Codonopsis lanceolata Water Extract Increases Hepatic Insulin Sensitivity in Rats with Experimentally-Induced Type 2 Diabetes
Abstract
:1. Introduction
2. Methods
2.1. Water Extracts of C. lanceolata
2.2. Animals and Ethics
2.3. Experimental Design
2.4. Energy Expenditure, Indirect Calorimetry
2.5. Euglycemic Hyperinsulinemic Clamp
2.6. Hyperglycemic Clamp
2.7. RNA Isolation and Reverse Transcription Polymerase Chain Reaction
2.8. Immunoblot Analysis
2.9. Immunohistochemistry
2.10. Statistical Analyses
3. Results
3.1. Total Polyphenols and Flavonoids in CLW
3.2. Body Composition and Energy Metabolism
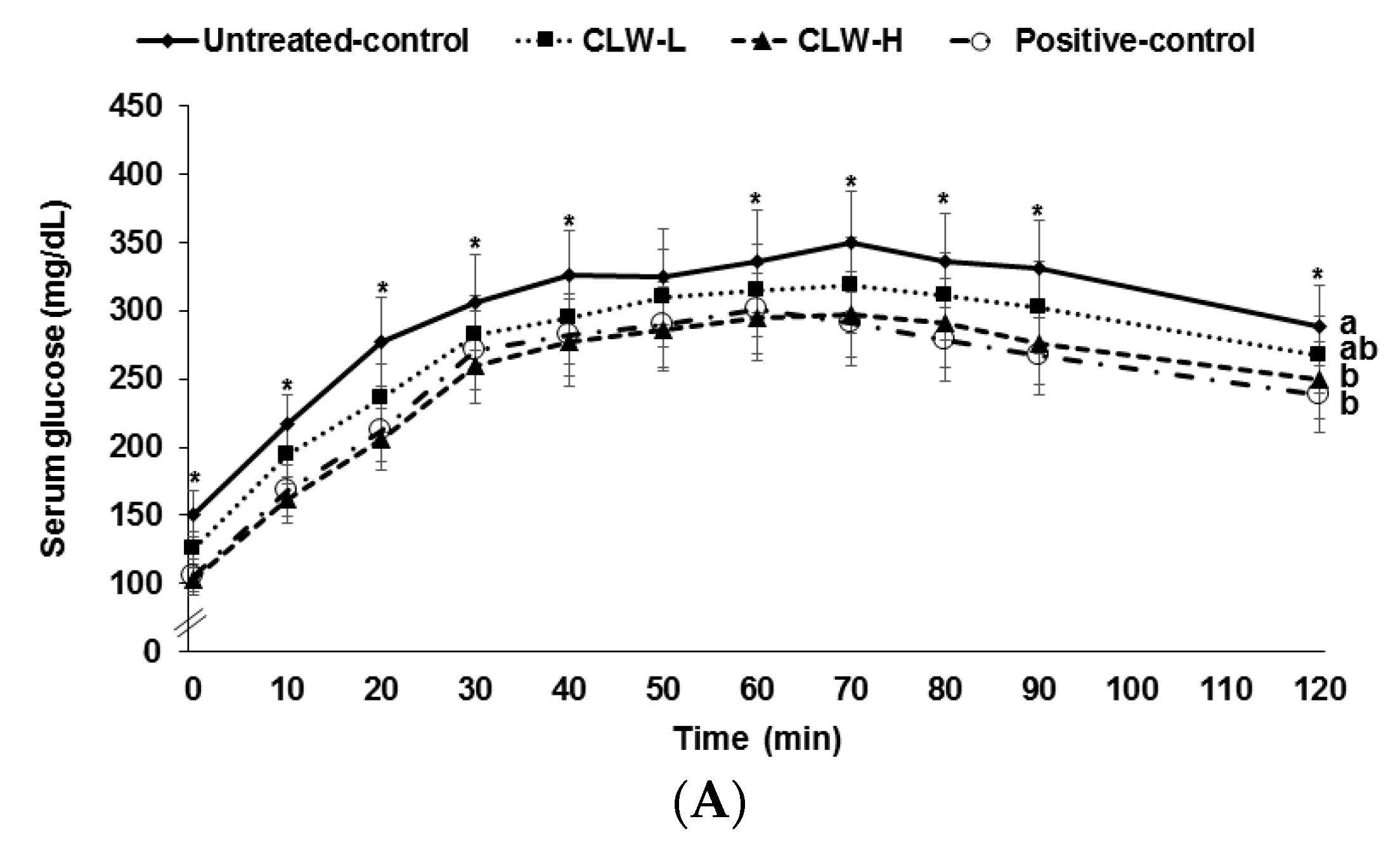
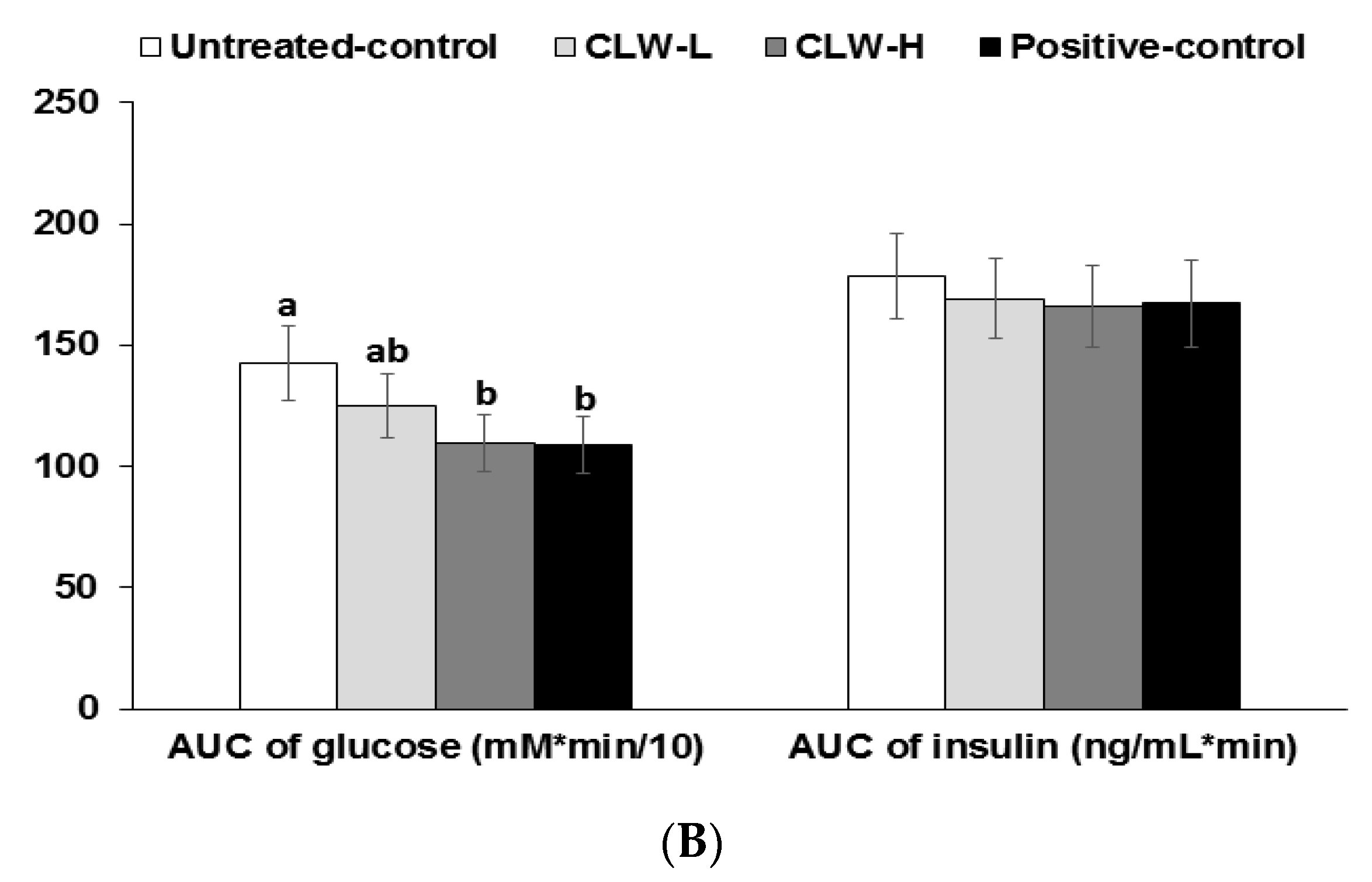
3.3. Glucose Tolerance
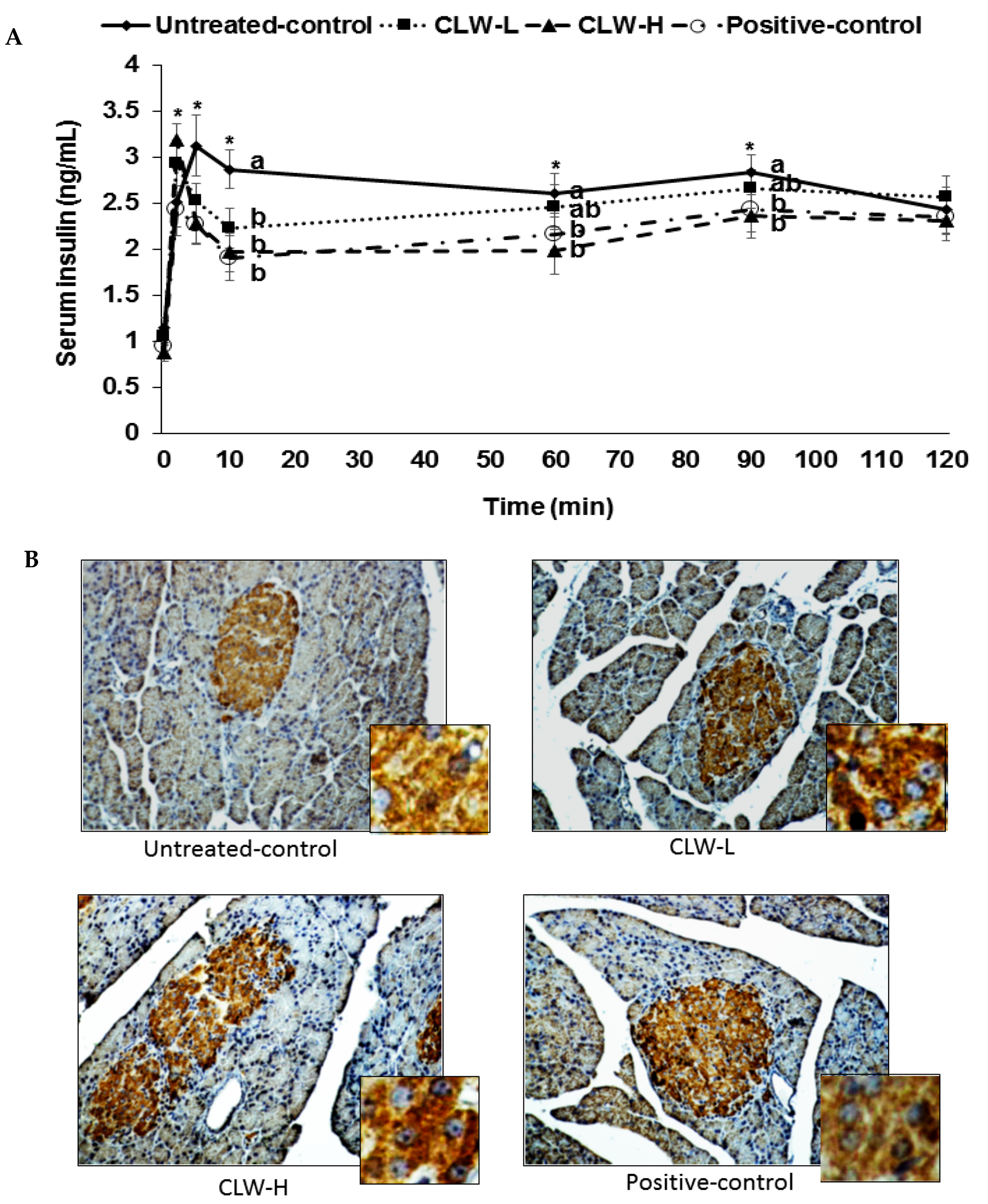
3.4. Insulin Secretion Capacity
3.5. β-Cell Mass
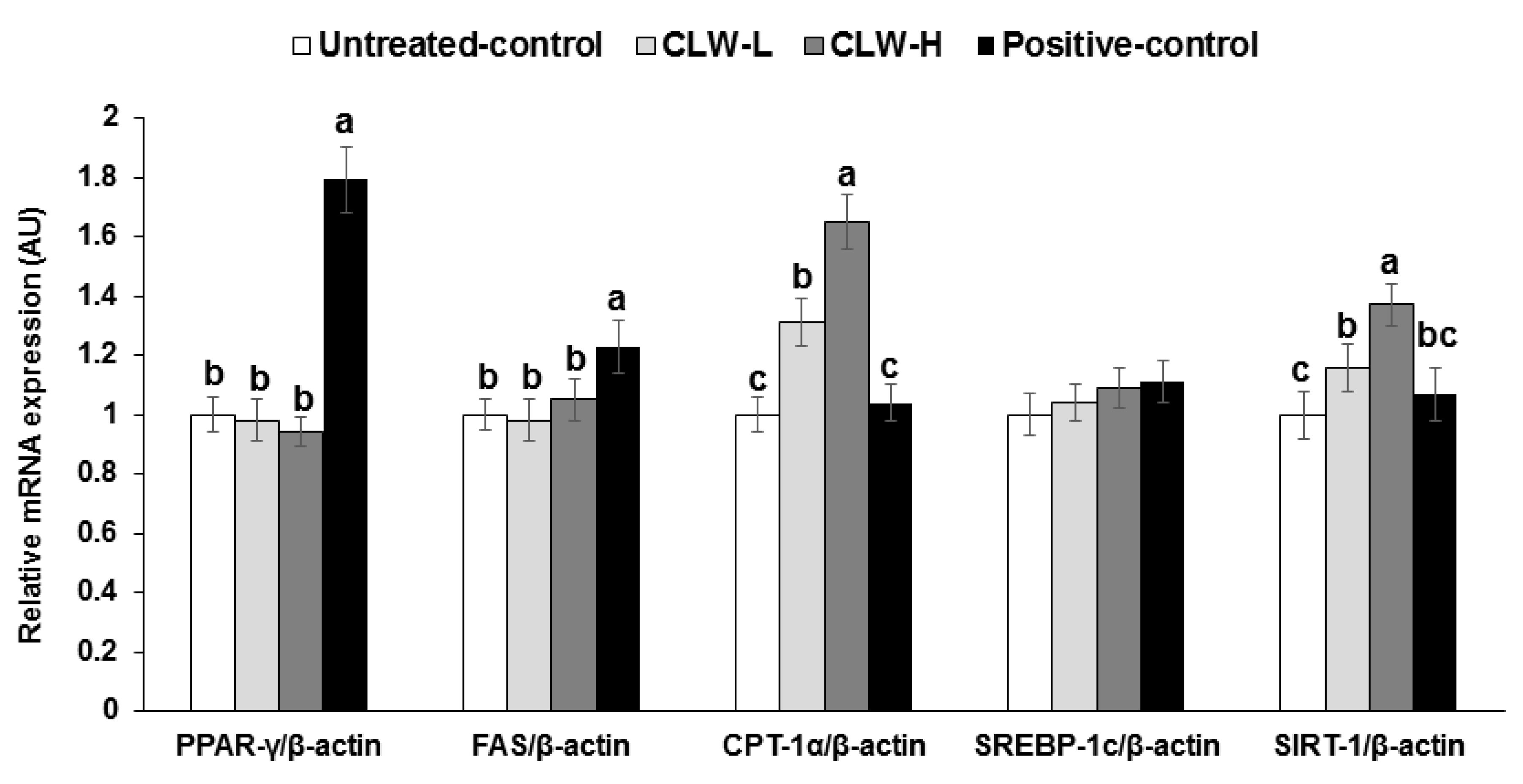
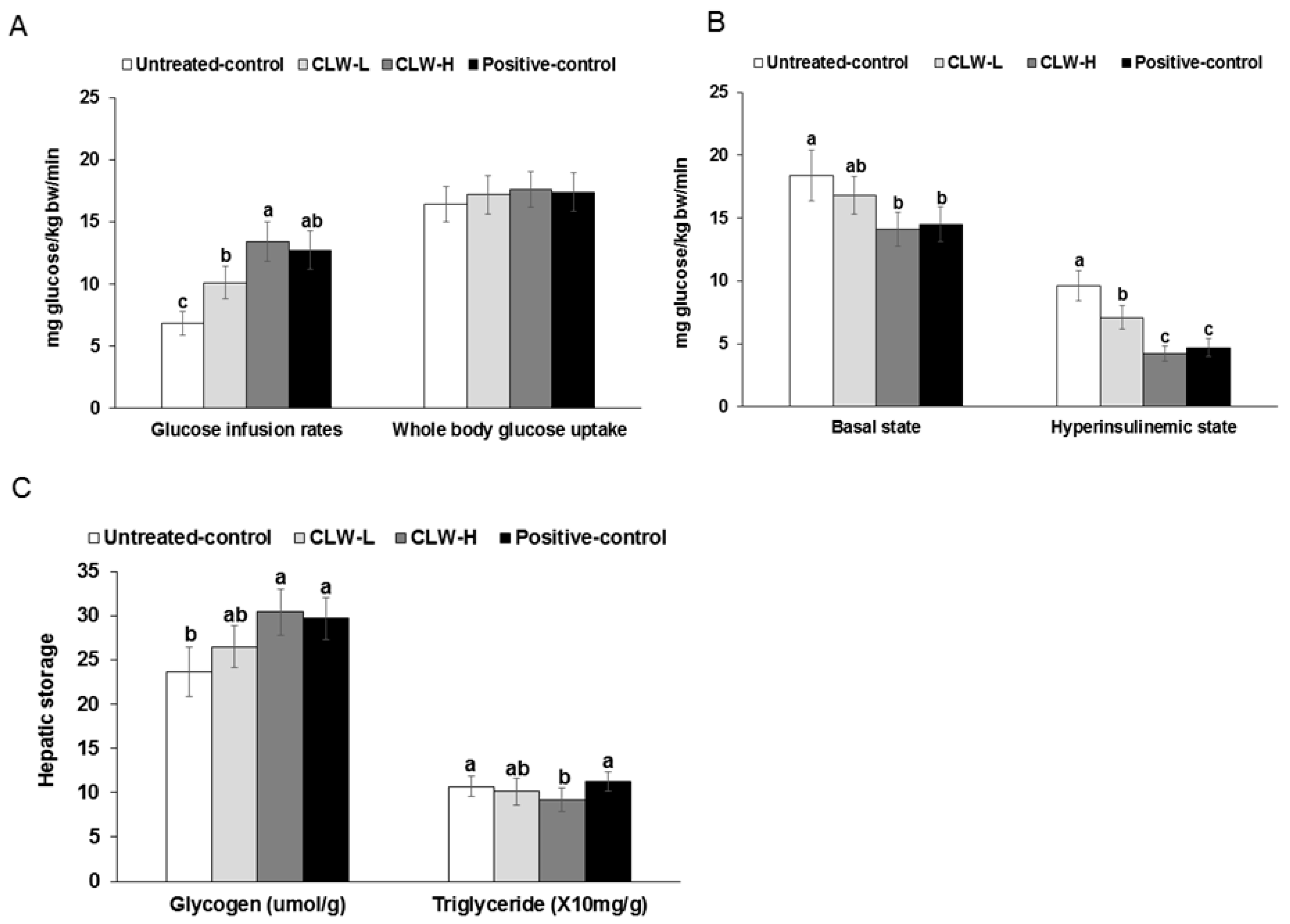
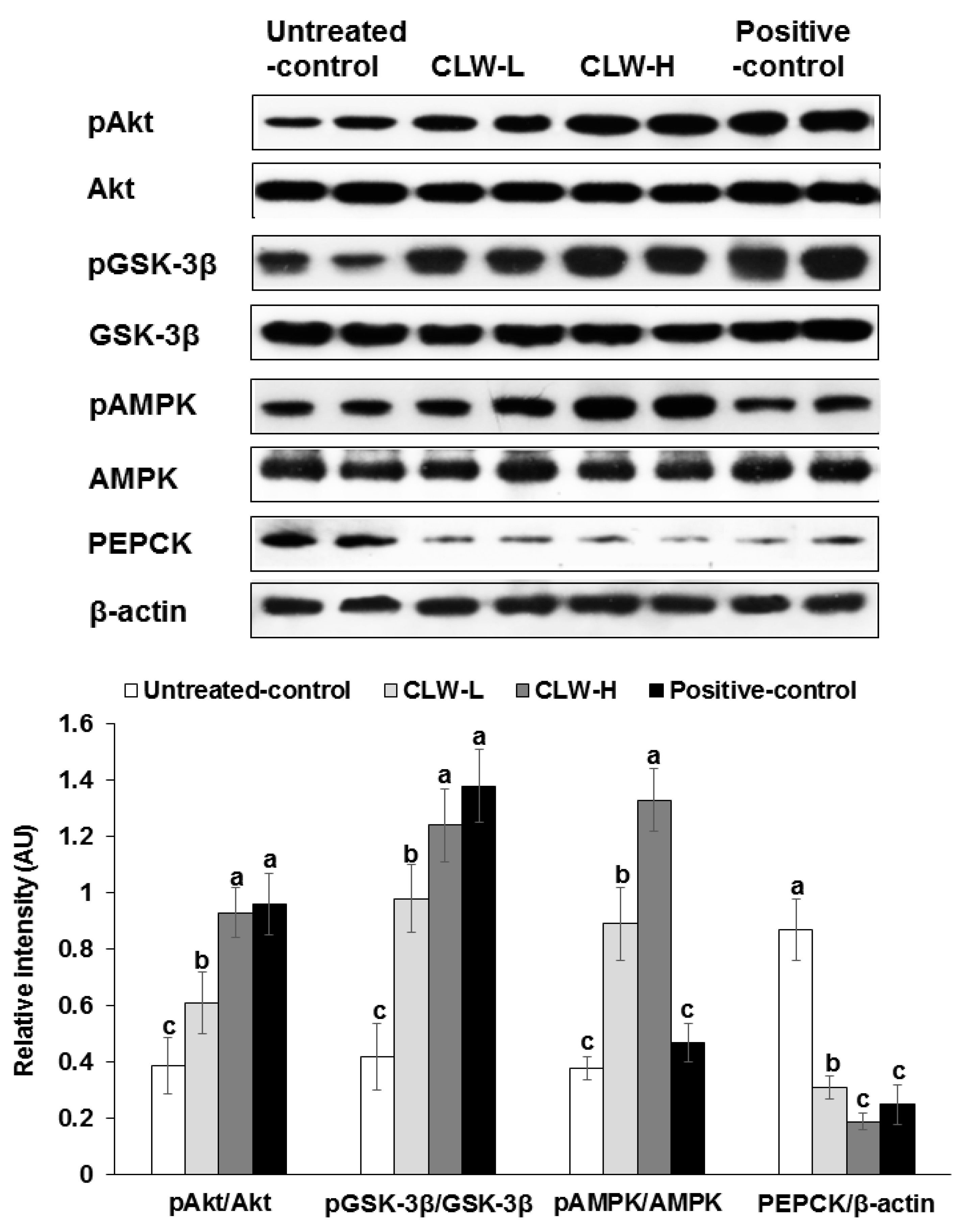
3.6. Insulin Sensitivity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CLW | Codonopsis lanceolata water extract |
| Px | pancreatectomized |
| OGTT | oral glucose tolerance test |
| VO2 | O2 consumption |
| VCO2 | CO2 production |
| REE | resting energy expenditure |
| RQ | respiratory quotient |
| CPT | carnitine palmitoyltransferase |
| SREBP | sterol regulatory element-binding protein |
| FAS | fatty acid synthase |
| PPAR | peroxisome proliferator-activated receptor |
| BrdU | bromodeoxyuridine |
| SIRT | Sirtuin |
| GSK | glycogen synthase kinase |
| AUC | areas under the curve |
| AMPK | AMP Kinase |
| JNK | c-Jun N-terminal kinases |
References
