Leptin and Physical Activity in Adult Patients with Anorexia Nervosa: Failure to Demonstrate a Simple Linear Association
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Population
2.3. Ethics
2.4. Clinical Data
2.5. Body Composition
2.6. Physical Activity
2.7. Leptin Level Analysis
2.8. Statistical Analysis
3. Results
3.1. Study Population
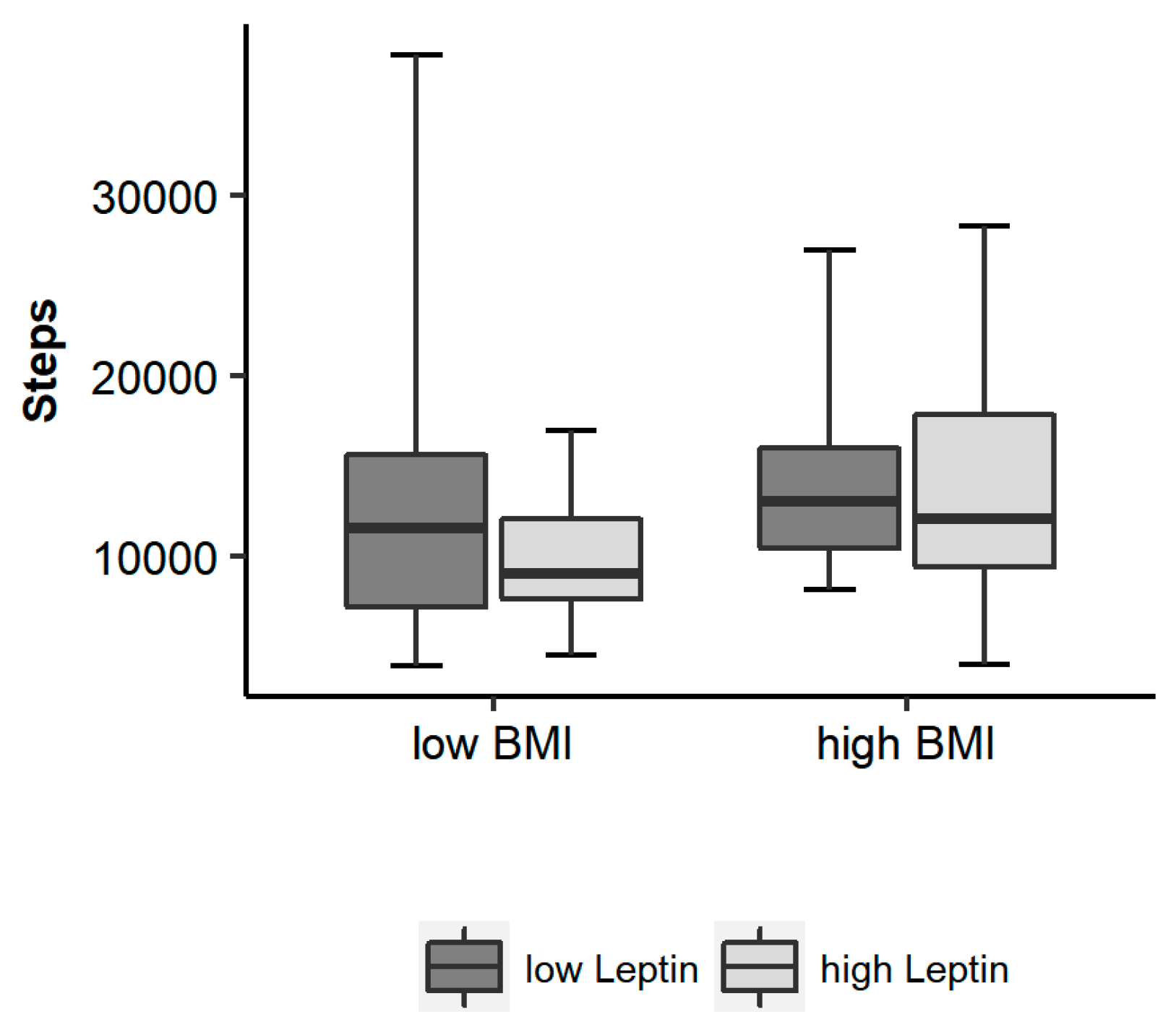
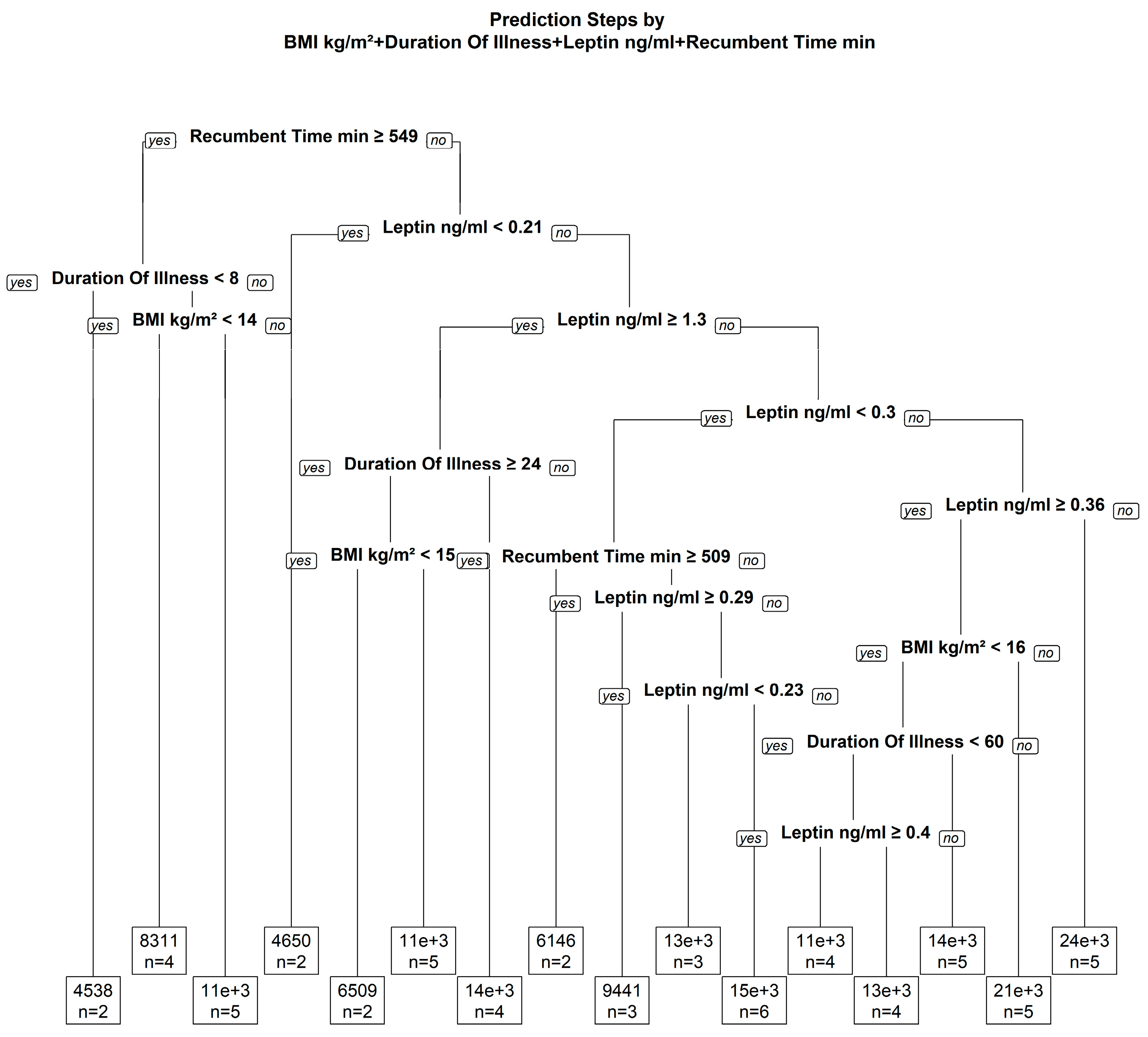
3.2. Relationship between Leptin, BMI, Body Composition and Physical Activity
3.3. Potential Moderators with an Effect on the Association of Leptin and Physical Activity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AN | anorexia nervosa |
| BMI | body mass index |
| PA | physical activity |
| SD | standard deviation |
References
- Carrera, O.; Adan, R.A.; Gutierrez, E.; Danner, U.N.; Hoek, H.W.; van Elburg, A.A.; Kas, M.J. Hyperactivity in anorexia nervosa: Warming up not just burning-off calories. PLoS ONE 2012, 7, e41851. [Google Scholar] [CrossRef] [PubMed]
- Schöll, M.; Herpertz-Dahlmann, B. Physical Activity in Adolescent Eating Disorders and Associated Psychopathological and Biological Conditions; Lehrstuhl für Kinder- und Jugendpsychiatrie und -Psychotherapie (535520-2); RWTH Aachen: Aachen, Germany, 2008. [Google Scholar]
- Zhang, Y.Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homolog. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Haas, V.; Onur, S.; Paul, T.; Nutzinger, D.O.; Bosy-Westphal, A.; Hauer, M.; Brabant, G.; Klein, H.; Muller, M.J. Leptin and body weight regulation in patients with anorexia nervosa before and during weight recovery. Am. J. Clin. Nutr. 2005, 81, 889–896. [Google Scholar] [PubMed]
- Haas, V.K.; Gaskin, K.J.; Kohn, M.R.; Clarke, S.D.; Muller, M.J. Different thermic effects of leptin in adolescent females with varying body fat content. Clin. Nutr. 2010, 29, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Exner, C.; Hebebrand, J.; Remschmidt, H.; Wewetzer, C.; Ziegler, A.; Herpertz, S.; Schweiger, U.; Blum, W.F.; Preibisch, G.; Heldmaier, G.; et al. Leptin suppresses semi-starvation induced hyperactivity in rats: implications for anorexia nervosa. Mol. Psychiatry 2000, 5, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, L.A.; Luijendijk, M.C.; Adan, R.A. Leptin reduces hyperactivity in an animal model for anorexia nervosa via the ventral tegmental area. Eur. Neuropsychopharmacol. 