Comparison of the Micellar Incorporation and the Intestinal Cell Uptake of Cholecalciferol, 25-Hydroxycholecalciferol and 1-α-Hydroxycholecalciferol
Abstract
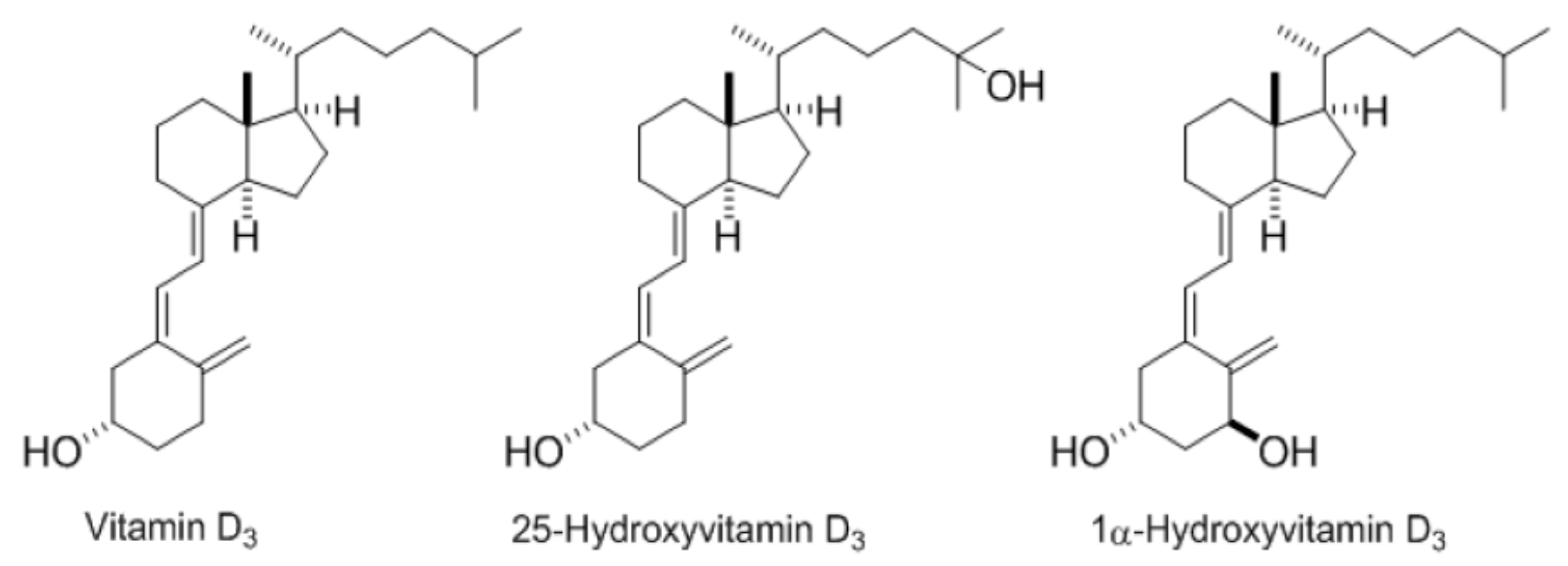
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Mixed Micelles for Micellar Incorporation Experiments and Cell Culture
2.3. Cell Culture
2.3.1. Caco-2 Cell Culture and Experiments
2.3.2. Griptite Cell Culture and Experiments
2.4. Vitamin D Form Extraction
2.5. HPLC Analysis
2.6. Statistical Analysis
3. Results
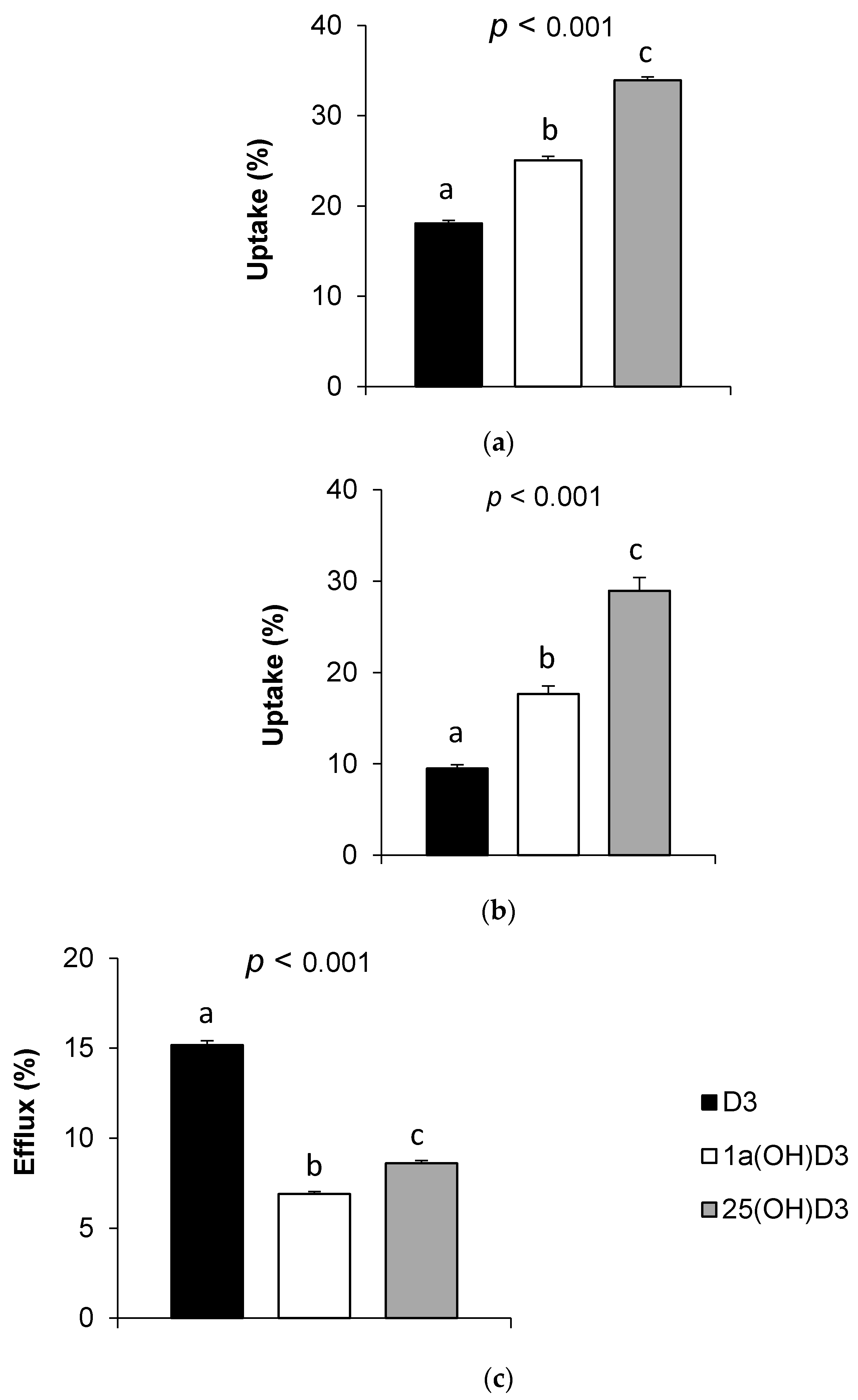
3.1. Incorporation of the Different Vitamin D Forms in Synthetic Mixed Micelles
3.2. Vitamin D Form Uptake by Caco-2 Cells
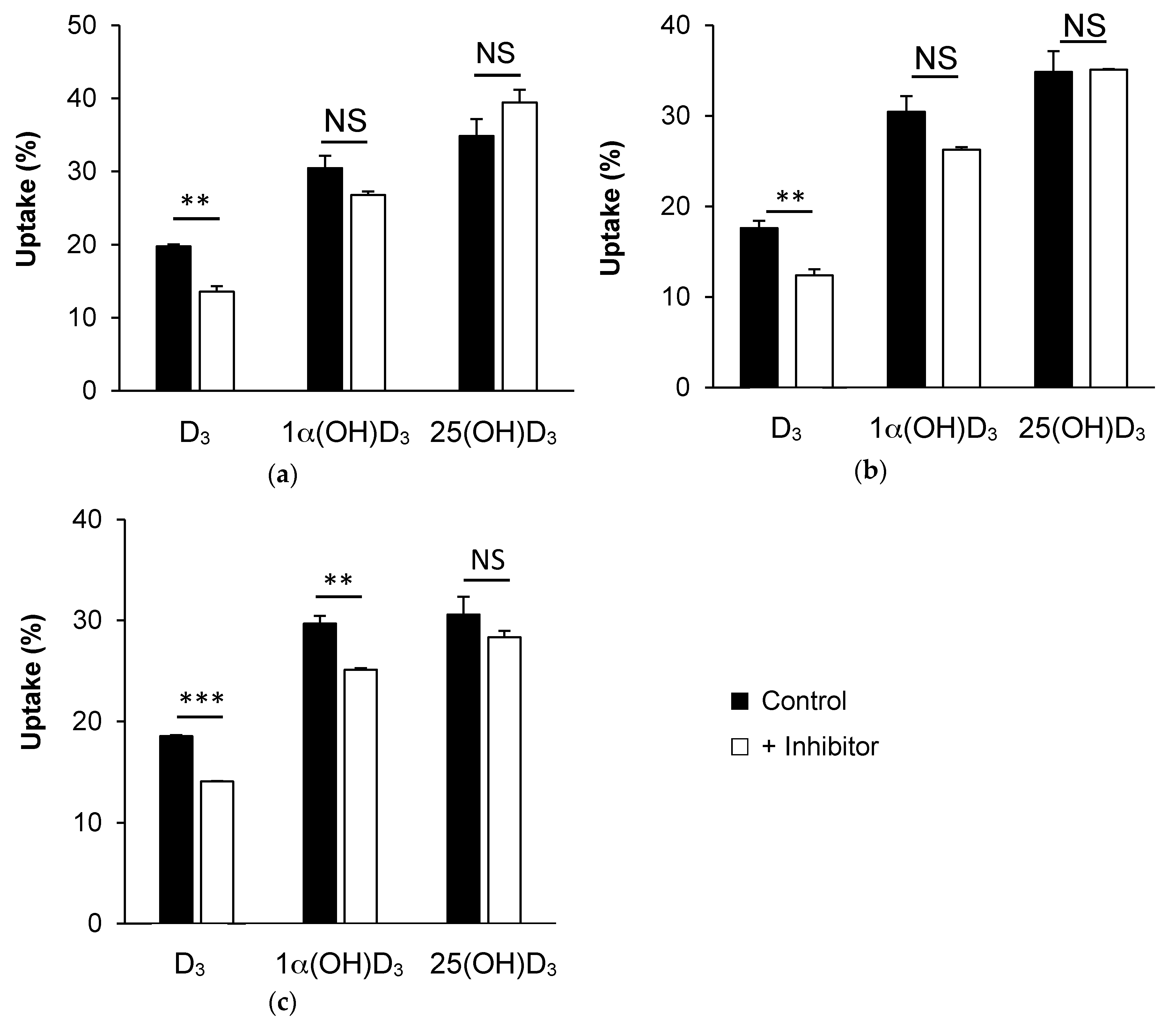
3.3. Effect of Membrane Protein Inhibition on Vitamin D Uptake by Caco-2 Cells
3.3.1. SR-BI Inhibition by BLT1
3.3.2. NPC1L1 Inhibition by Ezetimibe Glucuronide
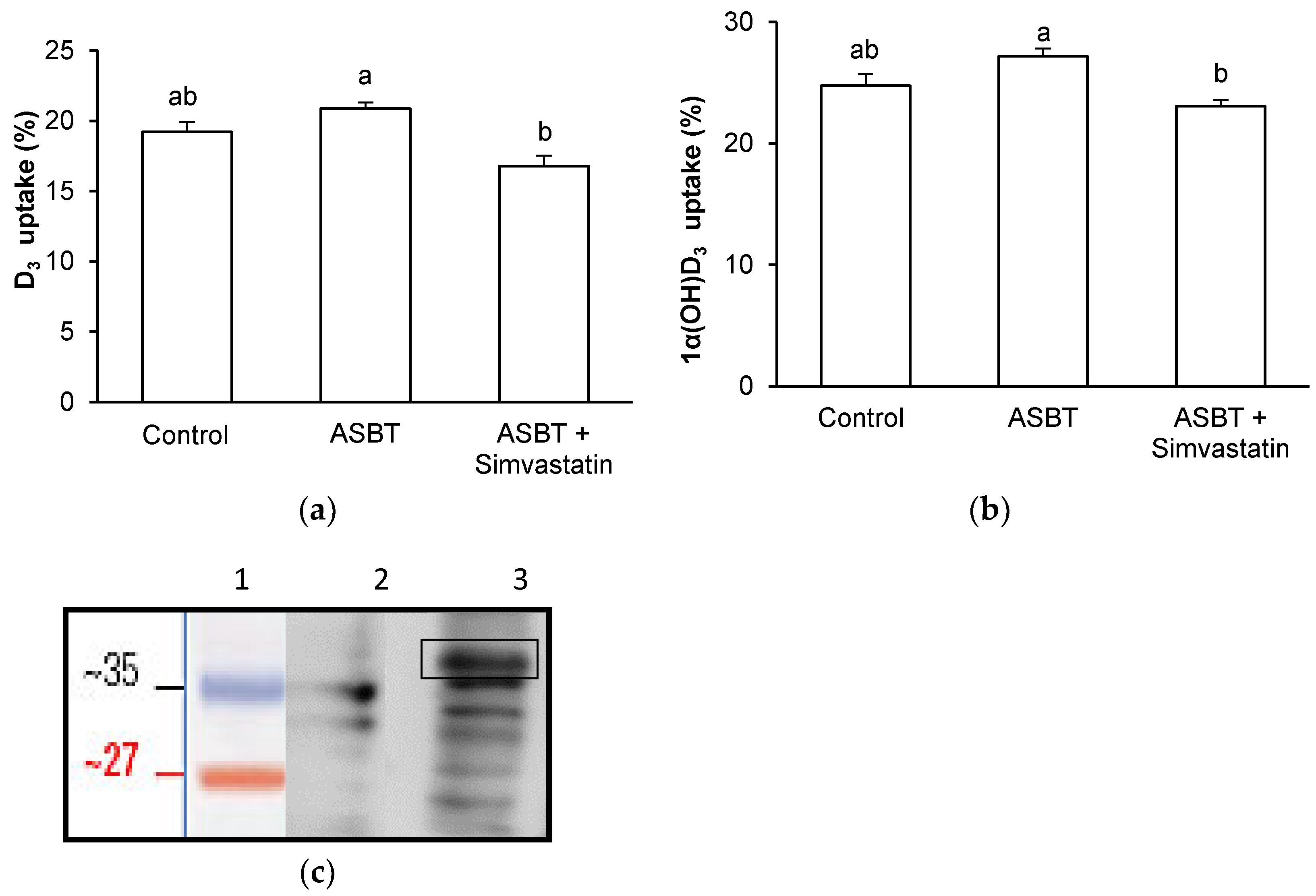
3.3.3. ASBT Inhibition by Simvastatin
3.4. Effect of ASBT Overexpression on D3 and 1α(OH)D3 Uptake by Griptite Cells
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grober, U.; Spitz, J.; Reichrath, J.; Kisters, K.; Holick, M.F. Vitamin D: Update 2013: From rickets prophylaxis to general preventive healthcare. Dermatoendocrinol 2014, 5, 331–347. [Google Scholar] [CrossRef]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef] [PubMed]
