Social Demography of Transitional Dietary Patterns in Thailand: Prospective Evidence from the Thai Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Selection
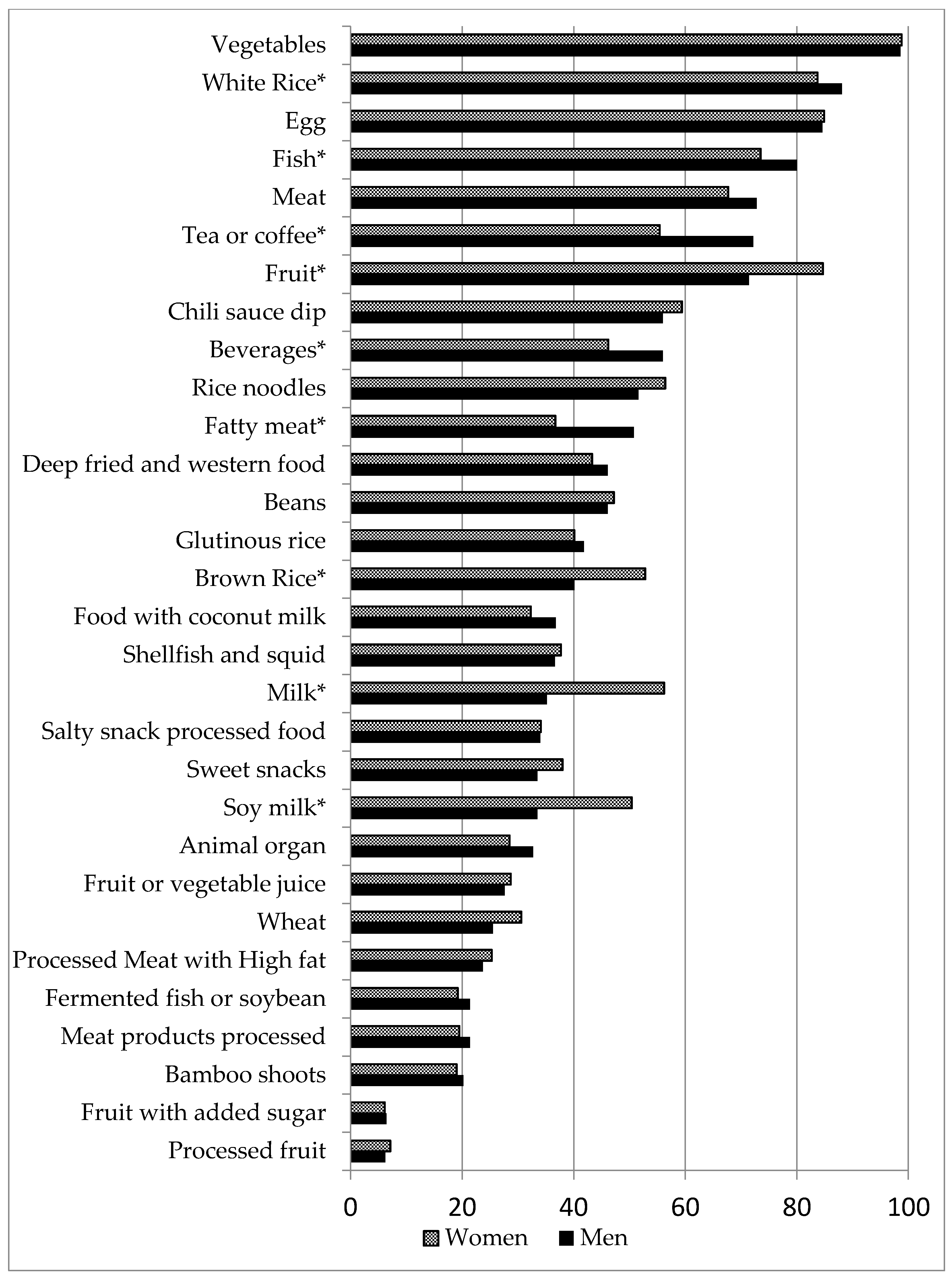
2.2. Dietary Intake
2.3. Socio-Economic Position
2.4. Demographic Factors
2.5. Statistical Methods
2.5.1. Dietary Patterns
2.5.2. Socio-Demographic Predictors of Dietary Patterns
2.5.3. Sensitivity Analysis
2.5.4. Sample Size
2.6. Ethics Approval
3. Results
3.1. Participants
3.2. Diet Patterns
3.3. Socio-Economic Position and Dietary Patterns
3.4. Urbanization and Dietary Patterns
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Health Status Statistics: Mortality. 2014. Available online: http://www.who.int/healthinfo/statistics/indhale/en/ (accessed on 22 September 2014).
