Dietary Factors Associated with Plasma Thyroid Peroxidase and Thyroglobulin Antibodies
Abstract
:1. Introduction
2. Materials and Methods
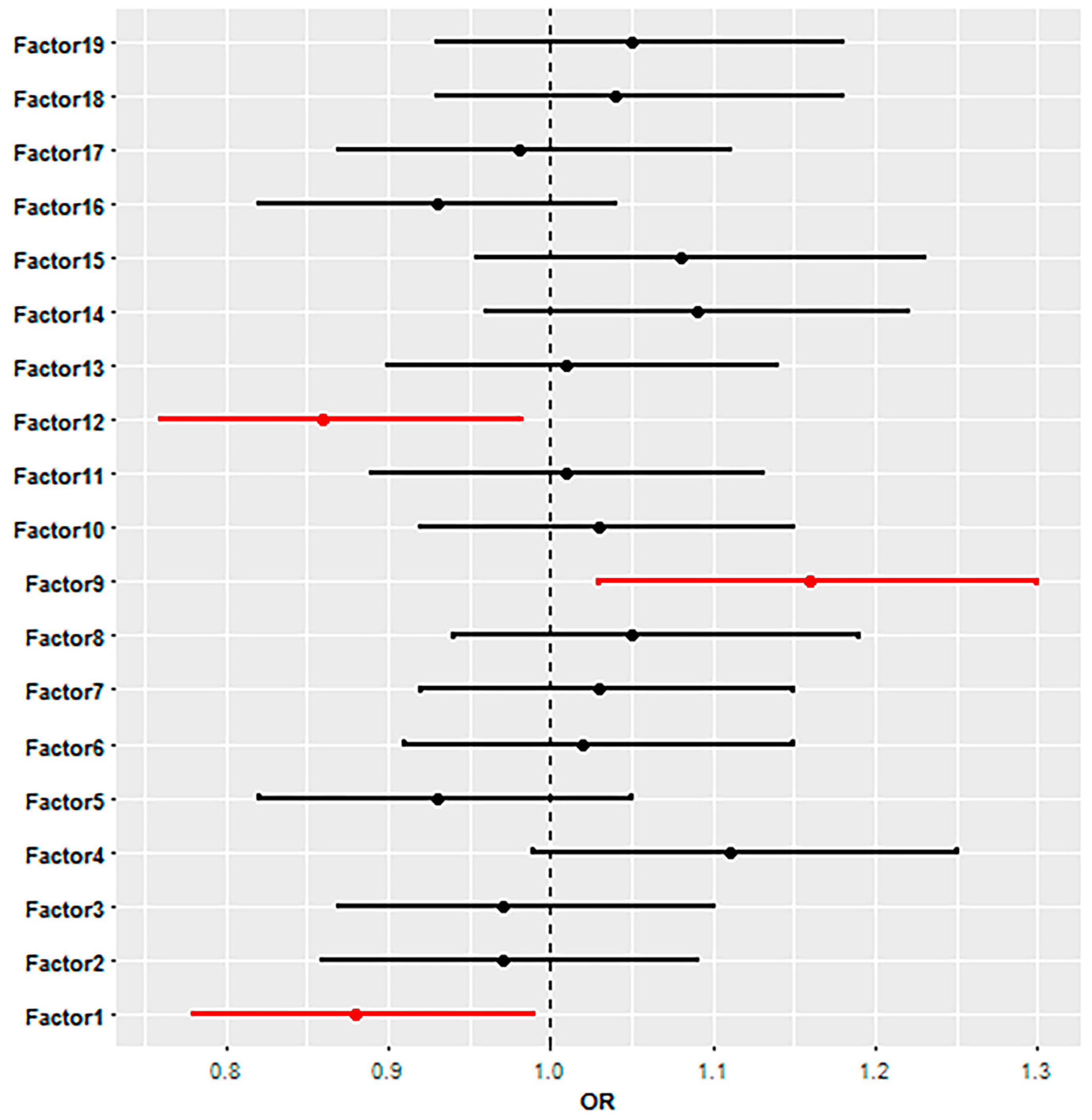
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wiersinga, W.M. Clinical relevance of environmental factors in the pathogenesis of autoimmune thyroid disease. Endocrinol. Metab. 2016, 31, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Effraimidis, G.; Tijssen, J.G.; Wiersinga, W.M. Discontinuation of smoking increases the risk for developing thyroid peroxidase antibodies and/or thyroglobulin antibodies: A prospective study. J. Clin. Endocrinol. Metab. 2009, 94, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Effraimidis, G.; Wiersinga, W.M. Mechanisms in endocrinology autoimmune thyroid disease: Old and new players. Eur. J. Endocrinol. 2014, 170, R241–R252. [Google Scholar] [CrossRef] [PubMed]
- Gaberscek, S.; Zaletel, K. Epidemiological trends of iodine-related thyroid disorders: An example from slovenia. Arch. Hig. Rada Toksikol. 2016, 67, 93–98. [Google Scholar]
- Pedersen, I.B.; Knudsen, N.; Carle, A.; Vejbjerg, P.; Jorgensen, T.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Laurberg, P. A cautious iodization programme bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin. Endocrinol. 2011, 75, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Guarneri, F. Molecular mimicry and autoimmune thyroid disease. Rev. Endocr. Metab. Disord. 2016, 17, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Nathan, E.; Oda, K.; Fraser, G. Vegan diets and hypothyroidism. Nutrients 2013, 5, 4642–4652. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Nathan, E.; Oda, K.; Fraser, G.E. Prevalence of hyperthyroidism according to type of vegetarian diet. Public Health Nutr. 2015, 18, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Rudan, I.; Marusic, A.; Jankovic, S.; Rotim, K.; Boban, M.; Lauc, G.; Grkovic, I.; Dogas, Z.; Zemunik, T.; Vatavuk, Z.; et al. “10 001 dalmatians”: Croatia launches its national biobank. Croat. Med. J. 2009, 50, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Polasek, O. Future of biobanks—Bigger, longer, and more dimensional. Croat. Med. J. 2013, 54, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Hollowell, J.G.; Staehling, N.W.; Flanders, W.D.; Hannon, W.H.; Gunter, E.W.; Spencer, C.A.; Braverman, L.E. Serum TSH, T(4), and thyroid antibodies in the united states population (1988 to 1994): National health and nutrition examination survey (NHANES III). J. Clin. Endocrinol. Metab. 2002, 87, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Kawicka, A.; Regulska-Ilow, B. Metabolic disorders and nutritional status in autoimmune thyroid diseases. Postepy Hig. Med. Dosw. 2015, 69, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Santini, F.; Marzullo, P.; Rotondi, M.; Ceccarini, G.; Pagano, L.; Ippolito, S.; Chiovato, L.; Biondi, B. Mechanisms in endocrinology: The crosstalk between thyroid gland and adipose tissue: Signal integration in health and disease. Eur. J. Endocrinol. 2014, 171, R137–R152. [Google Scholar] [CrossRef] [PubMed]