- Brereton, M.F.; Rohm, M.; Ashcroft, F.M. beta-Cell dysfunction in diabetes: A crisis of identity? Diabetes Obes. Metab. 2016, 18, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Nanditha, A.; Ma, R.C.; Ramachandran, A.; Snehalatha, C.; Chan, J.C.; Chia, K.S.; Shaw, J.E.; Zimmet, P.Z. Diabetes in Asia and the Pacific: Implications for the global epidemic. Diabetes Care 2016, 39, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.C.; Chan, J.C. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann. N. Y. Acad. Sci. 2013, 1281, 64–91. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kim, Y.S.; Hong, S.M.; Park, S. Long-term consumption of saponins derived from Platycodi radix (22 years old) enhances hepatic insulin sensitivity and glucose-stimulated insulin secretion in 90% pancreatectomized diabetic rats fed a high-fat diet. Br. J. Nutr. 2009, 101, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Dulaglutide for the treatment of type 2 diabetes. Expert Opin. Biol. Ther. 2017, 17, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Kim, M.Y.; Kim, J.H.; Cho, J.Y. Codonopsis lanceolata: A review of its therapeutic potentials. Phytother. Res. 2016, 30, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, Y.H.; Kim, D.B.; Shin, G.H.; Lee, J.H.; Cho, J.H.; Lee, B.Y.; Lee, O.H. Acute and subchronic (28 days) oral toxicity studies of Codonopsis lanceolata extract in Sprague-Dawley rats. Regul. Toxicol. Pharmacol. 2015, 71, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Hur, G.Y.; Choi, G.S.; Park, H.J.; Ye, Y.M.; Park, H.S. Anaphylactic shock induced by Codonopsis lanceolata, traditional Chinese medicine in a patient with allergic rhinitis. Allergy 2008, 63, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Kim, J.Y.; Lee, J.Y.; Byeon, S.E.; Hong, E.K.; Lee, J.; Rhee, M.H.; Park, H.J.; Cho, J.Y. Regulatory effects of Codonopsis lanceolata on macrophage-mediated immune responses. J. Ethnopharmacol. 2007, 112, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, K.J.; Kim, Y.H.; Kim, D.B.; Shin, G.H.; Cho, J.H.; Kim, B.K.; Lee, B.Y.; Lee, O.H. Codonopsis lanceolata extract prevents diet-induced obesity in C57BL/6 mice. Nutrients 2014, 6, 4663–4677. [Google Scholar] [CrossRef] [PubMed]
