W.; de Araujo, I.E. Nutrient selection in the absence of taste receptor signaling. J. Neurosci. 2010, 30, 8012–8023. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, I.E.; Oliveira-Maia, A.J.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G.; Nicolelis, M.A.; Simon, S.A. Food reward in the absence of taste receptor signaling. Neuron 2008, 57, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Delaere, F.; Akaoka, H.; De Vadder, F.; Duchampt, A.; Mithieux, G. Portal glucose influences the sensory, cortical and reward systems in rats. Eur. J. Neurosci. 2013, 38, 3476–3486. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Maia, A.J.; Roberts, C.D.; Walker, Q.D.; Luo, B.; Kuhn, C.; Simon, S.A.; Nicolelis, M.A. Intravascular food reward. PLoS ONE 2011, 6, e24992. [Google Scholar] [CrossRef] [PubMed]
- Karnani, M.; Burdakov, D. Multiple hypothalamic circuits sense and regulate glucose levels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R47–R55. [Google Scholar] [CrossRef] [PubMed]
- Cansell, C.; Castel, J.; Denis, R.G.; Rouch, C.; Delbes, A.S.; Martinez, S.; Mestivier, D.; Finan, B.; Maldonado-Aviles, J.G.; Rijnsburger, M.; et al. Dietary triglycerides act on mesolimbic structures to regulate the rewarding and motivational aspects of feeding. Mol. Psychiatry 2014, 19, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, J.A.; Icaza-Cukali, D.; Tayabali, H.; Sampson, C.; Galanopoulos, V.; Bamshad, D.; Touzani, K.; Sclafani, A.; Bodnar, R.J. Roles of dopamine d1 and d2 receptors in the acquisition and expression of fat-conditioned flavor preferences in rats. Neurobiol. Learn. Mem. 2012, 97, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Hankir, M.K.; Seyfried, F.; Hintschich, C.A.; Diep, T.A.; Kleberg, K.; Kranz, M.; Deuther-Conrad, W.; Tellez, L.A.; Rullmann, M.; Patt, M.; et al. Gastric bypass surgery recruits a gut ppar-alpha-striatal d1r pathway to reduce fat appetite in obese rats. Cell Metab. 2017, 25, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tabuchi, M.; Liu, S.; Kodama, L.; Horiuchi, W.; Daniels, J.; Chiu, L.; Baldoni, D.; Wu, M.N. Branch-specific plasticity of a bifunctional dopamine circuit encodes protein hunger. Science 2017, 356, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, A.; Melka, M.G.; Bernard, M.; Abrahamowicz, M.; Leonard, G.T.; Richer, L.; Perron, M.; Veillette, S.; Xu, C.J.; Greenwood, C.M.; et al. Opioid receptor mu 1 gene, fat intake and obesity in adolescence. Mol. Psychiatry 2014, 19, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Bhakthavatsalam, P.; Leibowitz, S.F. Morphine-elicited feeding: Diurnal rhythm, circulating corticosterone and macronutrient selection. Pharmacol. Biochem. Behav. 1986, 24, 911–917. [Google Scholar] [CrossRef]
- Zhang, M.; Gosnell, B.A.; Kelley, A.E. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J. Pharmacol. Exp. Ther. 1998, 285, 908–914. [Google Scholar] [PubMed]
- Sakamoto, K.; Okahashi, T.; Matsumura, S.; Okafuji, Y.; Adachi, S.; Tsuzuki, S.; Inoue, K.; Fushiki, T. The opioid system majorly contributes to preference for fat emulsions but not sucrose solutions in mice. Biosci. Biotechnol. Biochem. 2014, 79, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Matsumura, S.; Okafuji, Y.; Eguchi, A.; Yoneda, T.; Mizushige, T.; Tsuzuki, S.; Inoue, K.; Fushiki, T. The opioid system contributes to the acquisition of reinforcement for dietary fat but is not required for its maintenance. Physiol. Behav. 2015, 138, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Hankir, M.K.; Patt, M.; Patt, J.T.; Becker, G.A.; Rullmann, M.; Kranz, M.; Deuther-Conrad, W.; Schischke, K.; Seyfried, F.; Brust, P.; et al. Suppressed fat appetite after roux-en-y gastric bypass surgery associates with reduced brain mu-opioid receptor availability in diet-induced obese male rats. Front. Neurosci. 2017, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Z.; Wang, H.; Yang, M.; Liang, L.; Fu, J.; Wang, C.; Ling, J.; Zhang, Y.; Zhang, S.; et al. Interactions between obesity-related copy number variants and dietary behaviors in childhood obesity. Nutrients 2015, 7, 3054–3066. [Google Scholar] [CrossRef] [PubMed]
- Khandekar, N.; Berning, B.A.; Sainsbury, A.; Lin, S. The role of pancreatic polypeptide in the regulation of energy homeostasis. Mol. Cell. Endocrinol. 2015, 418, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F.; Elbers, C.C.; Adan, R.A.; Loos, R.J.; Onland-Moret, N.C.; Grobbee, D.E.; van Vliet-Ostaptchouk, J.V.; Wijmenga, C.; van der Schouw, Y.T. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am. J. Clin. Nutr. 2009, 90, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.Y.; Workalemahu, T.; Paynter, N.P.; Rose, L.M.; Giulianini, F.; Tanaka, T.; Ngwa, J.S.; Qi, Q.; Curhan, G.C.; Rimm, E.B.; et al. Novel locus including fgf21 is associated with dietary macronutrient intake. Hum. Mol. Genet. 2013, 22, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ngwa, J.S.; van Rooij, F.J.; Zillikens, M.C.; Wojczynski, M.K.; Frazier-Wood, A.C.; Houston, D.K.; Kanoni, S.; Lemaitre, R.N.; Luan, J.; et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am. J. Clin. Nutr. 2013, 97, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the fto gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Brunkwall, L.; Ericson, U.; Hellstrand, S.; Gullberg, B.; Orho-Melander, M.; Sonestedt, E. Genetic variation in the fat mass and obesity-associated gene (fto) in association with food preferences in healthy adults. Food Nutr. Res. 2013, 57. [Google Scholar] [CrossRef] [PubMed]
