The Dietary Furocoumarin Imperatorin Increases Plasma GLP-1 Levels in Type 1-Like Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of Diabetic Rats
2.4. Determination of Blood Glucose, Insulin and GLP-1 Levels in Diabetic Rats
2.5. Cell Cultures
2.6. TGR5 Transfection into CHO-K1 Cells
2.7. Measurement of 2-NBDG Uptake in Cells
2.8. Determination of Intracellular Calcium Concentrations
2.9. GPR119 Silencing in NCI-H716 Cells
2.10. Assay of GLP-1 Secretion from Cells
2.11. Determination of cAMP Levels in Cells
2.12. Statistical Analysis
3. Results
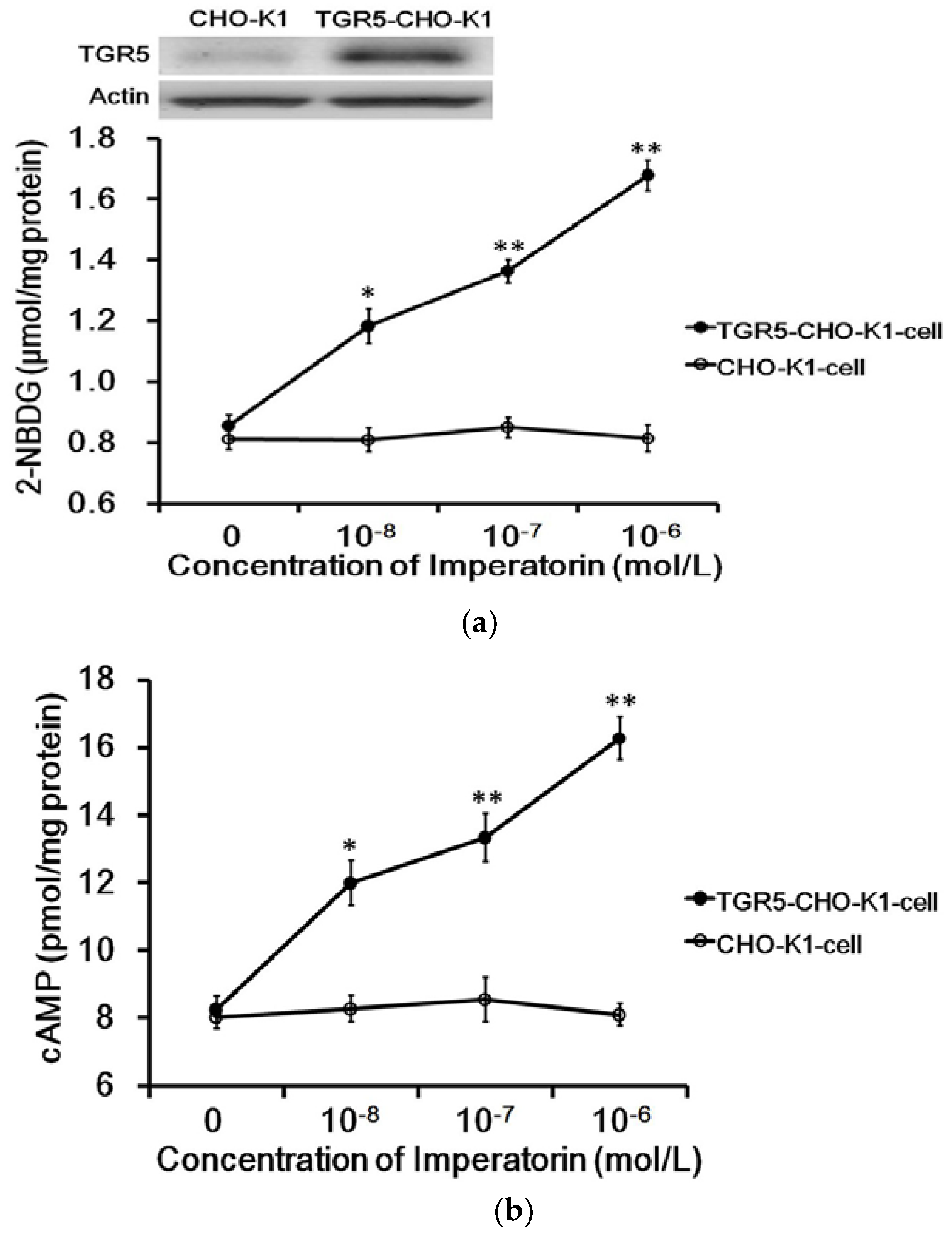
3.1. Imperatorin Activates TGR5 in TGR5-CHO-K1 Cells
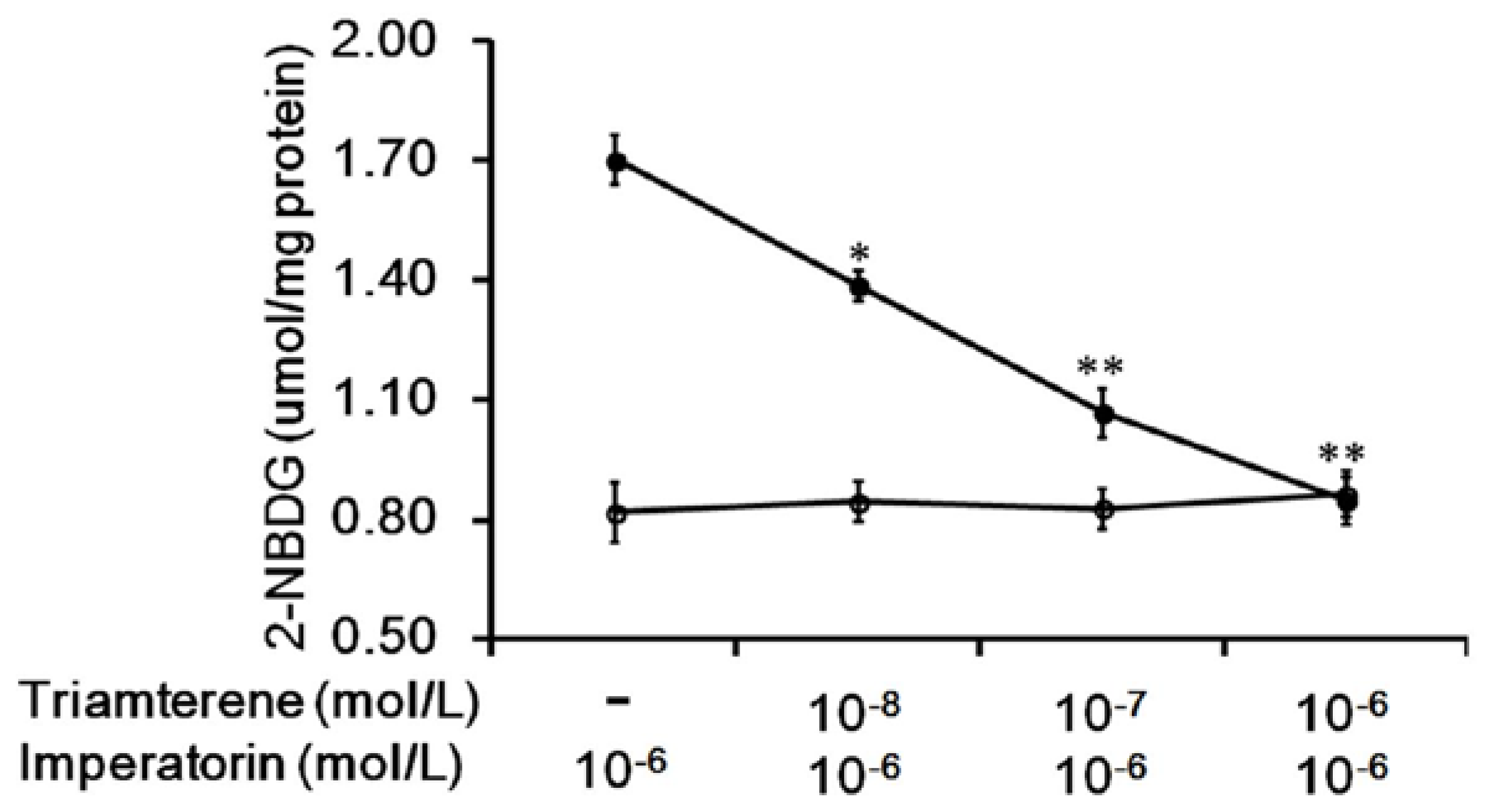
3.2. Effects of Triamterene-Mediated TGR5 Inhibition on Imperatorin-Induced Glucose Uptake in Cells Expressing TGR5
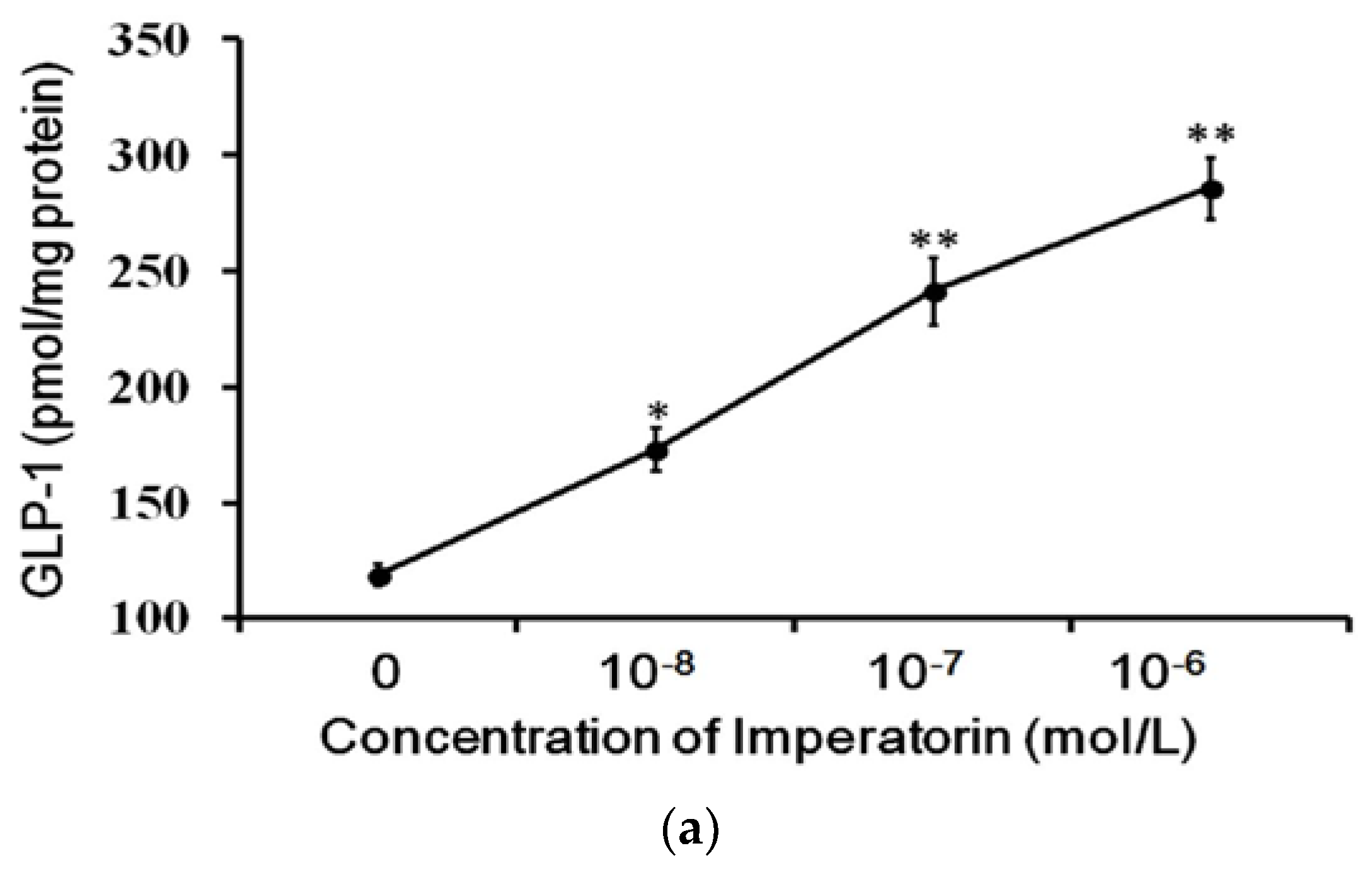
3.3. Effects of Imperatorin on GLP-1 Secretion in Cultured Cells
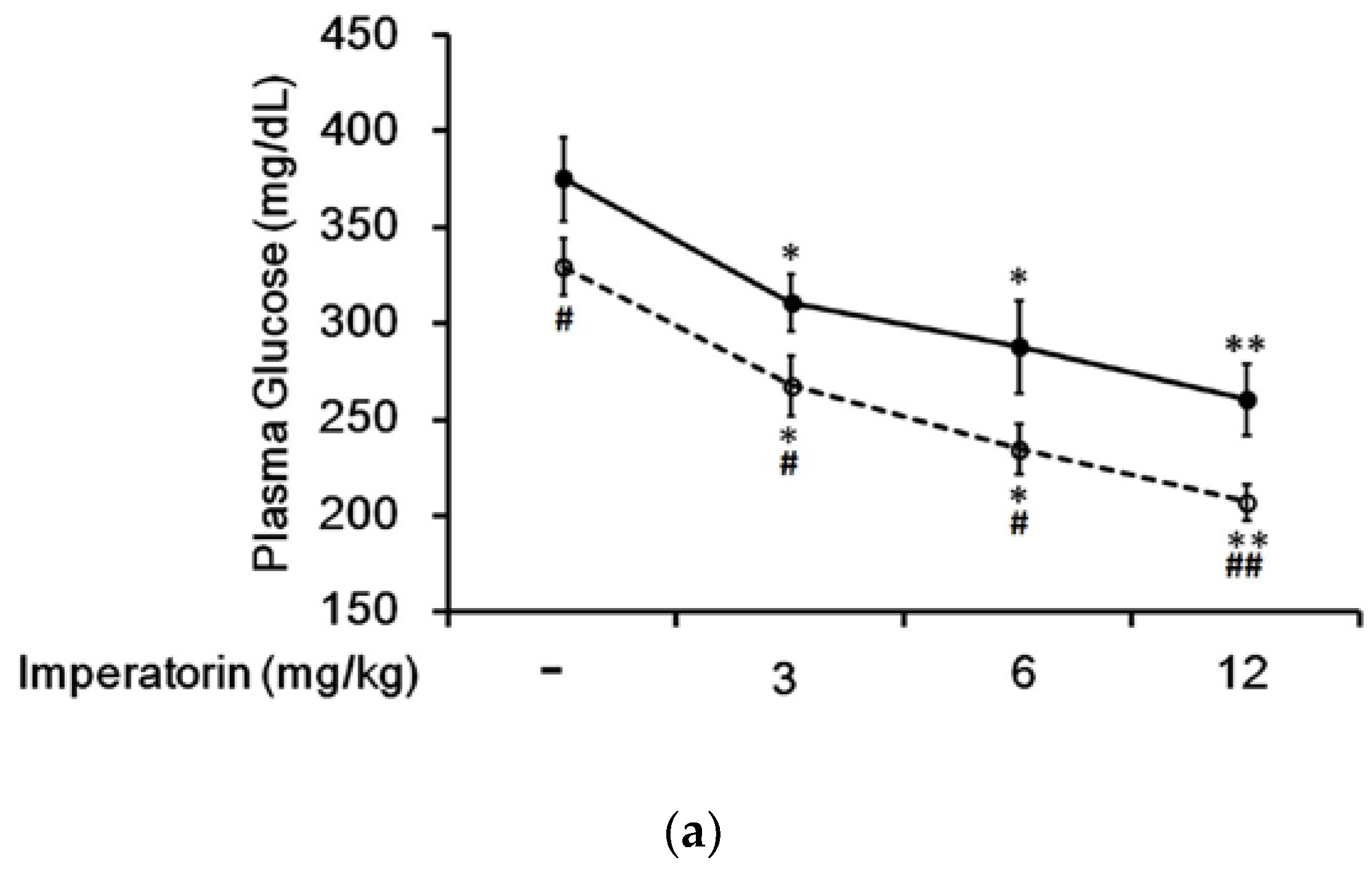
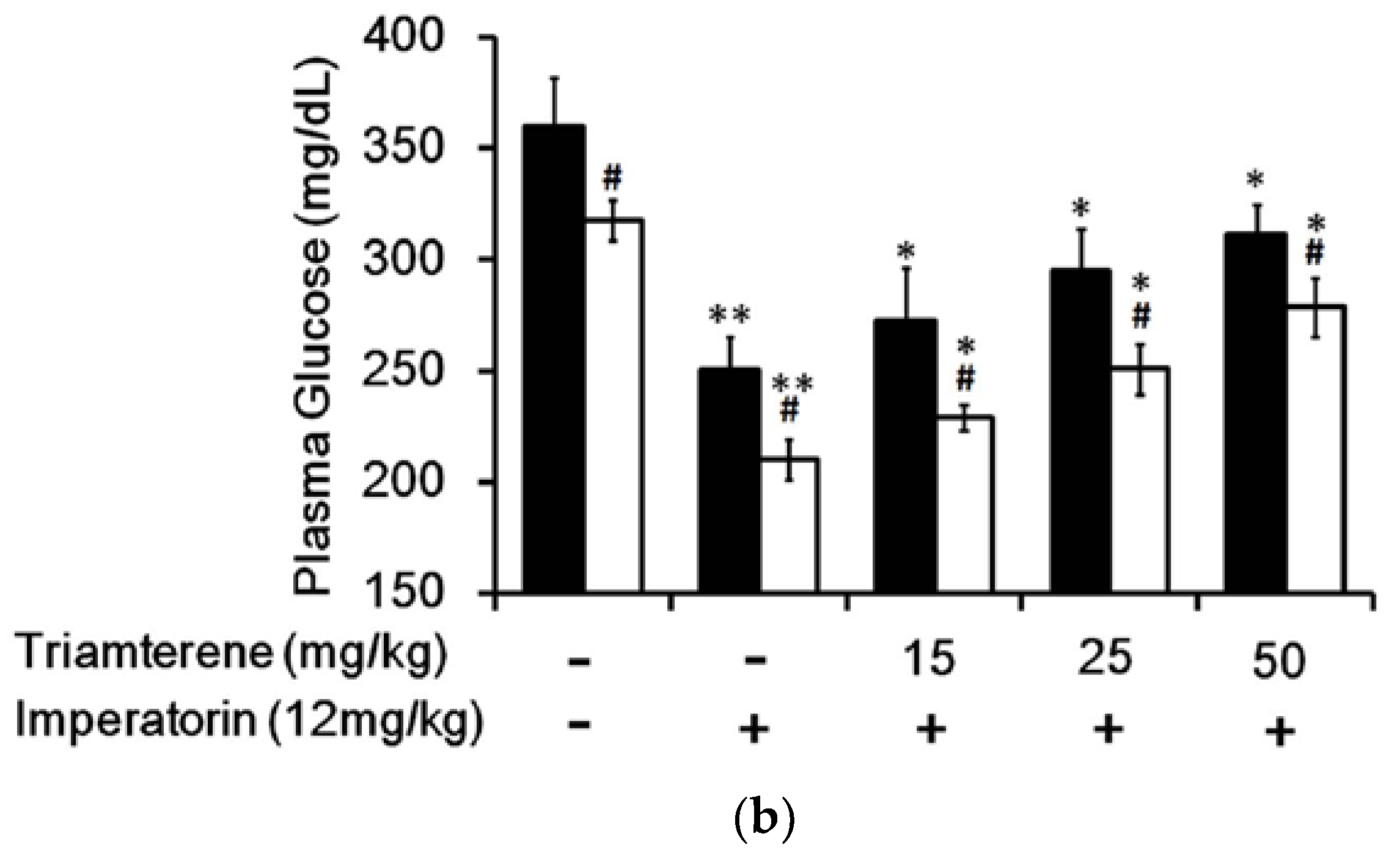
3.4. Effect of Imperatorin on Plasma Glucose Levels in Diabetic Rats
3.5. Effects of Imperatorin on Plasma GLP-1 Levels in Diabetic Rats
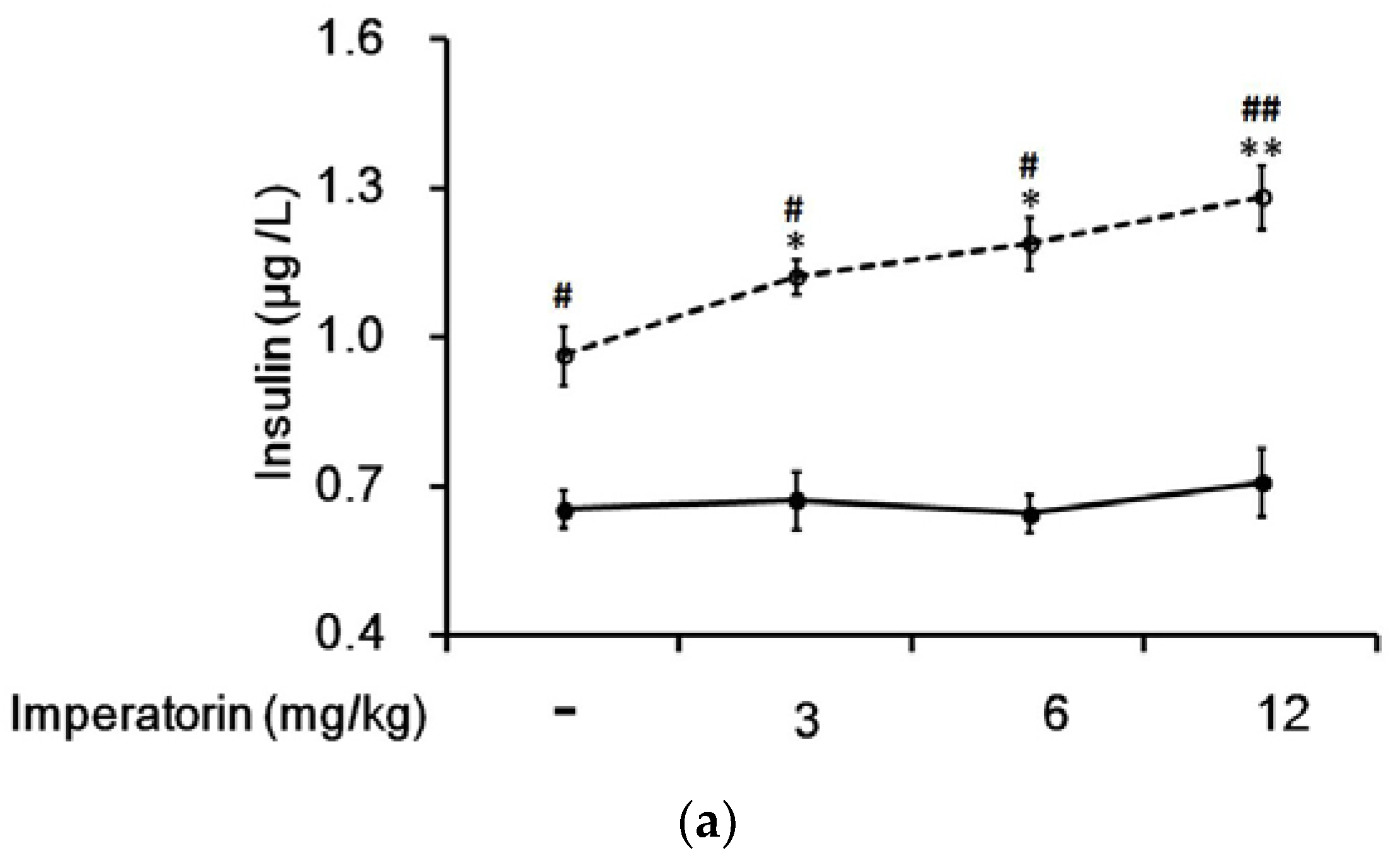
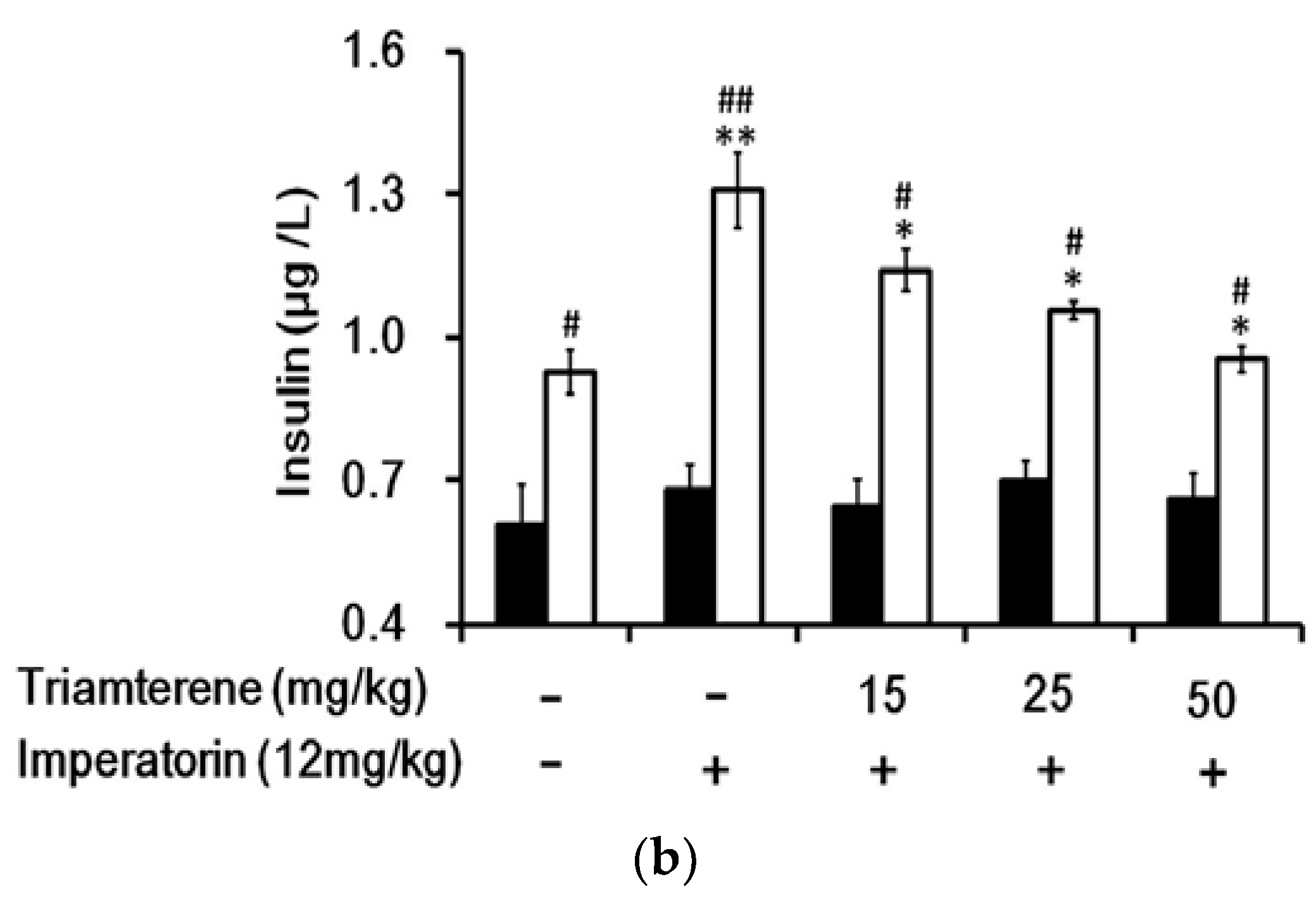
3.6. Effects of Imperatorin on Plasma Insulin Levels in Diabetic Rats
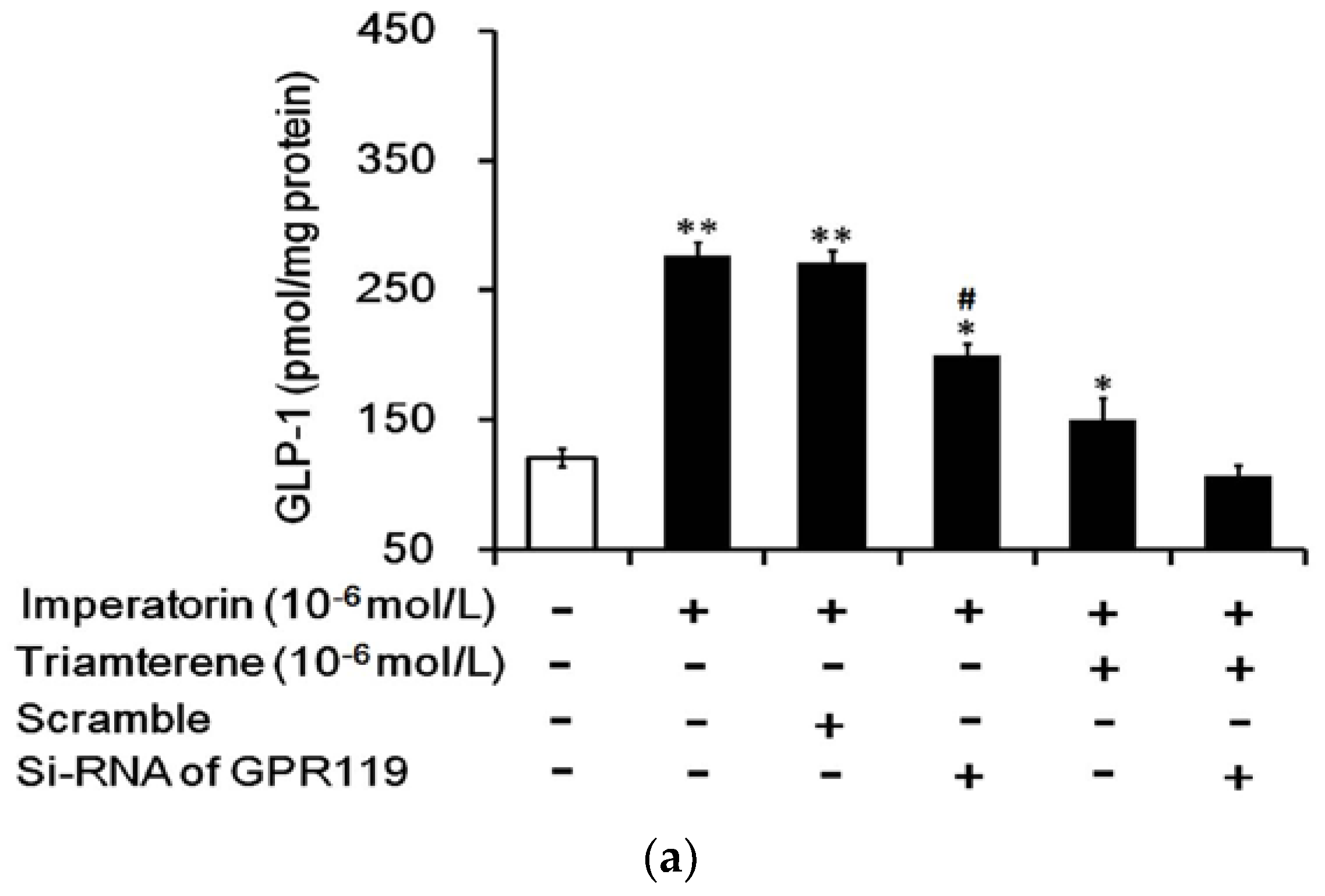