2011, 21, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.F.; Matthys, D.; Hryhorczuk, C.; Sharma, S.; Mogra, S.; Alquier, T.; Fulton, S. Leptin Suppresses the Rewarding Effects of Running via STAT3 Signaling in Dopamine Neurons. Cell Metab. 2015, 22, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, J.P.; Maraninchi, M.; Lorec, A.M.; Corroller, A.B.; Nicolay, A.; Gaudart, J.; Portugal, H.; Barone, R.; Vialettes, B.; Valero, R. Specific adipocytokines profiles in patients with hyperactive and/or binge/purge form of anorexia nervosa. Eur. J. Clin. Nutr. 2010, 64, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Van Elburg, A.A.; Kas, M.J.; Hillebrand, J.J.; Eijkemans, R.J.; van, E.H. The impact of hyperactivity and leptin on recovery from anorexia nervosa. J. Neural Transm. 2007, 114, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, K.; Herpertz-Dahlmann, B.; Hebebrand, K.; Mika, C.; Kratzsch, J.; Hebebrand, J. Physical activity and restlessness correlate with leptin levels in patients with adolescent anorexia nervosa. Biol. Psychiatry 2006, 60, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, S.; Burghardt, R.; Schneider, N.; Broecker-Preuss, M.; Weiss, D.; Merle, J.V.; Craciun, E.M.; Pfeiffer, E.; Mann, K.; Lehmkuhl, U.; et al. The role of leptin and cortisol in hyperactivity in patients with acute and weight-recovered anorexia nervosa. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Holtkamp, K.; Herpertz-Dahlmann, B.; Mika, C.; Heer, M.; Heussen, N.; Fichter, M.; Herpertz, S.; Senf, W.; Blum, W.F.; Schweiger, U.; et al. Elevated physical activity and low leptin levels co-occur in patients with anorexia nervosa. J. Clin. Endocrinol. Metab. 2003, 88, 5169–5174. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa, E.; van Elburg, A.A.; Sanders, N.; Sternheim, L.; Adan, R.A.; Kas, M.J. Longitudinal changes in the physical activity of adolescents with anorexia nervosa and their influence on body composition and leptin serum levels after recovery. PLoS ONE 2013, 8, e78251. [Google Scholar] [CrossRef] [PubMed]