- Steemburgo, T.; Azevedo, M.J.; Gross, J.L.; Milagro, F.I.; Campion, J.; Martinez, J.A. The rs9939609 polymorphism in the fto gene is associated with fat and fiber intakes in patients with type 2 diabetes. J. Nutrigenet. Nutrigenomics 2013, 6, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Sonestedt, E.; Roos, C.; Gullberg, B.; Ericson, U.; Wirfalt, E.; Orho-Melander, M. Fat and carbohydrate intake modify the association between genetic variation in the fto genotype and obesity. Am. J. Clin. Nutr. 2009, 90, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Gerken, T.; Girard, C.A.; Tung, Y.C.; Webby, C.J.; Saudek, V.; Hewitson, K.S.; Yeo, G.S.; McDonough, M.A.; Cunliffe, S.; McNeill, L.A.; et al. The obesity-associated fto gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007, 318, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.G.; et al. N6-methyladenosine in nuclear rna is a major substrate of the obesity-associated fto. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Mauer, J.; Luo, X.; Blanjoie, A.; Jiao, X.; Grozhik, A.V.; Patil, D.P.; Linder, B.; Pickering, B.F.; Vasseur, J.J.; Chen, Q.; et al. Reversible methylation of m6am in the 5′ cap controls mrna stability. Nature 2017, 541, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, P.K.; Fredriksson, R.; Eriksson, J.D.; Mitra, A.; Radomska, K.J.; Gosnell, B.A.; Solvang, M.N.; Levine, A.S.; Schioth, H.B. Fto colocalizes with a satiety mediator oxytocin in the brain and upregulates oxytocin gene expression. Biochem. Biophys. Res. Commun. 2011, 408, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Karra, E.; O’Daly, O.G.; Choudhury, A.I.; Yousseif, A.; Millership, S.; Neary, M.T.; Scott, W.R.; Chandarana, K.; Manning, S.; Hess, M.E.; et al. A link between fto, ghrelin, and impaired brain food-cue responsivity. J. Clin. Investig. 2013, 123, 3539–3551. [Google Scholar] [CrossRef] [PubMed]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Sassone-Corsi, P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.S.; Tsai, J.Y.; Villegas-Montoya, C.; Boland, B.B.; Blasier, Z.; Egbejimi, O.; Kueht, M.; Young, M.E. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int. J. Obes. (Lond.) 2010, 34, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Melanson, E.L.; Higgins, J.; Connick, E.; Moehlman, T.M.; Stothard, E.R.; Wright, K.P., Jr. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. USA 2014, 111, 17302–17307. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef] [PubMed]
- Timlin, M.T.; Pereira, M.A.; Story, M.; Neumark-Sztainer, D. Breakfast eating and weight change in a 5-year prospective analysis of adolescents: Project eat (eating among teens). Pediatrics 2008, 121, e638–e645. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gomez-Abellan, P.; Alburquerque-Bejar, J.J.; Lee, Y.C.; Ordovas, J.M.; Scheer, F.A. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. (Lond.) 2013, 37, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. Dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, T.; Tada, Y.; Hida, A.; Sunami, A.; Yokoyama, Y.; Yasuda, J.; Nakai, A.; Togo, F.; Kawano, Y. Effects of feeding schedule changes on the circadian phase of the cardiac autonomic nervous system and serum lipid levels. Eur. J. Appl. Physiol. 2013, 113, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Colles, S.L.; Dixon, J.B.; O’Brien, P.E. Night eating syndrome and nocturnal snacking: Association with obesity, binge eating and psychological distress. Int. J. Obes. (Lond.) 2007, 31, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Koopman, K.E.; Caan, M.W.; Nederveen, A.J.; Pels, A.; Ackermans, M.T.; Fliers, E.; la Fleur, S.E.; Serlie, M.J. Hypercaloric diets with increased meal frequency, but not meal size, increase intrahepatic triglycerides: A randomized controlled trial. Hepatology 2014, 60, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Zarrinpar, A.; Chaix, A.; Panda, S. Daily eating patterns and their impact on health and disease. Trends Endocrinol. Metab. 2016, 27, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Kettner, N.M.; Mayo, S.A.; Hua, J.; Lee, C.; Moore, D.D.; Fu, L. Circadian dysfunction induces leptin resistance in mice. Cell Metab. 2015, 22, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, K.; Sakata, T.; Yoshimatsu, H.; Fujimoto, K.; Uchimura, K.; Asano, C. Advance shift of feeding circadian rhythm induced by obesity progression in zucker rats. Am. J. Physiol. 1992, 263, R1169–R1175. [Google Scholar] [PubMed]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009, 17, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gomez-Abellan, P. Timing of food intake and obesity: A novel association. Physiol. Behav. 