- Sleigh, A.; Seubsman, S. Studying the Thai Health-Risk Transition. In Healthy People, Places and Planet; Butler, C., Dixon, J., Capon, A., Eds.; ANU Press: Canberra, Australia, 2015; pp. 166–176. [Google Scholar]
- Popkin, B. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 2006, 84, 289–298. [Google Scholar] [PubMed]
- Du, S.; Mroz, T.A.; Zhai, F.; Popkin, B.M. Rapid income growth adversely affects diet quality in China—Particularly for the poor! Soc. Sci. Med. 2004, 59, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yin, X.M.; Zhang, M.; Leslie, E.; Ware, R.; Owen, N. Family average income and diagnosed type 2 diabetes in urban and rural residents in regional mainland China. Diabet. Med. 2006, 23, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Mayén, A.-L.; Marques-Vidal, P.; Paccaud, F.; Bovet, P.; Stringhini, S. Socioeconomic determinants of dietary patterns in low-and middle-income countries: A systematic review. Am. J. Clin. Nutr. 2014, 100, 1520–1531. [Google Scholar] [CrossRef] [PubMed]
- Angkurawaranon, C.; Jiraporncharoen, W.; Chenthanakij, B.; Doyle, P.; Nitsch, D. Urbanization and non-communicable disease in Southeast Asia: A review of current evidence. Public Health 2014, 128, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Chen, Y.; Wang, F.; Wang, X.; Song, J.; Jiang, Q. High prevalence of hyperglycaemia and the impact of high household income in transforming Rural China. BMC Public Health 2011, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Stronks, K.; Arah, O.A. Global educational disparities in the associations between body mass index and diabetes mellitus in 49 low-income and middle-income countries. J. Epidemiol. Community Health 2014, 68, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Anjana, R.M.; Deepa, M.; Pradeepa, R.; Mahanta, J.; Narain, K.; Das, H.K.; Adhikari, P.; Rao, P.V.; Saboo, B.; Kumar, A.; et al. Prevalence of diabetes and prediabetes in 15 states of India: Results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol 2017. [Google Scholar] [CrossRef]
- Arruda, S.P.M.; Da Silva, A.A.; Kac, G.; Goldani, M.Z.; Bettiol, H.; Barbieri, M.A. Socioeconomic and demographic factors are associated with dietary patterns in a cohort of young Brazilian adults. BMC Public Health 2014, 14, 654. [Google Scholar] [CrossRef] [PubMed]
- Agardh, E.; Allebeck, P.; Hallqvist, J.; Moradi, T.; Sidorchuk, A. Type 2 diabetes incidence and socio-economic position: A systematic review and meta-analysis. Int. J. Epidemiol. 2011, 40, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Bertrais, S.; Péneau, S.; Estaquio, C.; Dauchet, L.; Vergnaud, A.C.; Czernichow, S.; Galan, P.; Hercberg, S.; Bellisle, F. Dietary patterns and their sociodemographic and behavioural correlates in French middle-aged adults from the SU.VI.MAX cohort. Eur. J. Clin. Nutr. 2009, 63, 521–528. [Google Scholar] [CrossRef] [PubMed]
- World Bank Group. Thailand: GNI per Capita, Atlas Method (Current US$); World Bank Group: Washington, DC, USA, 2016. [Google Scholar]
- Papier, K.; Jordan, S.; D’Este, C.; Bain, C.; Peungson, J.; Banwell, C.; Yiengprugsawan, V.; Seubsman, S.; Sleigh, A. Incidence and risk factors for type 2 diabetes mellitus in transitional Thailand: Results from the Thai cohort study. BMJ Open 2016, 6, e014102. [Google Scholar] [CrossRef] [PubMed]