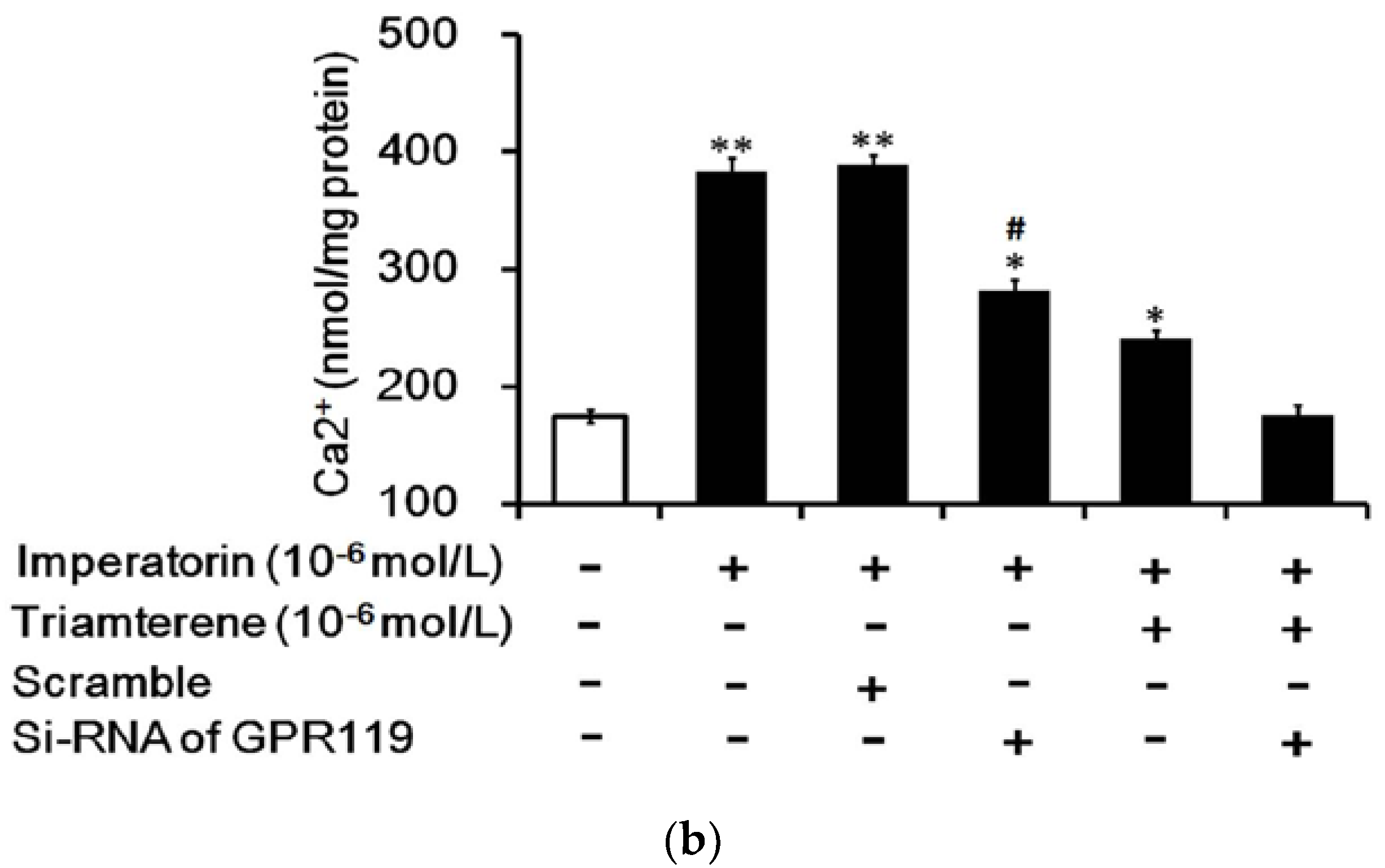
3.7. Role of GPR119 in Increased GLP-1 Secretion Induced by Imperatorin in Cells
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akkati, S.; Sam, K.G.; Tungha, G. Emergence of promising therapies in diabetes mellitus. J. Clin. Pharmacol. 2011, 51, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Prokopenko, I.; McCarthy, M.I.; Lindgren, C.M. Type 2 diabetes: New genes, new understanding. Trends Genet. 2008, 24, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Egan, J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 2008, 60, 470–512. [Google Scholar] [CrossRef] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of incretins: Glp-1 and gip. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Russell-Jones, D.; Gough, S. Recent advances in incretin-based therapies. Clin. Endocrinol. (Oxf) 2012, 77, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Preeti Sen, K.S.; Prasad, P.; Chandrakar, S.; Sahu, R.K.; Roy, A. Approach to phytochemistry and mechaniasm of action of plants having antidiabetic activity. UK J. Pharm. Biosci. 2016, 4, 82–120. [Google Scholar]