2014, 134, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Sherman, H.; Genzer, Y.; Cohen, R.; Chapnik, N.; Madar, Z.; Froy, O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012, 26, 3493–3502. [Google Scholar] [CrossRef] [PubMed]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Fuse, Y.; Hirao, A.; Kuroda, H.; Otsuka, M.; Tahara, Y.; Shibata, S. Differential roles of breakfast only (one meal per day) and a bigger breakfast with a small dinner (two meals per day) in mice fed a high-fat diet with regard to induced obesity and lipid metabolism. J. Circadian Rhythms 2012, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Delgado, R.; Angeles-Castellanos, M.; Saderi, N.; Buijs, R.M.; Escobar, C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology 2010, 151, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Sun, L.; ZhuGe, F.; Guo, X.; Zhao, Z.; Tang, R.; Chen, Q.; Chen, L.; Kato, H.; Fu, Z. Differential roles of breakfast and supper in rats of a daily three-meal schedule upon circadian regulation and physiology. Chronobiol. Int. 2011, 28, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.; Shikata, N.; Seki, S.; Koyama, N.; Noguchi, Y. Early nocturnal meal skipping alters the peripheral clock and increases lipogenesis in mice. Nutr. Metab. (Lond.) 2012, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Herzog, E.D.; Hermanstyne, T.; Smyllie, N.J.; Hastings, M.H. Regulating the suprachiasmatic nucleus (scn) circadian clockwork: Interplay between cell-autonomous and circuit-level mechanisms. Cold Spring Harb. Perspect. Biol. 2017, 9, a027706. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y.; Lenn, N.J. A retinohypothalamic projection in the rat. J. Comp. Neurol. 1972, 146, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, A.E.; Wagoner, N.; Cowan, W.M. An autoradiographic and electron microscopic study of retino-hypothalamic connections. Z. Zellforsch. Mikrosk. Anat. 1972, 135, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Provencio, I.; Tu, D.C.; Pires, S.S.; Rollag, M.D.; Castrucci, A.M.; Pletcher, M.T.; Sato, T.K.; Wiltshire, T.; Andahazy, M.; et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science 2003, 301, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Sato, T.K.; Castrucci, A.M.; Rollag, M.D.; DeGrip, W.J.; Hogenesch, J.B.; Provencio, I.; Kay, S.A. Melanopsin (opn4) requirement for normal light-induced circadian phase shifting. Science 2002, 298, 2213–2216. [Google Scholar] [CrossRef] [PubMed]
- Provencio, I.; Rodriguez, I.R.; Jiang, G.; Hayes, W.P.; Moreira, E.F.; Rollag, M.D. A novel human opsin in the inner retina. J. Neurosci. 2000, 20, 600–605. [Google Scholar] [PubMed]
- Provencio, I.; Rollag, M.D.; Castrucci, A.M. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature 2002, 415, 493. [Google Scholar] [CrossRef] [PubMed]
- Morin, L.P. Neuroanatomy of the extended circadian rhythm system. Exp. Neurol. 2013, 243, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.H.; Aston-Jones, G. Circuit projection from suprachiasmatic nucleus to ventral tegmental area: A novel circadian output pathway. Eur. J. Neurosci. 2009, 29, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Moorman, D.E.; Aston-Jones, G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: Diurnal influences. J. Neurosci. 2010, 30, 15585–15599. [Google Scholar] [CrossRef] [PubMed]
- Bourdy, R.; Barrot, M. A new control center for dopaminergic systems: Pulling the vta by the tail. Trends Neurosci. 2012, 35, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Chen, S.; Zhu, Y.; Oshinsky, M.L. A neural circuit for circadian regulation of arousal. Nat. Neurosci. 2001, 4, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Scammell, T.E.; Gooley, J.J.; Gaus, S.E.; Saper, C.B.; Lu, J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J. Neurosci. 2003, 23, 10691–10702. [Google Scholar] [PubMed]
- Buijs, R.M.; Hou, Y.X.; Shinn, S.; Renaud, L.P. Ultrastructural evidence for intra- and extranuclear projections of gabaergic neurons of the suprachiasmatic nucleus. J. Comp. Neurol. 1994, 340, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Vrang, N.; Larsen, P.J.; Mikkelsen, J.D. Direct projection from the suprachiasmatic nucleus to hypophysiotrophic corticotropin-releasing factor immunoreactive cells in the paraventricular nucleus of the hypothalamus demonstrated by means of phaseolus vulgaris-leucoagglutinin tract tracing. Brain Res. 1995, 684, 61–69. [Google Scholar] [CrossRef]
- Abrahamson, E.E.; Leak, R.K.; Moore, R.Y. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport 2001, 12, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Schibler, U.; Ripperger, J.; Brown, S.A. Peripheral circadian oscillators in mammals: Time and food. J. Biol. Rhythms 2003, 18, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Vollmers, C.; Gill, S.; DiTacchio, L.; Pulivarthy, S.R.; Le, H.D.; Panda, S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. USA 2009, 106, 21453–21458. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.L.; Patel, V.R.; de Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the circadian clock by nutritional challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; la Fleur, S.E.; Wortel, J.; Van Heyningen, C.; Zuiddam, L.; Mettenleiter, T.C.; Kalsbeek, A.; Nagai, K.; Niijima, A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 2003, 464, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.Y.; Bullock, C.M.; Li, C.; Lee, A.G.; Bermak, J.C.; Belluzzi, J.; Weaver, D.R.; Leslie, F.M.; Zhou, Q.Y. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 2002, 417, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Li, J.D.; Hu, W.P.; Boehmer, L.; Cheng, M.Y.; Lee, A.G.; Jilek, A.; Siegel, J.M.; Zhou, Q.Y. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J. Neurosci. 2006, 26, 11615–11623. [Google Scholar] [CrossRef] [PubMed]