- Aekplakorn, W.; Inthawong, R.; Kessomboon, P.; Sangthong, R.; Chariyalertsak, S.; Putwatana, P.; Taneepanichskul, S. Prevalence and trends of obesity and association with socioeconomic status in Thai adults: National health examination surveys, 1991–2009. J. Obes. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Aekplakorn, W.; Satheannoppakao, W.; Putwatana, P.; Taneepanichskul, S.; Kessomboon, P.; Chongsuvivatwong, V.; Chariyalertsak, S. Dietary pattern and metabolic syndrome in Thai adults. J. Nutr. Metab. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Sleigh, A.; Seubsman, S.; Bain, C.; The Thai Cohort Study Team. Cohort Profile: The Thai Cohort of 87 134 Open University students. Int. J. Epidemiol. 2008, 37, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Papier, K.; Jordan, S.; Bain, C.; D’este, C.; Thawornchaisit, P.; Seubsman, S.; Sleigh, A. Validity of Self-Reported Diabetes in a Cohort of Thai Adults. Glob. J. Health Sci. 2016, 9, 1. [Google Scholar] [CrossRef]
- Boontaveeyuwat, N. Validity of Food Consumption and Nutrition Survey Questionnnaire for the National Health Examination Survey IV; National Health Exmaination Survey Office: Bangkok, Thailand, 2008. [Google Scholar]
- Willett, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2013; p. 529. [Google Scholar]
- Hyun, H.S. Occupational segregation and gender discrimination in labor markets: Thailand and Viet Nam. In Poverty, Inequality, and Inclusive Growth in Asia Measurement, Policy Issues, and Country Studies; Ju, Z., Ed.; Anthem Press: London, UK, 2010; pp. 409–430. [Google Scholar]
- Rimpeekool, W.; Kirk, M.; Yiengprugsawan, V.; Banwell, C.; Seubsman, S.; Sleigh, A. Nutrition label experience and consumption of transitional foods among a nationwide cohort of 42,750 Thai adults. Br. Food J. 2017, 119, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.E.; Marshall, J.R.; Sechrest, L. Invited commentary: Factor analysis and the search for objectivity. Am. J. Epidemiol. 1998, 148, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Tinsley, H.E.A.; Tinsely, D.J. Uses of Factor Analysis in Counseling Psychology Research. J. Couns. Psychol. 1987, 34, 414–424. [Google Scholar] [CrossRef]
- Mayén, A.-L.; Bovet, P.; Marti-Soler, H.; Viswanathan, B.; Gedeon, J.; Paccaud, F.; Marques-Vidal, P.; Stringhini, S. Socioeconomic differences in dietary patterns in an East African Country: Evidence from the Republic of Seychelles. PLoS ONE 2016, 11, e0155617. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, D.; Das, N.; Saha, I.; Biswas, P.; Datta, S.; Mukhopadhyay, B.; Chaudhuri, D.; Ghosh, S.; Dey, S. Major dietary patterns and their associations with cardiovascular risk factors among women in West Bengal, India. Br. J. Nutr. 2011, 105, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Mayén, A.-L.; Stringhini, S.; Ford, N.D.; Martorell, R.; Stein, A.D.; Paccaud, F.; Marques-Vidal, P. Socioeconomic predictors of dietary patterns among Guatemalan adults. Int. J. Public Health 2016, 61, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Kell, K.; Judd, S.E.; Pearson, K.E.; Shikany, J.M.; Fernández, J.R. Associations between socio-economic status and dietary patterns in US black and white adults. Br. J. Nutr. 2015, 113, 1792. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, A.; Rashidkhani, B.; Omidvar, N. Association of major dietary patterns with socioeconomic and lifestyle factors of adult women living in Tehran, Iran. Nutrition 2010, 26, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Rimm, E.B.; Spiegelman, D.; Rifai, N.; Tofler, G.H.; Willett, W.C.; Hu, F.B. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am. J. Clin. Nutr. 2001, 73, 61–67. [Google Scholar] [PubMed]