- Piao, X.L.; Park, I.H.; Baek, S.H.; Kim, H.Y.; Park, M.K.; Park, J.H. Antioxidative activity of furanocoumarins isolated from angelicae dahuricae. J. Ethnopharmacol. 2004, 93, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Ni, H.; Wu, M.; Tang, Z.; Halim, M.; Shi, D. Ros-mediated glucose metabolic reprogram induces insulin resistance in type 2 diabetes. Biochem. Biophys. Res. Commun. 2016, 476, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Overton, H.A.; Fyfe, M.C.; Reynet, C. Gpr119, a novel g protein-coupled receptor target for the treatment of type 2 diabetes and obesity. Br. J. Pharmacol. 2008, 153 (Suppl. S1), S76–S81. [Google Scholar] [CrossRef] [PubMed]
- Park, E.Y.; Kim, E.H.; Kim, C.Y.; Kim, M.H.; Choung, J.S.; Oh, Y.S.; Moon, H.S.; Jun, H.S. Angelica dahurica extracts improve glucose tolerance through the activation of gpr119. PLoS ONE 2016, 11, e0158796. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yuan, Z.Y.; Yan, X.J.; Lei, F.; Jiang, J.F.; Yu, X.; Yang, X.W.; Xing, D.M.; Du, L.J. Effects of angelica dahurica on obesity and fatty liver in mice. Chin. J. Nat. Med. 2016, 14, 641–652. [Google Scholar] [CrossRef]
- Adebajo, A.C.; Iwalewa, E.O.; Obuotor, E.M.; Ibikunle, G.F.; Omisore, N.O.; Adewunmi, C.O.; Obaparusi, O.O.; Klaes, M.; Adetogun, G.E.; Schmidt, T.J.; et al. Pharmacological properties of the extract and some isolated compounds of clausena lansium stem bark: Anti-trichomonal, antidiabetic, anti-inflammatory, hepatoprotective and antioxidant effects. J. Ethnopharmacol. 