- Prosser, H.M.; Bradley, A.; Chesham, J.E.; Ebling, F.J.; Hastings, M.H.; Maywood, E.S. Prokineticin receptor 2 (prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc. Natl. Acad. Sci. USA 2007, 104, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Buijs, R.M.; van Heerikhuize, J.J.; Arts, M.; van der Woude, T.P. Vasopressin-containing neurons of the suprachiasmatic nuclei inhibit corticosterone release. Brain Res. 1992, 580, 62–67. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Fliers, E.; Hofman, M.A.; Swaab, D.F.; Buijs, R.M. Vasopressin and the output of the hypothalamic biological clock. J. Neuroendocrinol. 2010, 22, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Kraves, S.; Weitz, C.J. A role for cardiotrophin-like cytokine in the circadian control of mammalian locomotor activity. Nat. Neurosci. 2006, 9, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Buijs, R.M. Peptidergic transmitters of the suprachiasmatic nuclei and the control of circadian rhythmicity. Prog. Brain Res. 1992, 92, 321–333. [Google Scholar] [PubMed]
- Kantor, S.; Mochizuki, T.; Janisiewicz, A.M.; Clark, E.; Nishino, S.; Scammell, T.E. Orexin neurons are necessary for the circadian control of rem sleep. Sleep 2009, 32, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Mieda, M.; Williams, S.C.; Sinton, C.M.; Richardson, J.A.; Sakurai, T.; Yanagisawa, M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J. Neurosci. 2004, 24, 10493–10501. [Google Scholar] [CrossRef] [PubMed]
- Webb, I.C.; Patton, D.F.; Hamson, D.K.; Mistlberger, R.E. Neural correlates of arousal-induced circadian clock resetting: Hypocretin/orexin and the intergeniculate leaflet. Eur. J. Neurosci. 2008, 27, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Colwell, C.S.; Michel, S.; Itri, J.; Rodriguez, W.; Tam, J.; Lelievre, V.; Hu, Z.; Waschek, J.A. Selective deficits in the circadian light response in mice lacking pacap. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1194–R1201. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, J.; Georg, B.; Fahrenkrug, J. Altered circadian food anticipatory activity rhythms in pacap receptor 1 (pac1) deficient mice. PLoS ONE 2016, 11, e0146981. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, C.; Tanaka, K.; Isojima, Y.; Shintani, N.; Hashimoto, H.; Baba, A.; Nagai, K. Changes in light-induced phase shift of circadian rhythm in mice lacking pacap. Biochem. Biophys. Res. Commun. 2003, 310, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sankrithi, N.; Davis, F.C. Transforming growth factor-alpha is expressed in astrocytes of the suprachiasmatic nucleus in hamster: Role of glial cells in circadian clocks. Neuroreport 2002, 13, 2143–2147. [Google Scholar] [CrossRef] [PubMed]
- Engel, L.; Lorenzkowski, V.; Langer, C.; Rohleder, N.; Spessert, R. The photoperiod entrains the molecular clock of the rat pineal. Eur. J. Neurosci. 2005, 21, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.Y. Neural control of the pineal gland. Behav. Brain Res. 1996, 73, 125–130. [Google Scholar] [CrossRef]
- Kalsbeek, A.; van der Vliet, J.; Buijs, R.M. Decrease of endogenous vasopressin release necessary for expression of the circadian rise in plasma corticosterone: A reverse microdialysis study. J. Neuroendocrinol. 1996, 8, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; van Heerikhuize, J.J.; Wortel, J.; Buijs, R.M. A diurnal rhythm of stimulatory input to the hypothalamo-pituitary-adrenal system as revealed by timed intrahypothalamic administration of the vasopressin v1 antagonist. J. Neurosci. 1996, 16, 5555–5565. [Google Scholar] [PubMed]
- Boulos, Z.; Rosenwasser, A.M.; Terman, M. Feeding schedules and the circadian organization of behavior in the rat. Behav. Brain Res. 1980, 1, 39–65. [Google Scholar] [CrossRef]
- Stephan, F.K.; Swann, J.M.; Sisk, C.L. Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav. Neural Biol. 1979, 25, 346–363. [Google Scholar] [CrossRef]
- Honma, K.; Honma, S. The scn-independent clocks, methamphetamine and food restriction. Eur. J. Neurosci. 2009, 30, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Pevet, P.; Felder-Schmittbuhl, M.P.; Bailly, Y.; Challet, E. The cerebellum harbors a circadian oscillator involved in food anticipation. J. Neurosci. 2010, 30, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Landry, G.J.; Kent, B.A.; Patton, D.F.; Jaholkowski, M.; Marchant, E.G.; Mistlberger, R.E. Evidence for time-of-day dependent effect of neurotoxic dorsomedial hypothalamic lesions on food anticipatory circadian rhythms in rats. PLoS ONE 2011, 6, e24187. [Google Scholar] [CrossRef] [PubMed]
- Mieda, M.; Williams, S.C.; Richardson, J.A.; Tanaka, K.; Yanagisawa, M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc. Natl. Acad. Sci. USA 2006, 103, 12150–12155. [Google Scholar] [CrossRef] [PubMed]