- Weon, J.B.; Eom, M.R.; Jung, Y.S.; Hong, E.H.; Ko, H.J.; Lee, H.Y.; Park, D.S.; Ma, C.J. Steamed and fermented ethanolic extract from Codonopsis lanceolata attenuates amyloid-beta-induced memory impairment in mice. Evid. Based Complement. Alternat. Med. 2016, 2016, 1473801. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim. Biophys. Acta 2017, 186, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Prabhakar, M.; Ju, J.; Long, H.; Zhou, H.W. Effect of inulin-type fructans on blood lipid profile and glucose level: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2017, 71, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hameed, E.-S.S. Total phenolic contents and free radical scavenging activity of certain egyptian ficus species leaf samples. Food Chem. 2009, 114, 1271–1277. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis. Method Association of Official Analytical Communities, 19th ed.; AOAC International: Arlington, TX, USA, 2012. [Google Scholar]
- Woo, S.W.; Shin, H.Y.; Kang, K.S. Chemistry and pharmacognosy of flavone-c-glycosides from Ziziphus seeds. Korean J. Pharmacogn. 1980, 11, 141–148. [Google Scholar]
- Hosokawa, Y.A.; Hosokawa, H.; Chen, C.; Leahy, J.L. Mechanism of impaired glucose-potentiated insulin secretion in diabetic 90% pancreatectomy rats. Study using glucagonlike peptide-1 (7–37). J. Clin. Investig. 1996, 97, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