- Treasure, J.; Zipfel, S.; Micali, N.; Wade, T.; Stice, E.; Claudino, A.; Schmidt, U.; Frank, G.K.; Bulik, C.M.; Wentz, E. Anorexia nervosa. Nat. Rev. Dis. Primers 2015, 1, 15074. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Statistical Classification of diseaSes and Related Health Problems, 10th Revision (ICD-10); World Health Organization: New York, NY, USA, 2008. [Google Scholar]
- Hofmann, T.; Elbelt, U.; Haas, V.; Ahnis, A.; Klapp, B.F.; Rose, M.; Stengel, A. Plasma kisspeptin and ghrelin levels are independently correlated with physical activity in patients with anorexia nervosa. Appetite 2017, 108, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; Razak, F.; Blankenberg, S.; Vuksan, V.; Davis, A.D.; Miller, R.; Teo, K.; Gerstein, H.; Sharma, A.M.; Yusuf, S.; et al. Ethnic variation in adiponectin and leptin levels and their association with adiposity and insulin resistance. Diabetes Care 2010, 33, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Perez-Bravo, F.; Albala, C.; Santos, J.L.; Yanez, M.; Carrasco, E. Leptin levels distribution and ethnic background in two populations from Chile: Caucasian and Mapuche groups. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.S.; Chumlea, W.C.; Heymsfield, S.B.; Lukaski, H.C.; Schoeller, D.; Friedl, K.; Kuczmarski, R.J.; Flegal, K.M.; Johnson, C.L.; Hubbard, V.S. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. Am. J. Clin. Nutr. 2003, 77, 331–340. [Google Scholar] [PubMed]
- Elbelt, U.; Schuetz, T.; Hoffmann, I.; Pirlich, M.; Strasburger, C.J.; Lochs, H. Differences of energy expenditure and physical activity patterns in subjects with various degrees of obesity. Clin. Nutr. 2010, 29, 766–772. [Google Scholar] [CrossRef] [PubMed]
- David Andre. The Development of the SenseWear® Armband, a Revolutionary Energy Assessment Device to Assess Physical Activity and Lifestyle; BodyMedia Inc.: Pittsburgh, PA, USA, 2006. [Google Scholar]