- Gooley, J.J.; Schomer, A.; Saper, C.B. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat. Neurosci. 2006, 9, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.M.; Darvas, M.; Oviatt, M.; Chang, C.H.; Michalik, M.; Huddy, T.F.; Meyer, E.E.; Shuster, S.A.; Aguayo, A.; Hill, E.M.; et al. Dopamine receptor 1 neurons in the dorsal striatum regulate food anticipatory circadian activity rhythms in mice. eLife 2014, 3, e03781. [Google Scholar] [CrossRef] [PubMed]
- Verwey, M.; Amir, S. Food-entrainable circadian oscillators in the brain. Eur. J. Neurosci. 2009, 30, 1650–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, J.; Angeles-Castellanos, M.; Escobar, C. Entrainment by a palatable meal induces food-anticipatory activity and c-fos expression in reward-related areas of the brain. Neuroscience 2005, 133, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, D.A.; Loudon, A.S. Hypothalamic clocks and rhythms in feeding behaviour. Trends Neurosci. 2013, 36, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.M.; Perez-Tilve, D.; Nogueiras, R.; Fang, J.; Kim, J.K.; Cone, R.D.; Gimble, J.M.; Tschop, M.H.; Butler, A.A. The melanocortin-3 receptor is required for entrainment to meal intake. J. Neurosci. 2008, 28, 12946–12955. [Google Scholar] [CrossRef] [PubMed]
- Landry, G.J.; Yamakawa, G.R.; Webb, I.C.; Mear, R.J.; Mistlberger, R.E. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J. Biol. Rhythms 2007, 22, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Solis, R.; Ramadori, G.; Coppari, R.; Sassone-Corsi, P. Sirt1 relays nutritional inputs to the circadian clock through the sf1 neurons of the ventromedial hypothalamus. Endocrinology 2015, 156, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Solis, R.; Aguilar-Arnal, L.; Murakami, M.; Peruquetti, R.; Ramadori, G.; Coppari, R.; Sassone-Corsi, P. The circadian clock in the ventromedial hypothalamus controls cyclic energy expenditure. Cell Metab. 2016, 23, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kikuchi, O.; Shimpuku, M.; Susanti, V.Y.; Yokota-Hashimoto, H.; Taguchi, R.; Shibusawa, N.; Sato, T.; Tang, L.; Amano, K.; et al. Hypothalamic sirt1 prevents age-associated weight gain by improving leptin sensitivity in mice. Diabetologia 2014, 57, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Kim, H.J.; Kobayashi, M.; Kitamura, Y.I.; Yokota-Hashimoto, H.; Shiuchi, T.; Minokoshi, Y.; Kitamura, T. Induction of hypothalamic sirt1 leads to cessation of feeding via agouti-related peptide. Endocrinology 2010, 151, 2556–2566. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Brace, C.S.; Ben-Josef, G.; West, T.; Wozniak, D.F.; Holtzman, D.M.; Herzog, E.D.; Imai, S. Sirt1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J. Neurosci. 2010, 30, 10220–10232. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Steele, A.D.; Lindquist, S.; Guarente, L. Increase in activity during calorie restriction requires sirt1. Science 2005, 310, 1641. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Clesse, D.; Pevet, P.; Challet, E. Food-reward signalling in the suprachiasmatic clock. J. Neurochem. 2010, 112, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.N.; Patton, D.F.; Michalik, M.; Opiol, H.; Mistlberger, R.E. Dopaminergic regulation of circadian food anticipatory activity rhythms in the rat. PLoS ONE 2013, 8, e82381. [Google Scholar] [CrossRef] [PubMed]
- Mieda, M.; Sakurai, T. Bmal1 in the nervous system is essential for normal adaptation of circadian locomotor activity and food intake to periodic feeding. J. Neurosci. 2011, 31, 15391–15396. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, J.S.; Oda, G.A.; Niswender, K.D.; Yamazaki, S. Period determination in the food-entrainable and methamphetamine-sensitive circadian oscillator(s). Proc. Natl. Acad. Sci. USA 2012, 109, 14218–14223. [Google Scholar] [CrossRef] [PubMed]
- Pitts, S.; Perone, E.; Silver, R. Food-entrained circadian rhythms are sustained in arrhythmic clk/clk mutant mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R57–R67. [Google Scholar] [CrossRef] [PubMed]
- Dudley, C.A.; Erbel-Sieler, C.; Estill, S.J.; Reick, M.; Franken, P.; Pitts, S.; McKnight, S.L. Altered patterns of sleep and behavioral adaptability in npas2-deficient mice. Science 2003, 301, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Yamaguchi, S.; van der Horst, G.T.; Bonnefont, X.; Okamura, H.; Shibata, S. Altered food-anticipatory activity rhythm in cryptochrome-deficient mice. Neurosci. Res. 2005, 52, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, E.A.; Havekes, R.; Barf, R.P.; Hut, R.A.; Nijholt, I.M.; Jacobs, E.H.; Gerkema, M.P. Circadian time-place learning in mice depends on cry genes. Curr. Biol. 2008, 18, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, J.S.; Nakamura, W.; Friday, R.C.; Hatanaka, F.; Takumi, T.; Yamazaki, S. Robust food anticipatory activity in bmal1-deficient mice. PLoS ONE 2009, 4, e4860. [Google Scholar] [CrossRef] [PubMed]
- Storch, K.F.; Weitz, C.J. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc. Natl. Acad. Sci. USA 2009, 106, 6808–6813. [Google Scholar] [CrossRef] [PubMed]
- Mistlberger, R.; Rusak, B. Palatable daily meals entrain anticipatory activity rhythms in free-feeding rats: Dependence on meal size and nutrient content. Physiol. Behav. 1987, 41, 219–226. [Google Scholar] [CrossRef]