- Wyness, L. The role of red meat in the diet: Nutrition and health benefits. Proc. Nutr. Soc. 2016, 75, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Brassard, D.; Tessier-Grenier, M.; Allaire, J.; Rajendiran, E.; She, Y.; Ramprasath, V.; Gigleux, I.; Talbot, D.; Levy, E.; Tremblay, A.; et al. Comparison of the impact of SFAs from cheese and butter on cardiometabolic risk factors: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Sobiecki, J.G.; Appleby, P.N.; Bradbury, K.E.; Key, T.J. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: Results from the European prospective investigation into cancer and nutrition-oxford study. Nutr. Res. 2016, 36, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.J.; Lim, K.; Park, S.Y.; Jung, M.Y.; Lim, H.S.; Jeon, M.G.; Lee, S.I.; Park, B.H. Endogenous conversion of n-6 to n-3 polyunsaturated fatty acids attenuates K/BxN serum-transfer arthritis in fat-1 mice. J. Nutr. Biochem. 2015, 26, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Fan, Y.Y.; Monk, J.M.; Hou, T.Y.; Barhoumi, R.; McMurray, D.N.; Chapkin, R.S. n-3 PUFAs reduce T-helper 17 cell differentiation by decreasing responsiveness to interleukin-6 in isolated mouse splenic CD4(+) T cells. J. Nutr. 2014, 144, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Shoda, H.; Yanai, R.; Yoshimura, T.; Nagai, T.; Kimura, K.; Sobrin, L.; Connor, K.M.; Sakoda, Y.; Tamada, K.; Ikeda, T.; et al. Dietary omega-3 fatty acids suppress experimental autoimmune uveitis in association with inhibition of Th1 and Th17 cell function. PLoS ONE 2015, 10, e0138241. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrian, S.; Costa, A.G.; Navas-Carretero, S.; Zabala, M.; Laiglesia, L.M.; Martinez, J.A.; Moreno-Aliaga, M.J. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J. Physiol. Biochem. 2015, 71, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Vines, L.L.; Bates, M.A.; He, K.; Langohr, I. Comparative effects of n-3, n-6 and n-9 unsaturated fatty acid-rich diet consumption on lupus nephritis, autoantibody production and CD4+ T cell-related gene responses in the autoimmune NZBWF1 mouse. PLoS ONE 2014, 9, e0100255. [Google Scholar] [CrossRef] [PubMed]