- SMT Medical Technology GmbH&Co.KG W. Das Armband Kompendium, Aktivitätsmonitoring und Lebensstilanalyse mit dem Bodymedia Sensewear Armband; SMT Medical Technology GmbH&Co.KG W.: Würzburg, Germany, 2006. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Elbelt, U.; Ahnis, A.; Kobelt, P.; Rose, M.; Stengel, A. Irisin Levels are Not Affected by Physical Activity in Patients with Anorexia Nervosa. Front. Endocrinol. 2014, 4, 202. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Hebebrand, J.; Muller, T.D.; Holtkamp, K.; Herpertz-Dahlmann, B. The role of leptin in anorexia nervosa: Clinical implications. Mol. Psychiatry 2007, 12, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Hebebrand, J.; Exner, C.; Hebebrand, K.; Holtkamp, C.; Casper, R.C.; Remschmidt, H.; Herpertz-Dahlmann, B.; Klingenspor, M. Hyperactivity in patients with anorexia nervosa and in semistarved rats: evidence for a pivotal role of hypoleptinemia. Physiol. Behav. 2003, 79, 25–37. [Google Scholar] [CrossRef]
- El Ghoch, M.; Calugi, S.; Pellegrini, M.; Milanese, C.; Busacchi, M.; Battistini, N.C.; Bernabe, J.; Grave, R.D. Measured physical activity in anorexia nervosa: features and treatment outcome. Int. J. Eat. Disord. 2013, 46, 709–712. [Google Scholar]
- El Ghoch, M.; Calugi, S.; Pellegrini, M.; Chignola, E.; Grave, R.D. Physical activity, body weight, and resumption of menses in anorexia nervosa. Psychiatry Res. 2016, 246, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Farr, O.M.; Gavrieli, A.; Mantzoros, C.S. Leptin applications in 2015: What have we learned about leptin and obesity? Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Scheers, T.; Philippaerts, R.; Lefevre, J. SenseWear-determined physical activity and sedentary behavior and metabolic syndrome. Med. Sci. Sports Exerc. 2013, 45, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Knaeps, S.; De, B.S.; Bourgois, J.; Mertens, E.; Charlier, R.; Lefevre, J. Substituting Sedentary Time With Light And Moderate-to-Vigorous Physical Activity is Associated With Better Cardio-Metabolic Health. J. Phys. Act. Health 2017, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Haas, V.; Riedl, A.; Hofmann, T.; Nischan, A.; Burghardt, R.; Boschmann, M.; Klapp, B. Bioimpedance and bioimpedance vector analysis in patients with anorexia nervosa. Eur. Eat. Disord. Rev. 2012, 20, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Jagielska, G.; Kacperska, I. Outcome, comorbidity and prognosis in anorexia nervosa. Psychiatr. Pol. 2017, 51, 205–218. [Google Scholar] [CrossRef] [PubMed]