- Webb, I.C.; Baltazar, R.M.; Lehman, M.N.; Coolen, L.M. Bidirectional interactions between the circadian and reward systems: Is restricted food access a unique zeitgeber? Eur. J. Neurosci. 2009, 30, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, J.S.; Branecky, K.L.; Yang, W.; Ellacott, K.L.; Niswender, K.D.; Yamazaki, S. High-fat diet acutely affects circadian organisation and eating behavior. Eur. J. Neurosci. 2013, 37, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Webb, I.C.; Lehman, M.N.; Coolen, L.M. Diurnal and circadian regulation of reward-related neurophysiology and behavior. Physiol. Behav. 2015, 143, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Lee, E.J.; Yun, S.; Choe, H.K.; Park, S.B.; Son, H.J.; Kim, K.S.; Dluzen, D.E.; Lee, I.; Hwang, O.; et al. Impact of circadian nuclear receptor rev-erbalpha on midbrain dopamine production and mood regulation. Cell 2014, 157, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Webb, I.C.; Baltazar, R.M.; Wang, X.; Pitchers, K.K.; Coolen, L.M.; Lehman, M.N. Diurnal variations in natural and drug reward, mesolimbic tyrosine hydroxylase, and clock gene expression in the male rat. J. Biol. Rhythms 2009, 24, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Olson, R.J.; Justice, J.B., Jr. Quantitative microdialysis of dopamine in the striatum: Effect of circadian variation. J. Neurosci. Methods 1992, 44, 33–41. [Google Scholar] [CrossRef]
- Paulson, P.E.; Robinson, T.E. Relationship between circadian changes in spontaneous motor activity and dorsal versus ventral striatal dopamine neurotransmission assessed with on-line microdialysis. Behav. Neurosci. 1994, 108, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.; Cassidy, P.; Cossette, M.P.; Weigl, Y.; Verwey, M.; Robinson, B.; Stewart, J.; Amir, S. Endogenous dopamine regulates the rhythm of expression of the clock protein per2 in the rat dorsal striatum via daily activation of d2 dopamine receptors. J. Neurosci. 2010, 30, 14046–14058. [Google Scholar] [CrossRef] [PubMed]
- Ferris, M.J.; Espana, R.A.; Locke, J.L.; Konstantopoulos, J.K.; Rose, J.H.; Chen, R.; Jones, S.R. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc. Natl. Acad. Sci. USA 2014, 111, E2751–E2759. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, T.R.; de Prado, B.M.; Prieto, D.; Mora, F. Circadian rhythms of dopamine, glutamate and gaba in the striatum and nucleus accumbens of the awake rat: Modulation by light. J. Pineal Res. 2004, 36, 177–185. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.D.; Fillenz, M. Simultaneous monitoring of dopamine release in rat frontal cortex, nucleus accumbens and striatum: Effect of drugs, circadian changes and correlations with motor activity. Neuroscience 1985, 16, 49–55. [Google Scholar] [CrossRef]
- O’Neill, R.D. Uric acid levels and dopamine transmission in rat striatum: Diurnal changes and effects of drugs. Brain Res. 1990, 507, 267–272. [Google Scholar] [CrossRef]
- Hampp, G.; Ripperger, J.A.; Houben, T.; Schmutz, I.; Blex, C.; Perreau-Lenz, S.; Brunk, I.; Spanagel, R.; Ahnert-Hilger, G.; Meijer, J.H.; et al. Regulation of monoamine oxidase a by circadian-clock components implies clock influence on mood. Curr. Biol. 2008, 18, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, R.M.; Coolen, L.M.; Webb, I.C. Diurnal rhythms in neural activation in the mesolimbic reward system: Critical role of the medial prefrontal cortex. Eur. J. Neurosci. 2013, 38, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Sleipness, E.P.; Sorg, B.A.; Jansen, H.T. Diurnal differences in dopamine transporter and tyrosine hydroxylase levels in rat brain: Dependence on the suprachiasmatic nucleus. Brain Res. 2007, 1129, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Challet, E. Circadian insights into dopamine mechanisms. Neuroscience 2014, 282, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Coque, L.; Cao, J.L.; Kumar, J.; Chakravarty, S.; Asaithamby, A.; Graham, A.; Gordon, E.; Enwright, J.F., 3rd; DiLeone, R.J.; et al. Knockdown of clock in the ventral tegmental area through rna interference results in a mixed state of mania and depression-like behavior. Biol. Psychiatry 2010, 68, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Strother, W.N.; Norman, A.B.; Lehman, M.N. D1-dopamine receptor binding and tyrosine hydroxylase-immunoreactivity in the fetal and neonatal hamster suprachiasmatic nucleus. Brain Res. Dev. Brain Res. 1998, 106, 137–144. [Google Scholar] [CrossRef]
- Rivkees, S.A.; Lachowicz, J.E. Functional d1 and d5 dopamine receptors are expressed in the suprachiasmatic, supraoptic, and paraventricular nuclei of primates. Synapse 1997, 26, 1–10. [Google Scholar] [CrossRef]
- Smyllie, N.J.; Chesham, J.E.; Hamnett, R.; Maywood, E.S.; Hastings, M.H. Temporally chimeric mice reveal flexibility of circadian period-setting in the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA 2016, 113, 3657–3662. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Honma, K.; Hiroshige, T. Methamphetamine induced locomotor rhythm entrains to restricted daily feeding in scn lesioned rats. Physiol. Behav. 1989, 45, 1057–1065. [Google Scholar] [CrossRef]
- Ono, M.; Watanabe, A.; Matsumoto, Y.; Fukushima, T.; Nishikawa, Y.; Moriya, T.; Shibata, S.; Watanabe, S. Methamphetamine modifies the photic entraining responses in the rodent suprachiasmatic nucleus via serotonin release. Neuroscience 1996, 72, 213–224. [Google Scholar] [CrossRef]