2009, 122, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A g protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Miyamoto, Y.; Nakamura, T.; Tamai, Y.; Okada, H.; Sugiyama, E.; Nakamura, T.; Itadani, H.; Tanaka, K. Identification of membrane-type receptor for bile acids (m-bar). Biochem. Biophys. Res. Commun. 2002, 298, 714–719. [Google Scholar] [CrossRef]
- Pols, T.W.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor tgr5 as an emerging target in metabolism and inflammation. J. Hepatol. 2011, 54, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Pierce, K.L.; Premont, R.T.; Lefkowitz, R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell. Biol. 2002, 3, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. Tgr5-mediated bile acid sensing controls glucose homeostasis. Cell. Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.T.; Liu, I.M.; Chi, T.C.; Tzeng, T.F.; Lu, F.H.; Chang, C.J. Plasma glucose-lowering effect of tramadol in streptozotocin-induced diabetic rats. Diabetes 2001, 50, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.H.; Cheng, K.C.; Li, Y.X.; Chang, C.H.; Cheng, J.T.; Lee, K.S. Development of betulinic acid as an agonist of tgr5 receptor using a new in vitro assay. Drug Des. Dev. Ther. 2016, 10, 2669–2676. [Google Scholar]
- Yamada, K.; Saito, M.; Matsuoka, H.; Inagaki, N. A real-time method of imaging glucose uptake in single, living mammalian cells. Nat. Protoc. 2007, 2, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Blodgett, A.B.; Kothinti, R.K.; Kamyshko, I.; Petering, D.H.; Kumar, S.; Tabatabai, N.M. A fluorescence method for measurement of glucose transport in kidney cells. Diabetes Technol. Ther. 2011, 13, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Henke, W.; Cetinsoy, C.; Jung, K.; Loening, S. Non-hyperbolic calcium calibration curve of fura-2: Implications for the reliability of quantitative ca2+ measurements. Cell Calcium 1996, 20, 287–292. [Google Scholar] [CrossRef]
- Lin, J.W.; Tsai, C.C.; Chen, L.J.; Niu, H.S.; Chang, C.K.; Niu, C.S. Characterization of musclin as a new target for treatment of hypertension. Biomed. Res. Int. 2014, 2014, 354348. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhang, S.Y.; Li, J.; Liu, H.N.; Xie, X.; Nan, F.J. Highly lipophilic 3-epi-betulinic acid derivatives as potent and selective tgr5 agonists with improved cellular efficacy. Acta Pharmacol. Sin. 2014, 35, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Anini, Y.; Brubaker, P.L. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 2003, 52, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef] [PubMed]