- Park, S.; Kim, D.S.; Kang, S. Gastrodia elata Blume water extracts improve insulin resistance by decreasing body fat in diet-induced obese rats: Vanillin and 4-hydroxybenzaldehyde are the bioactive candidates. Eur. J. Nutr. 2011, 50, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.S.; Kim, D.S.; Kang, S.; Ryuk, J.A.; Park, S. Prunus mume and Lithospermum erythrorhizon extracts synergistically prevent visceral adiposity by improving energy metabolism through potentiating hypothalamic leptin and insulin signalling in ovariectomized rats. Evid. Based Complement. Alternat. Med. 2013, 2013, 750986. [Google Scholar] [CrossRef] [PubMed]
- Granato, L.; Brandes, A.; Bruni, C.; Greco, A.V.; Mingrone, G. VO2, VCO2, and RQ in a respiratory chamber: Accurate estimation based on a new mathematical model using the Kalman-Bucy method. J. Appl. Physiol. 2004, 96, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Labayen, I.; Forga, L.; Martinez, J.A. Nutrient oxidation and metabolic rate as affected by meals containing different proportions of carbohydrate and fat, in healthy young women. Eur. J. Nutr. 1999, 38, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kim, Y.S.; Ryu, S.Y.; Cha, M.R.; Yon, G.H.; Yang, H.J.; Kim, M.J.; Kang, S.; Park, S. Capsiate improves glucose metabolism by improving insulin sensitivity better than capsaicin in diabetic rats. J. Nutr. Biochem. 2013, 24, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, Y.J.; Fillmore, J.J.; Chen, Y.; Moore, I.; Lee, J.; Yuan, M.; Li, Z.W.; Karin, M.; Perret, P.; et al. Prevention of fat-induced insulin resistance by salicylate. J. Clin. Investig. 2001, 108, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Jang, J.S.; Park, S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology 2005, 146, 4786–4794. [Google Scholar] [CrossRef] [PubMed]