- Xu, X.; Hall, J.; Byles, J.; Shi, Z. Dietary pattern is associated with obesity in older people in China: Data from China Health and Nutrition Survey (CHNS). Nutrients 2015, 7, 8170–8188. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zheng, W.; Xiang, Y.B.; Xu, W.H.; Yang, G.; Li, H.; Shu, X.O. Dietary patterns and their correlates among middle-aged and elderly Chinese men: A report from the Shanghai Men’s Health Study. Br. J. Nutr. 2007, 98, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, M.G.; Milte, C.M.; Crawford, D.; McNaughton, S.A. A comparison of the dietary patterns derived by principal component analysis and cluster analysis in older Australians. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Park, S.J.; Kwack, H.K.; Kim, M.K.; Ko, K.P.; Kim, S.S. Rice-eating pattern and the risk of metabolic syndrome especially waist circumference in Korean Genome and Epidemiology Study (KoGES). BMC Public Health 2013, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- De Munter, J.S.; Hu, F.B.; Spiegelman, D.; Franz, M.; Van Dam, R.M. Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Med. 2007, 4, e261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas-Salvadó, J.; Martinez-González, M.Á.; Bulló, M.; Ros, E. The role of diet in the prevention of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2011, 21, B32–B48. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Gordon-Larsen, P. The nutrition transition: Worldwide obesity dynamics and their determinants. Int. J. Obes. 2004, 28, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Seubsman, S.; Kelly, M.; Yuthapornpinit, P.; Sleigh, A.C. Cultural resistance to fast-food consumption? A study of youth in North Eastern Thailand. Int. J. Consumer Stud. 2009, 33, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, P.; Drewnowski, A. Lower-energy-density diets are associated with higher monetary costs per kilocalorie and are consumed by women of higher socioeconomic status. J. Am. Diet. Assoc. 2009, 109, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.C.; Verly Junior, E.; Junger, W.L.; Sichieri, R. Independent associations of income and education with nutrient intakes in Brazilian adults: 2008–2009 National Dietary Survey. Public Health Nutr. 2014, 17, 2740–2752. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Conde, W.L.; Popkin, B.M. Independent effects of income and education on the risk of obesity in the Brazilian adult population. J. Nutr. 2001, 131, 881S–886S. [Google Scholar] [PubMed]
- Naja, F.; Nasreddine, L.; Itani, L.; Chamieh, M.C.; Adra, N.; Sibai, A.M.; Hwalla, N. Dietary patterns and their association with obesity and sociodemographic factors in a national sample of Lebanese adults. Public Health Nutr. 2011, 14, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Olinto, M.T.; Willett, W.C.; Gigante, D.P.; Victora, C.G. Sociodemographic and lifestyle characteristics in relation to dietary patterns among young Brazilian adults. Public Health Nutr. 2011, 14, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Haase, A.M.; Steptoe, A. Body image and weight control in young adults: International comparisons in university students from 22 countries. Int. J. Obes. 2006, 30, 644. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.A.; Moura, E.C.; Conde, W.L.; Popkin, B.M. Socioeconomic status and obesity in adult populations of developing countries: A review. Bull. World Health Organ. 2004, 82, 940–946. [Google Scholar] [PubMed]