- Cohen, S.; Vainer, E.; Matar, M.A.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Mathe, A.A.; Cohen, H. Diurnal fluctuations in hpa and neuropeptide y-ergic systems underlie differences in vulnerability to traumatic stress responses at different zeitgeber times. Neuropsychopharmacology 2015, 40, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Akabayashi, A.; Levin, N.; Paez, X.; Alexander, J.T.; Leibowitz, S.F. Hypothalamic neuropeptide y and its gene expression: Relation to light/dark cycle and circulating corticosterone. Mol. Cell. Neurosci. 1994, 5, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Kalra, P.S.; Farmerie, W.G.; Kalra, S.P. Daily changes in hypothalamic gene expression of neuropeptide y, galanin, proopiomelanocortin, and adipocyte leptin gene expression and secretion: Effects of food restriction. Endocrinology 1999, 140, 2868–2875. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Honma, S.; Honma, K. Effects of restricted daily feeding on neuropeptide y release in the rat paraventricular nucleus. Am. J. Physiol. 1996, 270, E589–E595. [Google Scholar] [PubMed]
- Wiater, M.F.; Mukherjee, S.; Li, A.J.; Dinh, T.T.; Rooney, E.M.; Simasko, S.M.; Ritter, S. Circadian integration of sleep-wake and feeding requires npy receptor-expressing neurons in the mediobasal hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1569–R1583. [Google Scholar] [CrossRef] [PubMed]
- Gunapala, K.M.; Gallardo, C.M.; Hsu, C.T.; Steele, A.D. Single gene deletions of orexin, leptin, neuropeptide y, and ghrelin do not appreciably alter food anticipatory activity in mice. PLoS ONE 2011, 6, e18377. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.X.; van der Vliet, J.; Dai, J.; Yin, G.; Ru, L.; Buijs, R.M. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 2006, 147, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Card, J.P.; Moore, R.Y. Ventral lateral geniculate nucleus efferents to the rat suprachiasmatic nucleus exhibit avian pancreatic polypeptide-like immunoreactivity. J. Comp. Neurol. 1982, 206, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Saderi, N.; Cazarez-Marquez, F.; Buijs, F.N.; Salgado-Delgado, R.C.; Guzman-Ruiz, M.A.; del Carmen Basualdo, M.; Escobar, C.; Buijs, R.M. The npy intergeniculate leaflet projections to the suprachiasmatic nucleus transmit metabolic conditions. Neuroscience 2013, 246, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Wiater, M.F.; Oostrom, M.T.; Smith, B.R.; Wang, Q.; Dinh, T.T.; Roberts, B.L.; Jansen, H.T.; Ritter, S. Leptin-sensitive neurons in the arcuate nuclei contribute to endogenous feeding rhythms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R1313–R1326. [Google Scholar] [CrossRef] [PubMed]
- Tiesjema, B.; Adan, R.A.; Luijendijk, M.C.; Kalsbeek, A.; la Fleur, S.E. Differential effects of recombinant adeno-associated virus-mediated neuropeptide y overexpression in the hypothalamic paraventricular nucleus and lateral hypothalamus on feeding behavior. J. Neurosci. 2007, 27, 14139–14146. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, L.; Lozeva, V.; Valjakka, A.; Lecklin, A. Modifying effects of histamine on circadian rhythms and neuronal excitability. Behav. Brain Res. 2001, 124, 129–135. [Google Scholar] [CrossRef]
- Yu, X.; Zecharia, A.; Zhang, Z.; Yang, Q.; Yustos, R.; Jager, P.; Vyssotski, A.L.; Maywood, E.S.; Chesham, J.E.; Ma, Y.; et al. Circadian factor bmal1 in histaminergic neurons regulates sleep architecture. Curr. Biol. 2014, 24, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, H. Hypothalamic neuronal histamine regulates body weight through the modulation of diurnal feeding rhythm. Nutrition 2008, 24, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Pevet, P.; Challet, E. High-fat feeding alters the clock synchronization to light. J. Physiol. 2008, 586, 5901–5910. [Google Scholar] [CrossRef] [PubMed]
- Branecky, K.L.; Niswender, K.D.; Pendergast, J.S. Disruption of daily rhythms by high-fat diet is reversible. PLoS ONE 2015, 10, e0137970. [Google Scholar] [CrossRef] [PubMed]
- Mifune, H.; Tajiri, Y.; Nishi, Y.; Hara, K.; Iwata, S.; Tokubuchi, I.; Mitsuzono, R.; Yamada, K.; Kojima, M. Voluntary exercise contributed to an amelioration of abnormal feeding behavior, locomotor activity and ghrelin production concomitantly with a weight reduction in high fat diet-induced obese rats. Peptides 2015, 71, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Velazquez, A.; Mendoza, J.; Garcia, A.N.; la Fleur, S.E. Diet-induced obesity and circadian disruption of feeding behavior. Front. Neurosci. 2017, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Vgontzas, A.N.; Bixler, E.O.; Fang, J. Sleep is increased by weight gain and decreased by weight loss in mice. Sleep 2008, 31, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.B.; Omori, T.; Guan, Z.; Vgontzas, A.N.; Bixler, E.O.; Fang, J. Sleep is increased in mice with obesity induced by high-fat food. Physiol. Behav. 2006, 87, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Luppi, M.; Cerri, M.; Martelli, D.; Tupone, D.; Del Vecchio, F.; Di Cristoforo, A.; Perez, E.; Zamboni, G.; Amici, R. Waking and sleeping in the rat made obese through a high-fat hypercaloric diet. Behav. Brain Res. 2014, 258, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.P.; Yi, C.X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Posey, K.A.; Clegg, D.J.; Printz, R.L.; Byun, J.; Morton, G.J.; Vivekanandan-Giri, A.; Pennathur, S.; Baskin, D.G.; Heinecke, J.W.; Woods, S.C.; et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1003–E1012. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.L.; Omran, S.F.; Weir, J.; Meikle, P.J.; Watt, M.J. Consumption of a high-fat diet, but not regular endurance exercise training, regulates hypothalamic lipid accumulation in mice. J. Physiol. 2012, 590, 4377–4389. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef] [PubMed]