| Measurement Parameters | All Patients | Restrictive Subtype | Purging Subtype | p-Value |
|---|---|---|---|---|
| n (%) | 61 (100%) | 49 (80.3) | 12 (19.7) | |
| Demographic parameters | ||||
| Age (years) | 25 (21/30; 18–52) | 24 (19/32; 18–52) | 26 (21/29; 18–32) | 0.643 |
| Height (m) | 1.67 ± 0.07 (1.53–1.84) | 1.67 ± 0.07 (1.53–1.84) | 1.69 ± 0.06 (1.55–1.80) | 0.233 |
| Weight (kg) | 41 ± 7 (29–59) | 41 ± 7 (29–59) | 45 ± 4 (37–49) | 0.056 |
| BMI (kg/m2) | 14.8 ± 2.0 (10.5–19.3) | 14.6 ± 2.1 (10.5–19.3) | 15.7 ± 1.6 (13.3–18.4) | 0.085 |
| Duration of illness (months) | 44 (12/120; 1–312) | 33 (12/77; 1–312) | 84 (25/120; 4–180) | 0.243 |
| Body composition | ||||
| Fat-free mass (kg) | 37 ± 4 (30–48) | 37 ± 4 (30–48) | 40 ± 4 (32–48) | 0.016 |
| Fat mass (kg) | 4.3 ± 4.7 (−3.5–17.2) | 4.1 ± 5.2 (−3.5–17.2) | 4.9 ± 2.3 (0.6–7.3) | 0.599 |
| Fat mass (%) | 9.1 ± 10.2 (−10.7–29.5) | 8.6 ± 11.2 (−10.7–29.5) | 11.0 ± 5.1 (1.2–15.3) | 0.464 |
| Physical activity | ||||
| Steps | 12,841 ± 6408 (3956–37,750) | 13,069 ± 6717 (3956–37,750) | 11,908 ± 5091 (4534–18,505) | 0.578 |
| Recumbent time (min) | 480 (445/533; 333–666) | 473 (443/527; 333–581) | 502 (464/569; 407–666) | 0.174 |
| Leptin (ng/mL) | 0.4 (0.3/1.0; 0.2–8.8) | 0.4 (0.3/1.0; 0.2–8.8) | 0.5 (0.3/2.1; 0.3–4.9) | 0.246 |
| Log leptin (ng/mL) | −0.46 ± 1.04 (−1.62–2.19) | −0.51 ± 1.05 (−1.62–2.19) | −0.27 ± 1.01 (−1.27–1.59) | 0.480 |
| Reference | Subjects (n) | Mean Age (Year) (Range) | Mean BMI (kg/m2) (Range) | Mean Leptin (ng/mL) (Range) | Assessment of Physical Activity | Association Leptin-Physical Activity | Medication | Physical Activity Restriction |
|---|---|---|---|---|---|---|---|---|
| Exner 2000 | 30 | 16.4 ± 3.5 (12–31) | 14.5 ± 1.4 (11.8–16.8) | 0.65 ± 0.56 (0.02–1.62) | patient ratings of motor restlessness | upon attainment of maximal leptin; motor restlessness was ranked lower compared to baseline | n.r. | n.r |
| Holtkamp 2003 | 61 | 17.5 ± 4.6 (12–31) | 14.5 ± 1.5 (10.5–17.4) | 0.91 ± 1.37 (0.004–7.43) | self and expert rating | association of expert rating of motor restlessness and leptin | no | n.r. |