- Copple, B.L.; Li, T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol. Res. 2016, 104, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Patil, A.; Aware, U.; Deshmukh, P.; Darji, B.; Sasane, S.; Sairam, K.V.; Priyadarsiny, P.; Giri, P.; Patel, H.; et al. Discovery of a potent and orally efficacious tgr5 receptor agonist. ACS Med. Chem. Lett. 2016, 7, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.K.; Niu, C.; Lo, S.; Cheng, J.T.; Niu, H. Investigation of triamterene as an inhibitor of the tgr5 receptor: Identification in cells and animals. Drug Des. Dev. Ther. 2017, 11, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Genet, C.; Strehle, A.; Schmidt, C.; Boudjelal, G.; Lobstein, A.; Schoonjans, K.; Souchet, M.; Auwerx, J.; Saladin, R.; Wagner, A. Structure-activity relationship study of betulinic acid, a novel and selective tgr5 agonist, and its synthetic derivatives: Potential impact in diabetes. J. Med. Chem. 2010, 53, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Holst, J.J.; Kappe, C. The regulation of function, growth and survival of glp-1-producing l-cells. Clin.Sci. 2016, 130, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: Preclinical biology and mechanisms of action. Diabetes Care 2007, 30, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Aulinger, B.; Prigeon, R.L.; D’Alessio, D.A. Effect of endogenous glp-1 on insulin secretion in type 2 diabetes. Diabetes 2010, 59, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Tonneijck, L.; Muskiet, M.H.; Kramer, M.H.; Cahen, D.L.; van Raalte, D.H. Gastrointestinal actions of glucagon-like peptide-1-based therapies: Glycaemic control beyond the pancreas. Diabetes Obes. Metab. 2016, 18, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Dhanesha, N.; Joharapurkar, A.; Shah, G.; Dhote, V.; Kshirsagar, S.; Bahekar, R.; Jain, M. Exendin-4 reduces glycemia by increasing liver glucokinase activity: An insulin independent effect. Pharmacol. Rep. 2012, 64, 140–149. [Google Scholar] [CrossRef]
- Seghieri, M.; Rebelos, E.; Gastaldelli, A.; Astiarraga, B.D.; Casolaro, A.; Barsotti, E.; Pocai, A.; Nauck, M.; Muscelli, E.; Ferrannini, E. Direct effect of glp-1 infusion on endogenous glucose production in humans. Diabetologia 2013, 56, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.P.; Jones, K.L.; Annink, C.E.; Cousins, C.E.; Meier, J.J.; Chapman, M.J.; Horowitz, M.; Deane, A.M. Glucagon-like peptide 1 attenuates the acceleration of gastric emptying induced by hypoglycemia in healthy subjects. Diabetes Care 2014, 37, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Kramer, C.K.; Choi, H.; Swaminathan, B.; Zinman, B. Liraglutide and the preservation of pancreatic beta-cell function in early type 2 diabetes: The libra trial. Diabetes Care 2014, 37, 3270–3278. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Scheltema, M.J.; Sonne, D.P.; Hansen, J.S.; Sperling, M.; Rehfeld, J.F.; Holst, J.J.; Vilsboll, T.; Knop, F.K. Effect of chenodeoxycholic acid and the bile acid sequestrant colesevelam on glucagon-like peptide-1 secretion. Diabetes Obes. Metab. 2016, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-Y.; Cheng, K.-C.; Li, Y.; Niu, C.-S.; Cheng, J.-T.; Niu, H.-S. The Dietary Furocoumarin Imperatorin Increases Plasma GLP-1 Levels in Type 1-Like Diabetic Rats. Nutrients 2017, 9, 1192. https://doi.org/10.3390/nu9111192
Wang L-Y, Cheng K-C, Li Y, Niu C-S, Cheng J-T, Niu H-S. The Dietary Furocoumarin Imperatorin Increases Plasma GLP-1 Levels in Type 1-Like Diabetic Rats. Nutrients. 2017; 9(11):1192. https://doi.org/10.3390/nu9111192
Chicago/Turabian StyleWang, Lin-Yu, Kai-Chun Cheng, Yingxiao Li, Chiang-Shan Niu, Juei-Tang Cheng, and Ho-Shan Niu. 2017. "The Dietary Furocoumarin Imperatorin Increases Plasma GLP-1 Levels in Type 1-Like Diabetic Rats" Nutrients 9, no. 11: 1192. https://doi.org/10.3390/nu9111192