- Dobbins, R.L.; Szczepaniak, L.S.; Myhill, J.; Tamura, Y.; Uchino, H.; Giacca, A.; McGarry, J.D. The composition of dietary fat directly influences glucose-stimulated insulin secretion in rats. Diabetes 2002, 51, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hong, S.M.; Lee, J.E.; Sung, S.R. Exercise improves glucose homeostasis that has been impaired by a high-fat diet by potentiating pancreatic beta-cell function and mass through IRS2 in diabetic rats. J. Appl. Physiol. 2007, 103, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Joh, E.H.; Kim, D.H. Lancemaside A inhibits lipopolysaccharide-induced inflammation by targeting LPS/TLR4 complex. J. Cell. Biochem. 2010, 111, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.H.; Jang, S.E.; Joh, E.H.; Chung, J.; Han, M.J.; Kim, D.H. Lancemaside A isolated from Codonopsis lanceolata and its metabolite echinocystic acid ameliorate scopolamine-induced memory and learning deficits in mice. Phytomedicine 2012, 20, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ahn, I.S.; Kwon, D.Y.; Ko, B.S.; Jun, W.K. Ginsenosides Rb1 and Rg1 suppress triglyceride accumulation in 3T3-L1 adipocytes and enhance beta-cell insulin secretion and viability in Min6 cells via PKA-dependent pathways. Biosci. Biotechnol. Biochem. 2008, 72, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Jung, J.S.; Le, T.K.; Kim, D.H.; Kim, H.S. Lancemaside A inhibits microglial activation via modulation of JNK signaling pathway. Biochem. Biophys. Res. Commun. 2013, 431, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Giraud, J.; Davis, R.J.; White, M.F. c-Jun N-terminal kinase (JNK) mediates feedback inhibition of the insulin signaling cascade. J. Biol. Chem. 2003, 278, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Jang, J.S.; Hong, S.M.; Lee, J.E.; Sung, S.R.; Park, H.R.; Park, S. Long-term consumption of fermented soybean-derived Chungkookjang enhances insulinotropic action unlike soybeans in 90% pancreatectomized diabetic rats. Eur. J. Nutr. 2007, 46, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Wilson, R.D. Experimentally induced rodent models of type 2 diabetes. Methods Mol. Biol. 2012, 933, 161–174. [Google Scholar] [PubMed]