- Bacher, M.; Metz, C.N.; Calandra, T.; Mayer, K.; Chesney, J.; Lohoff, M.; Gemsa, D.; Donnelly, T.; Bucala, R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc. Natl. Acad. Sci. USA 1996, 93, 7849–7854. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Rolinski, J. Immune disorders in hashimoto’s thyroiditis: What do we know so far? J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Xu, B.C.; Wang, Y.; Guo, H.; Jiang, Y.F. A high frequency of circulating Th22 and Th17 cells in patients with new onset graves’ disease. PLoS ONE 2013, 8, e68446. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Monograph. Plant sterols and sterolins. Altern. Med. Rev. 2001, 6, 203–206. [Google Scholar]
- Devaraj, S.; Jialal, I.; Rockwood, J.; Zak, D. Effect of orange juice and beverage with phytosterols on cytokines and PAI-1 activity. Clin. Nutr. 2011, 30, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Feinstein, D.L.; Hejna, M.J.; Lorens, S.A.; McGuire, S.O. Beneficial effects of blueberries in experimental autoimmune encephalomyelitis. J. Agric. Food Chem. 2012, 60, 5743–5748. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.H.; Chung, S.J.; Lee, S.W.; Park, Y.B.; Lee, S.K.; Park, M.C. Gallic acid, a natural polyphenolic acid, induces apoptosis and inhibits proinflammatory gene expressions in rheumatoid arthritis fibroblast-like synoviocytes. Jt. Bone Spine Revue Rhum. 2013, 80, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Kuppan, G.; Balasubramanyam, J.; Monickaraj, F.; Srinivasan, G.; Mohan, V.; Balasubramanyam, M. Transcriptional regulation of cytokines and oxidative stress by gallic acid in human thp-1 monocytes. Cytokine 2010, 49, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Vigo, M.T.; Metro, D.; Granese, R.; Vita, R.; Le Donne, M. Type of fish consumed and thyroid autoimmunity in pregnancy and postpartum. Endocrine 2016, 52, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Soltan, S.S.; Gibson, R.A. Levels of omega 3 fatty acids in australian seafood. Asia Pac. J. Clin. Nutr. 2008, 17, 385–390. [Google Scholar] [PubMed]
- Milerova, J.; Cerovska, J.; Zamrazil, V.; Bilek, R.; Lapcik, O.; Hampl, R. Actual levels of soy phytoestrogens in children correlate with thyroid laboratory parameters. Clin. Chem. Lab. Med. 2006, 44, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Riccio, P.; Rossano, R. Nutrition facts in multiple sclerosis. ASN Neuro 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C. Lupus erythematosus and nutrition: A review of the literature. J. Ren. Nutr. 2000, 10, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Skoldstam, L.; Brudin, L.; Hagfors, L.; Johansson, G. Weight reduction is not a major reason for improvement in rheumatoid arthritis from lacto-vegetarian, vegan or mediterranean diets. Nutr. J. 2005, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarty, M.F. Upregulation of lymphocyte apoptosis as a strategy for preventing and treating autoimmune disorders: A role for whole-food vegan diets, fish oil and dopamine agonists. Med. Hypotheses 2001, 57, 258–275. [Google Scholar] [CrossRef] [PubMed]