- Banwell, C.; Dixon, J.; Seubsman, S.A.; Pangsap, S.; Kelly, M.; Sleigh, A. Evolving food retail environments in Thailand and implications for the health and nutrition transition. Public Health Nutr. 2013, 16, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Gorton, M.; Sauer, J.; Supatpongkul, P. Wet markets, supermarkets and the “big middle” for food retailing in developing countries: Evidence from Thailand. World Dev. 2011, 39, 1624–1637. [Google Scholar] [CrossRef]
- Kelly, M.; Seubsman, S.; Banwell, C.; Dixon, J.; Sleigh, A. Traditional, modern or mixed? Perspectives on social, economic, and health impacts of evolving food retail in Thailand. Agric. Hum. Values 2015, 32, 445–460. [Google Scholar] [CrossRef] [PubMed]
| Food Groups (Men) | Healthy Transitional | Fatty Western | Highly Processed | Traditional |
| Soy milk | 0.41 | - | - | - |
| Beans | 0.37 | - | - | - |
| Fruit | 0.34 | - | - | - |
| Milk | 0.32 | - | - | - |
| Brown rice | 0.30 | - | - | - |
| Wheat | 0.30 | - | - | - |
| Fatty meat | - | 0.38 | - | - |
| Deep fried and western food | - | 0.36 | - | - |
| Meat | - | 0.34 | - | - |
| Rice noodles | - | 0.33 | - | - |
| Food with coconut milk | - | 0.30 | - | - |
| Fruit with added sugar | - | - | 0.49 | - |
| Processed fruit | - | - | 0.44 | - |
| Sweet snacks | - | - | 0.38 | - |
| Meat products (processed) | - | - | 0.35 | - |
| Fermented fish or soybean | - | - | - | 0.53 |
| Glutinous rice | - | - | - | 0.47 |
| Bamboo shoots | - | - | - | 0.40 |
| Chilli dipping sauce | - | - | - | 0.33 |
| Dietary variance explained % | 10.9 | 10.8 | 8.5 | 6.7 |
| Food groups (Women) | Fatty Western | Healthy transitional | Highly processed | Traditional |
| Deep fried and western food | 0.35 | - | - | - |
| Fatty meat | 0.35 | - | - | - |
| Food with coconut milk | 0.31 | - | - | - |
| Soy milk | - | 0.37 | - | - |
| Beans | - | 0.37 | - | - |
| Fish | - | 0.36 | - | - |
| Milk | - | 0.30 | - | - |
| Processed fruit | - | - | 0.44 | - |
| Wheat | - | - | 0.34 | - |
| Fruit or vegetable juice | - | - | 0.33 | - |
| Salty snacks | - | - | 0.31 | - |
| Fermented fish or soybean | - | - | - | 0.49 |
| Glutinous rice | - | - | - | 0.47 |
| Bamboo shoots | - | - | - | 0.46 |
| Chilli dipping sauce | - | - | - | 0.31 |
| Dietary variance explained % | 11.2 | 9.7 | 7.8 | 7.1 |
| Predictors | Beta Coefficients and 95% Confidence Intervals | |||
|---|---|---|---|---|
| Healthy Transitional | Fatty Western | Highly Processed | Traditional | |
| Income (Baht/month) | ||||
| ≤10,000 | reference | reference | ** | reference |
| 10,001–20,000 | −0.20 (−0.79, 0.40) | −0.09 (−0.68, 0.51) | 0.06 (−0.39, 0.53) | |
| 20,001–30,000 | −0.05 (−0.67, 0.57) | 0.06 (−0.55, 0.68) | 0.01 (−0.47, 0.49) | |
| ≥30,001 | 0.66 (−0.04, 1.36) | −0.16 (−0.86, 0.53) | −0.36 (−0.90, 0.18) | |
| Education | ||||
| University | −0.35 (−0.82, 0.11) | −0.24 (−0.70, 0.22) | 0.05 (−0.30, 0.41) | |
| Education level by income (Baht/month) | ||||
| Below university | - | - | reference | - |
| <10,000, university | - | - | −1.02 (−1.78, −0.25) | - |
| 10,001–20,000, university | - | - | 0.07 (−0.54, 0.69) | - |
| 20,001–30,000, university | - | - | −0.11 (−0.86, 0.64) | - |