- Choi, H.K.; Won, E.K.; Jang, Y.P.; Choung, S.Y. Antiobesity Effect of Codonopsis lanceolata in High-Calorie/High-Fat-Diet-Induced Obese Rats. Evid. Based Complement. Alternat. Med. 2013, 2013, 210297. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.S.; Kang, S.; Hong, S.M.; Shin, B.K. Effect of central AMPK activation on insulin secretion and insulin resistance in type 2 diabetic rats. Brain Res. Bull. 2014, 108C, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Cha, A.; Choi, Y.; Jin, Y.; Sung, M.K.; Koo, Y.C.; Lee, K.W.; Park, T. Antilipogenic and anti-inflammatory activities of Codonopsis lanceolata in mice hepatic tissues after chronic ethanol feeding. J. Biomed. Biotechnol. 2012, 2012, 141395. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Kim, S.J.; Park, S.H.; Kim, S.; Park, T. Protective effect of Codonopsis lanceolata root extract against alcoholic fatty liver in the rat. J. Med. Food 2009, 12, 1293–1301. [Google Scholar] [CrossRef] [PubMed]
- Jayanthy, G.; Roshana Devi, V.; Ilango, K.; Subramanian, S.P. Rosmarinic acid mediates mitochondrial biogenesis in insulin resistant skeletal muscle through activation of AMPK. J. Cell. Biochem. 2017, 118, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.T.; Lerin, C.; Haas, W.; Gygi, S.P.; Spiegelman, B.M.; Puigserver, P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005, 434, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dentin, R.; Chen, D.; Hedrick, S.; Ravnskjaer, K.; Schenk, S.; Milne, J.; Meyers, D.J.; Cole, P.; Yates, J., 3rd; et al. Fasting Inducible Switch Modulates Gluconeogenesis Via Activator-Coactivator Exchange. Nature 2008, 456, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Alcain, F.J.; Villalba, J.M. Sirtuin activators. Expert Opin. Ther. Pat. 2009, 19, 403–414. [Google Scholar] [CrossRef] [PubMed]