- Milanski, M.; Degasperi, G.; Coope, A.; Morari, J.; Denis, R.; Cintra, D.E.; Tsukumo, D.M.; Anhe, G.; Amaral, M.E.; Takahashi, H.K.; et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of tlr4 signaling in hypothalamus: Implications for the pathogenesis of obesity. J. Neurosci. 2009, 29, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic ikkbeta/nf-kappab and er stress link overnutrition to energy imbalance and obesity. Cell 2008, 135, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017, 26, 185–197.e3. [Google Scholar] [CrossRef] [PubMed]
- Douglass, J.D.; Dorfman, M.D.; Fasnacht, R.; Shaffer, L.D.; Thaler, J.P. Astrocyte ikkbeta/nf-kappab signaling is required for diet-induced obesity and hypothalamic inflammation. Mol. Metab. 2017, 6, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Cai, D. One step from prediabetes to diabetes: Hypothalamic inflammation? Endocrinology 2012, 153, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Jais, A.; Bruning, J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.; Del Olmo, N.; Valladolid-Acebes, I.; Fole, A.; Cano, V.; Merino, B.; Stucchi, P.; Ruggieri, D.; Lopez, L.; Alguacil, L.F.; et al. Shift of circadian feeding pattern by high-fat diets is coincident with reward deficits in obese mice. PLoS ONE 2012, 7, e36139. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.S.; Ahern, S.A.; Smith, L.C.; da Silva Santos, C.S.; Wager, T.T.; Bechtold, D.A. Targeting of the circadian clock via ck1delta/epsilon to improve glucose homeostasis in obesity. Sci. Rep. 2016, 6, 29983. [Google Scholar] [CrossRef] [PubMed]
- Guilding, C.; Hughes, A.T.; Brown, T.M.; Namvar, S.; Piggins, H.D. A riot of rhythms: Neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol. Brain 2009, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Oosterman, J.E.; Kalsbeek, A.; la Fleur, S.E.; Belsham, D.D. Impact of nutrients on circadian rhythmicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R337–R350. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.J.; Vincent, A.D.; Kennaway, D.J.; Wittert, G.A.; Page, A.J. High-fat diet-induced obesity ablates gastric vagal afferent circadian rhythms. J. Neurosci. 2016, 36, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Stucchi, P.; Hernandez-Nuno, F.; Cano, V.; Morales, L.; Chowen, J.A.; Del Olmo, N.; Ruiz-Gayo, M. Free-choice high-fat diet alters circadian oscillation of energy intake in adolescent mice: Role of prefrontal cortex. Eur. J. Nutr. 2017, 56, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- La Fleur, S.E.; Luijendijk, M.C.; van der Zwaal, E.M.; Brans, M.A.; Adan, R.A. The snacking rat as model of human obesity: Effects of a free-choice high-fat high-sugar diet on meal patterns. Int. J. Obes. (Lond.) 2013, 38, 643–649. [Google Scholar] [CrossRef] [PubMed]
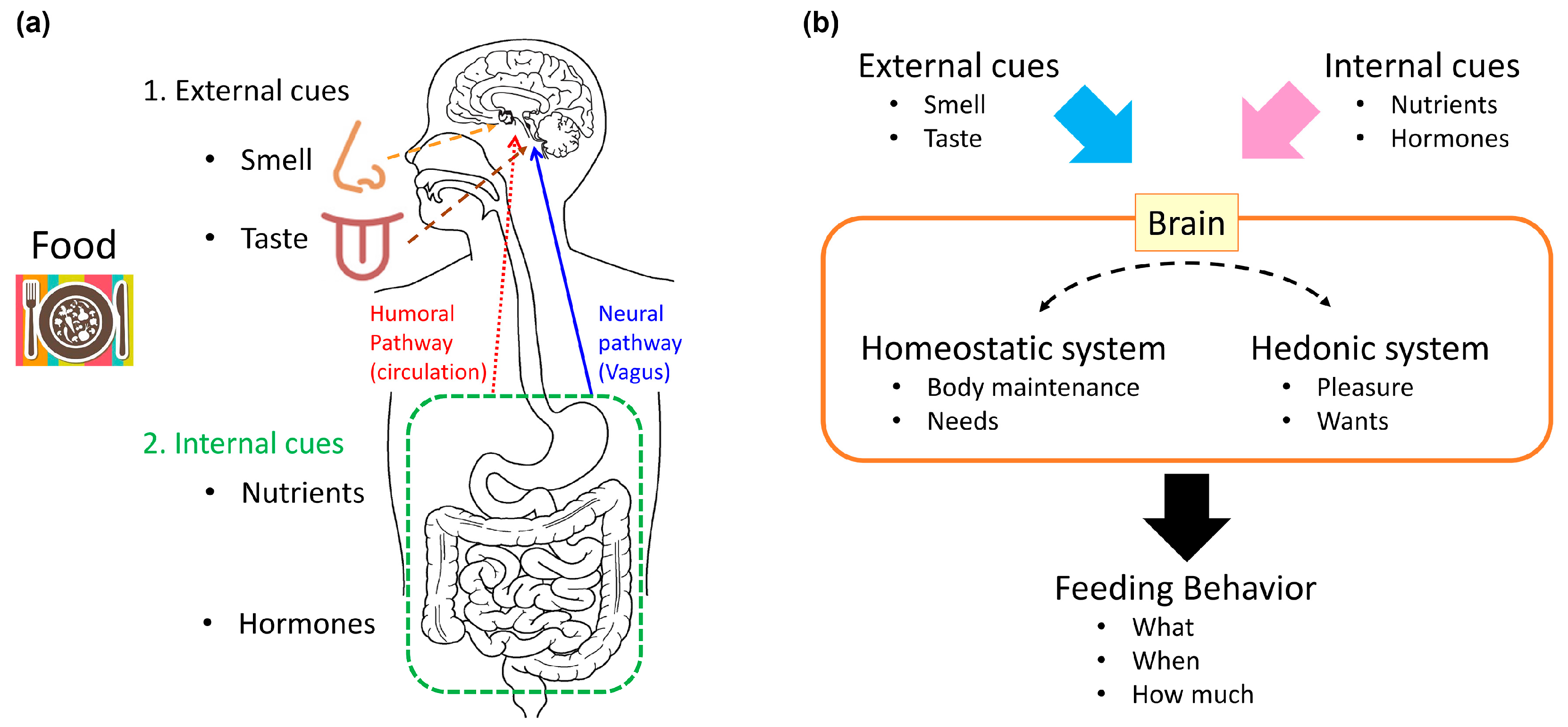
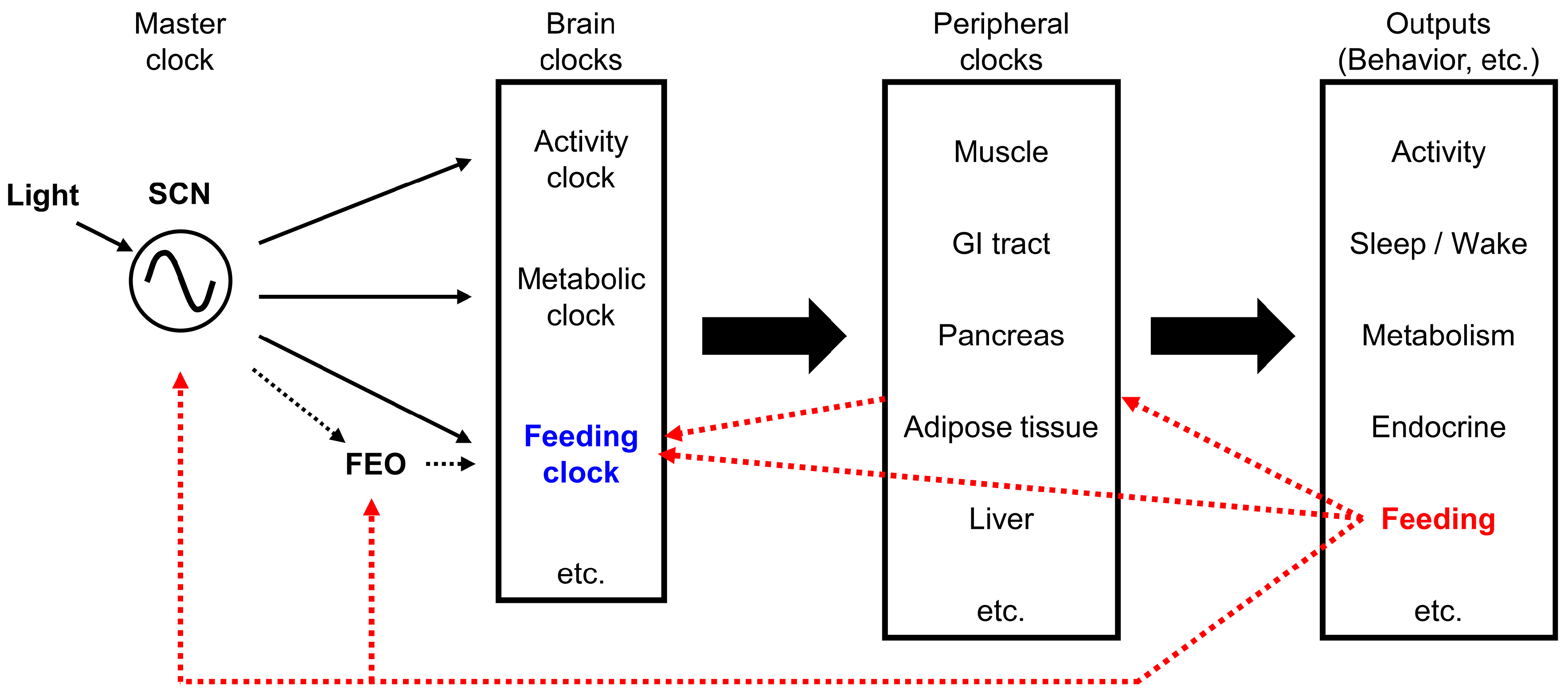
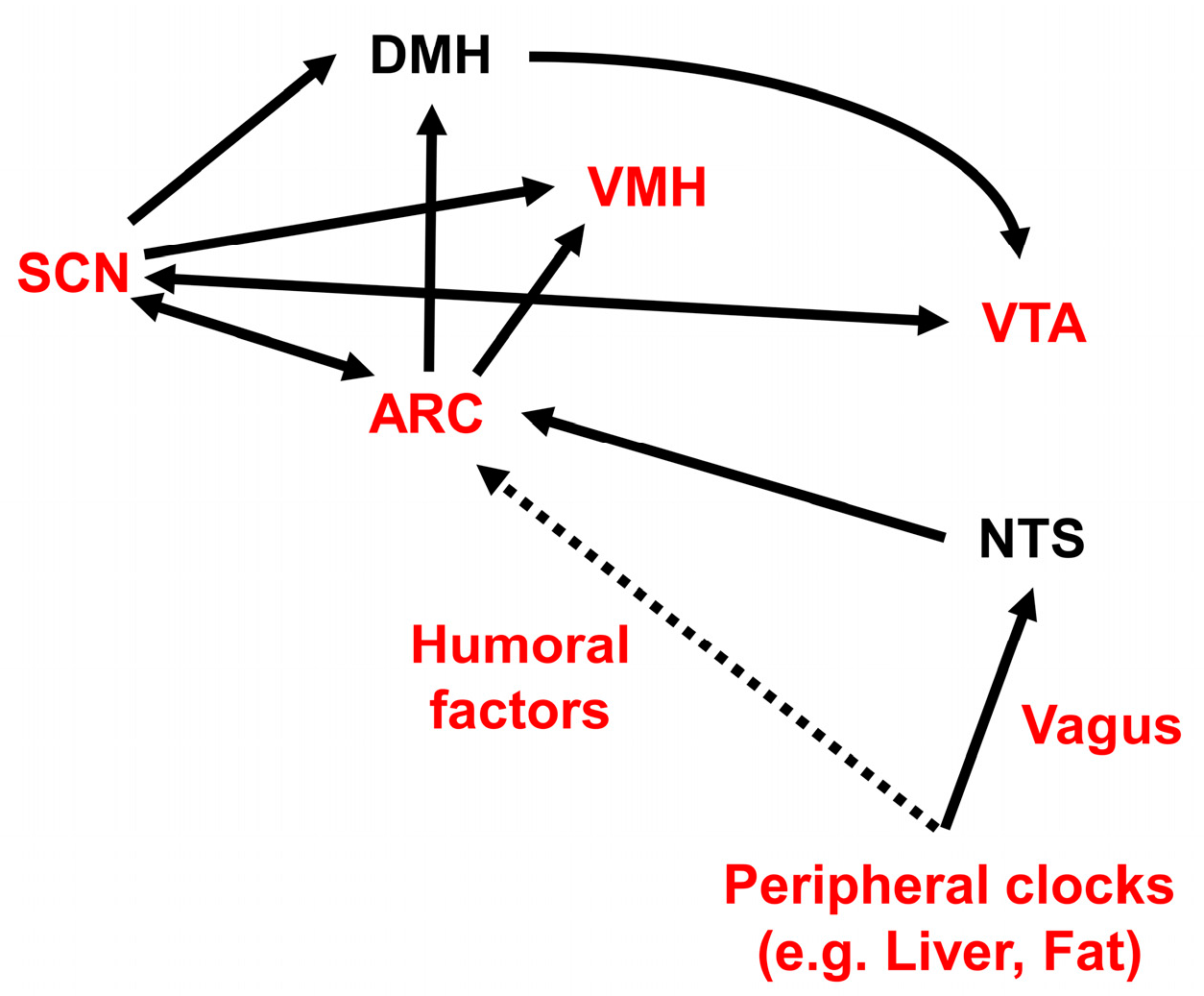
| “Wanting” | Appetitive (Before Ingestion) | “Want to Eat!” | Motivation Incentive Salience | Dopamine System |
|---|---|---|---|---|
| “Liking” | Consummatory (after ingestion) | “Delicious!” | Pleasure Hedonic impact | Opioid system |
| MC4R Mutation (Human) | Pomc-Null (Mice) | Ay/a (Mice) | |
|---|---|---|---|
| Fat preference | Increase | Increase | Increase |
| Carbohydrate preference | Decrease | N.D. 1 | Decrease |
| Macronutrient | Galanin | NMU | NPY | Oxt | MCH | CRH |
|---|---|---|---|---|---|---|
| Neuropeptide effect on fat preference | Increase (PVN) | Decrease (PVN) | Increase (NAc) | (-) | N.D. 1 | Increase (-) |
| Effect of fat ingestion on peptidergic function | Increase (PVN) | N.D. 1 | N.D. 1 | N.D. 1 | N.D. 1 | N.D. 1 |
| Neuropeptide effect on carbohydrate preference | (-) | (-) | Increase (PVN, ICV) | Decrease | Increase vs. (-) | Increase vs. decrease |
| Effect of carbohydrate ingestion on peptidergic function | Decrease (PVN) | N.D. 1 | Increase (ARC, PVN) | Increase (PVN) | N.D. 1 | N.D. 1 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, T. Neural and Molecular Mechanisms Involved in Controlling the Quality of Feeding Behavior: Diet Selection and Feeding Patterns. Nutrients 2017, 9, 1151. https://doi.org/10.3390/nu9101151
Sasaki T. Neural and Molecular Mechanisms Involved in Controlling the Quality of Feeding Behavior: Diet Selection and Feeding Patterns. Nutrients. 2017; 9(10):1151. https://doi.org/10.3390/nu9101151
Chicago/Turabian StyleSasaki, Tsutomu. 2017. "Neural and Molecular Mechanisms Involved in Controlling the Quality of Feeding Behavior: Diet Selection and Feeding Patterns" Nutrients 9, no. 10: 1151. https://doi.org/10.3390/nu9101151