| ≥30,001, university | - | - | 0.95 (−0.20, 2.09) | - |
| Occupation | ||||
| Manual worker | 0.09 (−0.48, 0.67) | 0.52 (−0.05, 1.09) | 0.34 (−0.14, 0.82) | 0.04 (−0.41, 0.48) |
| Office assistant | reference | reference | reference | reference |
| Skilled worker | 0.26 (−0.44, 0.96) | 0.19 (−0.50, 0.88) | 0.26 (−0.32, 0.84) | −0.01 (−0.54, 0.54) |
| Professional | 0.01 (−0.50, 0.51) | −0.07 (−0.57, 0.43) | 0.03 (−0.39, 0.45) | −0.06 (−0.45, 0.33) |
| Manager | 0.38 (−0.15, 0.92) | 0.19 (−0.34, 0.72) | 0.18 (−0.26, 0.63) | 0.25 (−0.16, 0.66) |
| Urban residence | ||||
| Rural-rural | reference | reference | reference | reference |
| Urban-rural | −0.17 (−0.98, 0.63) | −0.18 (−0.98, 0.62) | −0.20 (−0.87, 0.46) | −0.77 (−1.39, −0.15) |
| Rural-Urban | 0.44 (−0.18, 1.05) | 0.29 (−0.32, 0.90) | 0.24 (−0.26, 0.75) | −0.74 (−1.21, −0.26) |
| Urban-Urban | 0.19 (−0.21, 0.60) | 0.59 (0.20, 1.00) | 0.14 (−0.19, 0.48) | −1.00 (−1.31, −0.68) |
| Predictors | Beta Coefficients and 95% Confidence Intervals | |||
|---|---|---|---|---|
| Healthy Transitional | Fatty Western | Highly Processed | Traditional | |
| Income (Baht/month) | ||||
| ≤10,000 | reference | reference | reference | reference |
| 10,001–20,000 | −0.20 (−0.64, 0.24) | −0.01 (−0.45, 0.43) | −0.06 (−0.44, 0.31) | −0.20 (−0.55, 0.16) |
| 20,001–30,000 | −0.21 (−0.75, 0.33) | −0.22 (−0.76, 0.32) | 0.26 (−0.20, 0.72) | −0.62 (−1.06, −0.18) |
| ≥30,001 | −0.37 (−0.96, 0.22) | 0.03 (−0.56, 0.62) | 0.48 (−0.01, 0.98) | −0.67 (−1.15, −0.19) |
| Education | ||||
| University | −0.02 (−0.51, 0.46) | −0.04 (−0.52, 0.44) | −0.57 (−0.98, −0.17) | 0.42 (0.03, 0.81) |
| Occupation | ||||
| Manual worker | −0.22 (−0.76, 0.33) | −0.09 (−0.63, 0.46) | −0.15 (−0.61, 0.31) | −0.02 (−0.46, 0.42) |
| Office assistant | reference | reference | reference | reference |
| Skilled worker | 0.18 (−0.69, 1.05) | 0.09 (−0.78, 0.95) | 0.05 (−0.68, 0.78) | −0.01 (−0.71, 0.69) |
| Professional | 0.08 (−0.30, 0.47) | −0.48 (−0.86, −0.11) | 0.06 (−0.26, 0.38) | 0.08 (−0.23, 0.38) |
| Manager | 0.28 (−0.26, 0.83) | −0.60 (−1.14, −0.05) | −0.13 (−0.59, 0.33) | 0.26 (−0.18, 0.70) |
| Urban residence | ||||
| Rural-rural | reference | reference | reference | reference |
| Urban-rural | 0.12 (−0.47, 0.70) | 0.58 (−0.01, 1.16) | 0.12 (−0.37, 0.62) | −0.22 (−0.69, 0.25) |
| Rural-Urban | 0.08 (−0.46, 0.61) | 0.55 (0.02, 1.08) | 0.27 (−0.18, 0.72) | −0.60 (−1.04, −0.17) |
| Urban-Urban | −0.10 (−0.46, 0.27) | 0.68 (0.32, 1.04) | 0.44 (0.13, 0.75) | −0.68 (−0.98, −0.39) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papier, K.; Jordan, S.; D’Este, C.; Banwell, C.; Yiengprugsawan, V.; Seubsman, S.-a.; Sleigh, A. Social Demography of Transitional Dietary Patterns in Thailand: Prospective Evidence from the Thai Cohort Study. Nutrients 2017, 9, 1173. https://doi.org/10.3390/nu9111173
Papier K, Jordan S, D’Este C, Banwell C, Yiengprugsawan V, Seubsman S-a, Sleigh A. Social Demography of Transitional Dietary Patterns in Thailand: Prospective Evidence from the Thai Cohort Study. Nutrients. 2017; 9(11):1173. https://doi.org/10.3390/nu9111173
Chicago/Turabian StylePapier, Keren, Susan Jordan, Catherine D’Este, Cathy Banwell, Vasoontara Yiengprugsawan, Sam-ang Seubsman, and Adrian Sleigh. 2017. "Social Demography of Transitional Dietary Patterns in Thailand: Prospective Evidence from the Thai Cohort Study" Nutrients 9, no. 11: 1173. https://doi.org/10.3390/nu9111173