| Untreated Control (n = 16) | CLW-L (n = 16) | CLW-H (n = 16) | Positive Control (n = 16) | |
|---|---|---|---|---|
| Body weight (g) | 326 ± 19 b | 342 ± 29 a,b | 351 ± 37 a | 347 ± 28 a |
| Epididymal fat pads (g) | 2.8 ± 0.6 b | 3.5 ± 0.7 a,b | 4.0 ± 0.7 a | 3.8 ± 0.7 a |
| Caloric intake (kcal/day) | 87.7 ± 7.6 a | 84.6 ± 7.8 a,b | 80.2 ± 8.1 b | 79.2 ± 8.4 b |
| Intake of C. lanceolata (mg/day/kg bw) | 0 ± 0 c | 161 ± 17 b | 746 ± 73 a | 0 ± 0c |
| Caloric expenditure (kcal/kg bw0.75/day) | 104 ± 11 | 106 ± 13 | 114 ± 15 | 109 ± 12 |
| Carbohydrate oxidation (mg/kg bw0.75/min) | 3.3 ± 0.5 b | 3.6 ± 0.5 b | 4.3 ± 0.6 a | 4.3 ± 0.5 a |
| Fat oxidation (mg/kg bw0.75/min) | 7.8 ± 0.9 | 7.6 ± 0.9 | 7.9 ± 0.9 | 7.4 ± 0.8 |
| Locomotive activity (cm/min) | 60.6 ± 8.3 c | 71.5 ± 9.5 b | 80.2 ± 9.7 a | 77.8 ± 8.9 a,b |
| Urinary glucose | ++++ | ++ | + | + |
| Total cholesterol (mg/dL) | 101.7 ± 10.2 a | 99.2 ± 10.5 a | 93.3 ± 9.6 a,b | 90.8 ± 9.7 b |
| HDL cholesterol (mg/dL) | 22.2 ± 2.7 b | 24.1 ± 2.6 a,b | 25.9 ± 2.4 a | 26.3 ± 2.6 a |
| LDL cholesterol (mg/dL) | 62.8 ± 5.9 a | 62.4 ± 6.8 a | 56.7 ± 6.1 b | 52.3 ± 5.6 b |
| Triglyceride (mg/dL) | 78.4 ± 8.1 a | 63.6 ± 6.9 b | 53.6 ± 5.7 c | 60.9 ± 6.6 b |
| Untreated Control (n = 8) | CLW-L (n = 8) | CLW-H (n = 8) | Positive Control (n = 8) | |
|---|---|---|---|---|
| Overnight-fasted serum glucose (mg/dL) | 151 ± 18 a | 125 ± 17 b | 101 ± 13 c | 102 ± 16 c |
| Overnight-fasted serum insulin (ng/mL) | 1.14 ± 0.12 a | 1.05 ± 0.09 a,b | 0.88 ± 0.10 b | 0.94 ± 0.11 b |
| Serum glucose at 60–120 min (mg/dL) | 254 ± 12 a | 230 ± 10 b | 208 ± 9 c | 207 ± 10 c |