| Variable | Cases | Controls | p Value |
|---|---|---|---|
| Gender | <0.001 a | ||
| Males | 119 (25.8%) | 584 (42%) | |
| Females | 343 (74.2%) | 804 (58%) | |
| Age (year) | 53.66 ± 13.65 | 52.72 ± 14.93 | 0.45 b |
| BMI | 27.78 ± 7.29 | 27.95 ± 7.79 | 0.67 b |
| Factors | Food Items (Factor Loadings) |
|---|---|
| Factor 1 | Root vegetables (0.79), flower vegetables (0.76), leafy vegetables (0.74), fruity vegetables (0.70), legumes (0.54) |
| Factor 2 | Squid and octopus (0.78), sea-food (shells, crab) (0.71), blue fish (0.67), dried fish and salted sardines (0.56), white fish (0.49) |
| Factor 3 | Chocolate (0.78), cookies (0.76), cakes (0.69), bonbons (0.51) |
| Factor 4 | Salami (0.69), canned meat derivates (0.62), sausages (0.57), eggs (0.45), bacon (0.36) |
| Factor 5 | Cedevita (powder based vitamin juice) (0.68), fruits juices (0.62), refreshing non-alcoholic drinks (0.60) |
| Factor 6 | Bran bread (−0.81), white bread (0.73) |
| Factor 7 | Full-fat cheese (0.69), cottage cheese (0.63), hard cheese (0.52), sour cream (0.46) |
| Factor 8 | Venison (0.76), fish derivates (0.53) |
| Factor 9 | Butter (0.73), animal fats (0.70) |
| Factor 10 | Internal organs (0.73), lamb (0.52), pork (0.37) |
| Factor 11 | Mushrooms (0.64), canned and pickled vegetables (0.61), potato (−0.30) |
| Factor 12 | Muesli (0.70), dried fruit (0.56), nuts (0.44) |
| Factor 13 | Hard liquor (0.68), vegetables juices (0.57), powder soups (0.33) |
| Factor 14 | Tea (0.64), olive oil (0.40) |
| Factor 15 | Milk (0.70), coffee (0.51), yoghurt (0.49), fresh fruits (0.40) |
| Factor 16 | Chicken (0.68), turkey (0.68) |
| Factor 17 | Beef (0.73), veal (0.34) |
| Factor 18 | Macaroni or rice (0.63), jam and marmalade (0.37), fruit compote (0.33) |
| Factor 19 | Plant oil (0.77) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matana, A.; Torlak, V.; Brdar, D.; Popović, M.; Lozić, B.; Barbalić, M.; Perica, V.B.; Punda, A.; Polašek, O.; Hayward, C.; et al. Dietary Factors Associated with Plasma Thyroid Peroxidase and Thyroglobulin Antibodies. Nutrients 2017, 9, 1186. https://doi.org/10.3390/nu9111186
Matana A, Torlak V, Brdar D, Popović M, Lozić B, Barbalić M, Perica VB, Punda A, Polašek O, Hayward C, et al. Dietary Factors Associated with Plasma Thyroid Peroxidase and Thyroglobulin Antibodies. Nutrients. 2017; 9(11):1186. https://doi.org/10.3390/nu9111186
Chicago/Turabian StyleMatana, Antonela, Vesela Torlak, Dubravka Brdar, Marijana Popović, Bernarda Lozić, Maja Barbalić, Vesna Boraska Perica, Ante Punda, Ozren Polašek, Caroline Hayward, and et al. 2017. "Dietary Factors Associated with Plasma Thyroid Peroxidase and Thyroglobulin Antibodies" Nutrients 9, no. 11: 1186. https://doi.org/10.3390/nu9111186