- Pramyothin, P.; Holick, M.F. Vitamin D supplementation: Guidelines and evidence for subclinical deficiency. Curr. Opin. Gastroenterol. 2012, 28, 139–150. [Google Scholar] [CrossRef]
- Maillot, M.; Vieux, F.; Ferguson, E.F.; Volatier, J.L.; Amiot, M.J.; Darmon, N. To meet nutrient recommendations, most French adults need to expand their habitual food repertoire. J. Nutr. 2009, 139, 1721–1727. [Google Scholar] [CrossRef]
- Manson, J.E.; Brannon, P.M.; Rosen, C.J.; Taylor, C.L. Vitamin D Deficiency—Is There Really a Pandemic? N. Engl. J. Med. 2016, 375, 1817–1820. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Gonzalez-Gross, M.; Valtuena, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic and consequences for nonskeletal health: Mechanisms of action. Mol. Asp. Med. 2008, 29, 361–368. [Google Scholar] [CrossRef]
- Reboul, E. Intestinal absorption of vitamin D: From the meal to the enterocyte. Food Funct. 2015, 6, 356–362. [Google Scholar] [CrossRef]
- Reboul, E.; Goncalves, A.; Comera, C.; Bott, R.; Nowicki, M.; Landrier, J.F.; Jourdheuil-Rahmani, D.; Dufour, C.; Collet, X.; Borel, P. Vitamin D intestinal absorption is not a simple passive diffusion: Evidences for involvement of cholesterol transporters. Mol. Nutr. Food Res. 2011, 55, 691–702. [Google Scholar] [CrossRef]
- Thompson, G.R.; Lewis, B.; Booth, C.C. Absorption of vitamin D3–3H in control subjects and patients with intestinal malabsorption. J. Clin. Investig. 1966, 45, 94–102. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Borel, P.; Goncalves, A.; Kopec, R.; Nowicki, M.; Morange, S.; Lesavre, N.; Portugal, H.; Reboul, E. A Combination of Single-Nucleotide Polymorphisms Is Associated with Interindividual Variability in Cholecalciferol Bioavailability in Healthy Men. J. Nutr. 2016, 146, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C.; Halekoh, U.; Larsen, T.; Jensen, S.K. Reproductive performance and bone status markers of gilts and lactating sows supplemented with two different forms of vitamin D. J. Anim. Sci. 2010, 88, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Edwards, H.M., Jr. Studies on the efficacy of cholecalciferol and derivatives for stimulating phytate utilization in broilers. Poult. Sci. 2002, 81, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Zerwekh, J.E.; Hesse, R.H.; Rizzardo, E.; Pechet, M.M. Biological activity of 1alpha-hydroxycholecalciferol, a synthetic analog of the hormonal form of vitamin D3. Proc. Natl. Acad. Sci. USA 1973, 70, 2248–2252. [Google Scholar] [CrossRef] [PubMed]
- Boris, A.; Hurley, J.F.; Trmal, T. Relative activities of some metabolites and analogs of cholecalciferol in stimulation of tibia ash weight in chicks otherwise deprived of vitamin D. J. Nutr. 1977, 107, 194–198. [Google Scholar] [PubMed]
- Goncalves, A.; Gontero, B.; Nowicki, M.; Margier, M.; Masset, G.; Amiot, M.J.; Reboul, E. Micellar lipid composition affects micelle interaction with class B scavenger receptor extracellular loops. J. Lipid Res. 2015, 56, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, C.; Tourniaire, F.; Preveraud, D.P.; Samson-Kremser, C.; Crenon, I.; Rosilio, V.; Borel, P. The distribution and relative hydrolysis of tocopheryl acetate in the different matrices coexisting in the lumen of the small intestine during digestion could explain its low bioavailability. Mol. Nutr. Food Res. 2013, 57, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Gleize, B.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.J.; Reboul, E. Fatty acids affect micellar properties and modulate vitamin D uptake and basolateral efflux in Caco-2 cells. J. Nutr. Biochem. 2013, 24, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Margier, M.; Tagliaferri, C.; Lebecque, P.; George, S.; Wittrant, Y.; Coxam, V.; Amiot, M.J.; Reboul, E. Pinoresinol of olive oil decreases vitamin D intestinal absorption. Food Chem. 2016, 206, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Borel, P.; Lietz, G.; Goncalves, A.; Szabo de Edelenyi, F.; Lecompte, S.; Curtis, P.; Goumidi, L.; Caslake, M.J.; Miles, E.A.; Packard, C.; et al. CD36 and SR-BI Are Involved in Cellular Uptake of Provitamin A Carotenoids by Caco-2 and HEK Cells, and Some of Their Genetic Variants Are Associated with Plasma Concentrations of These Micronutrients in Humans. J. Nutr. 2013, 143, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Margier, M.; Roi, S.; Collet, X.; Niot, I.; Goupy, P.; Caris-Veyrat, C.; Reboul, E. Intestinal scavenger receptors are involved in vitamin K1 absorption. J. Biol. Chem. 2014, 289, 30743–30752. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Abou, L.; Mikail, C.; Ghiringhelli, O.; Andre, M.; Portugal, H.; Jourdheuil-Rahmani, D.; Amiot, M.J.; Lairon, D.; Borel, P. Lutein transport by Caco-2 TC-7 cells occurs partly by a facilitated process involving the scavenger receptor class B type I (SR-BI). Biochem. J. 2005, 387, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Margier, M.; Reboul, E. Assessment of the cytotoxicity of synthetic mixed micelles in Griptite cells. NORT Nutrition Obesity and Risk of Thrombosis, Aix-Marseille University, INRA, INSERM, 13385, Marseille, France. Unpublished work. 2017. [Google Scholar]
- Zheng, X.; Ekins, S.; Raufman, J.P.; Polli, J.E. Computational models for drug inhibition of the human apical sodium-dependent bile acid transporter. Mol. Pharm. 2009, 6, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Teegarden, D.; Nickel, K.P.; Shi, L. Characterization of 25-hydroxyvitamin D binding protein from intestinal cells. Biochem. Biophys. Res. Commun. 2000, 275, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Teegarden, D.; Meredith, S.C.; Sitrin, M.D. Determination of the affinity of vitamin D metabolites to serum vitamin D binding protein using assay employing lipid-coated polystyrene beads. Anal. Biochem. 1991, 199, 293–299. [Google Scholar] [CrossRef]