| AUC of serum insulin at first phase (ng/mL min) | 22.0 ± 2.9 a | 17.9 ± 1.9 b | 15.5 ± 1.8 c | 14.8 ± 1.6 c |
| AUC of serum insulin at second phase (ng/mL min) | 231 ± 24 a | 206 ± 22 b | 168 ± 18 c | 176 ± 19 c |
| Glucose infusion rate (mg/kg bw/min) | 7.3 ± 1.1 b | 8.1 ± 1.2 b | 9.5 ± 1.5 a | 9.8 ± 1.4 a |
| Insulin sensitivity (µmol glucose min−1 100 g−1 per µmol insulin/L) | 9.5 ± 1.6 c | 11.8 ± 2.0 b | 17.0 ± 2.6 a | 16.7 ± 2.3 a |
| Untreated Control (n = 8) | CLW-L (n = 8) | CLW-H (n = 8) | Positive Control (n = 8) | |
|---|---|---|---|---|
| Total β-cell area (%) | 5.7 ± 0.6 c | 6.8 ± 0.9 b | 8.4 ± 1.0 a | 7.3 ± 0.9 b |
| Individual β-cell size (μm2) | 238 ± 30 a | 216 ± 27 a,b | 195 ± 27 b | 187 ± 26 b |
| Absolute β-cell mass (mg) | 17.1 ± 2.8 c | 21.9 ± 3.9 b | 27.7 ± 3.7 a | 25.2 ± 3.2 a |
| BrdU+ cells (% BrdU+ cells of islets) | 0.87 ± 0.10 | 0.91 ± 0.11 | 0.94 ± 0.11 | 0.92 ± 0.12 |
| Apoptosis (% apoptotic bodies of islets) | 0.82 ± 0.09 a | 0.64 ± 0.08 b | 0.53 ± 0.07 c | 0.59 ± 0.08 b,c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-Y.; Kang, S.; Kim, D.S.; Park, S. Codonopsis lanceolata Water Extract Increases Hepatic Insulin Sensitivity in Rats with Experimentally-Induced Type 2 Diabetes. Nutrients 2017, 9, 1200. https://doi.org/10.3390/nu9111200
Jeong S-Y, Kang S, Kim DS, Park S. Codonopsis lanceolata Water Extract Increases Hepatic Insulin Sensitivity in Rats with Experimentally-Induced Type 2 Diabetes. Nutrients. 2017; 9(11):1200. https://doi.org/10.3390/nu9111200
Chicago/Turabian StyleJeong, Seong-Yeop, Suna Kang, Da Sol Kim, and Sunmin Park. 2017. "Codonopsis lanceolata Water Extract Increases Hepatic Insulin Sensitivity in Rats with Experimentally-Induced Type 2 Diabetes" Nutrients 9, no. 11: 1200. https://doi.org/10.3390/nu9111200