| 27 | 14.5 ± 1.3 (11.5–17.4) | 14.5 ± 1.3 (12.3–17.5) | 1.77 ± 1.06 (0.53–4.57) | SIAB-EX interview | association of expert rating of motor restlessness and leptin; leptin, but not BMI, explained the variance of PA | n.r. | ||
| Holtkamp 2006 | 26 | 15.6 ± 1.9 | 15.2 ± 1.6 | 1.3 ± 0.76 | SIAB-EX interview | leptin predicted all types of activity and restlessness | n.r. | n.r. |
| Van Elburg 2007 | 31 31 | 15.9 ±1.2 20.6 ± 2.9 | 15.4 ± 1.3 | 2.1 (0.5–13.3) | nurse evaluation | on admission: linear association between leptin/PA in adolescents, but not in adults | 57% on antipsychotics | n.r. |
| Ehrlich 2009 | 36 | 18.2 ± 3.3 (14–29) | 15.3 ± 1.3 | 1.4 ± 2.3 | SIAB-EX interview | inverse association in acute. but not in recovered AN; when samples were split according to age (22 adolescents, 14 adults): linear association remained significant in both groups | n.r. | |
| Nogueira 2010 | 24 | 22.8 ± 5.9 | 13.5 ± 1.2 | 0.75 ± 1.0 | SIAB-EX interview | leptin levels higher in high level PA patients | yes | n.r. |
| Kostrzewa 2013 | 37 | 15.2 (13–17.5) | 15.7 ± 1.4 (12.6–18.4) | n.r. | accelerometry | no significant difference in leptin between high and low PA patients | n.r. | yes |
| Haas 2017 | 61 | 25 (18–52) | 14.8 (10.5–19.3) | 1.2 | accelerometry | no association of leptin and steps | 15% | no |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stengel, A.; Haas, V.; Elbelt, U.; Correll, C.U.; Rose, M.; Hofmann, T. Leptin and Physical Activity in Adult Patients with Anorexia Nervosa: Failure to Demonstrate a Simple Linear Association. Nutrients 2017, 9, 1210. https://doi.org/10.3390/nu9111210
Stengel A, Haas V, Elbelt U, Correll CU, Rose M, Hofmann T. Leptin and Physical Activity in Adult Patients with Anorexia Nervosa: Failure to Demonstrate a Simple Linear Association. Nutrients. 2017; 9(11):1210. https://doi.org/10.3390/nu9111210
Chicago/Turabian StyleStengel, Andreas, Verena Haas, Ulf Elbelt, Christoph U. Correll, Matthias Rose, and Tobias Hofmann. 2017. "Leptin and Physical Activity in Adult Patients with Anorexia Nervosa: Failure to Demonstrate a Simple Linear Association" Nutrients 9, no. 11: 1210. https://doi.org/10.3390/nu9111210