- Han, J.C.; Chen, G.H.; Zhang, J.L.; Wang, J.G.; Qu, H.X.; Yan, Y.F.; Yang, X.J.; Cheng, Y.H. Relative biological value of 1alpha-hydroxycholecalciferol to 25-hydroxycholecalciferol in broiler chicken diets. Poult. Sci. 2017, 96, 2330–2335. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.J.; Reboul, E. Fat-soluble vitamin intestinal absorption: Absorption sites in the intestine and interactions for absorption. Food Chem. 2015, 172, 155–160. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desmarchelier, C.; Margier, M.; Prévéraud, D.P.; Nowicki, M.; Rosilio, V.; Borel, P.; Reboul, E. Comparison of the Micellar Incorporation and the Intestinal Cell Uptake of Cholecalciferol, 25-Hydroxycholecalciferol and 1-α-Hydroxycholecalciferol. Nutrients 2017, 9, 1152. https://doi.org/10.3390/nu9101152
Desmarchelier C, Margier M, Prévéraud DP, Nowicki M, Rosilio V, Borel P, Reboul E. Comparison of the Micellar Incorporation and the Intestinal Cell Uptake of Cholecalciferol, 25-Hydroxycholecalciferol and 1-α-Hydroxycholecalciferol. Nutrients. 2017; 9(10):1152. https://doi.org/10.3390/nu9101152
Chicago/Turabian StyleDesmarchelier, Charles, Marielle Margier, Damien P. Prévéraud, Marion Nowicki, Véronique Rosilio, Patrick Borel, and Emmanuelle Reboul. 2017. "Comparison of the Micellar Incorporation and the Intestinal Cell Uptake of Cholecalciferol, 25-Hydroxycholecalciferol and 1-α-Hydroxycholecalciferol" Nutrients 9, no. 10: 1152. https://doi.org/10.3390/nu9101152





