Pilot Study: Comparison of Sourdough Wheat Bread and Yeast-Fermented Wheat Bread in Individuals with Wheat Sensitivity and Irritable Bowel Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Study Breads
2.3. Determination of Monomeric, Dimeric and Tetrameric ATIs
2.4. Inflammatory Markers
2.5. Symptoms
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Dietary Intakes
3.3. ATIs in the Supplied Breads
3.4. Inflammatory Markers
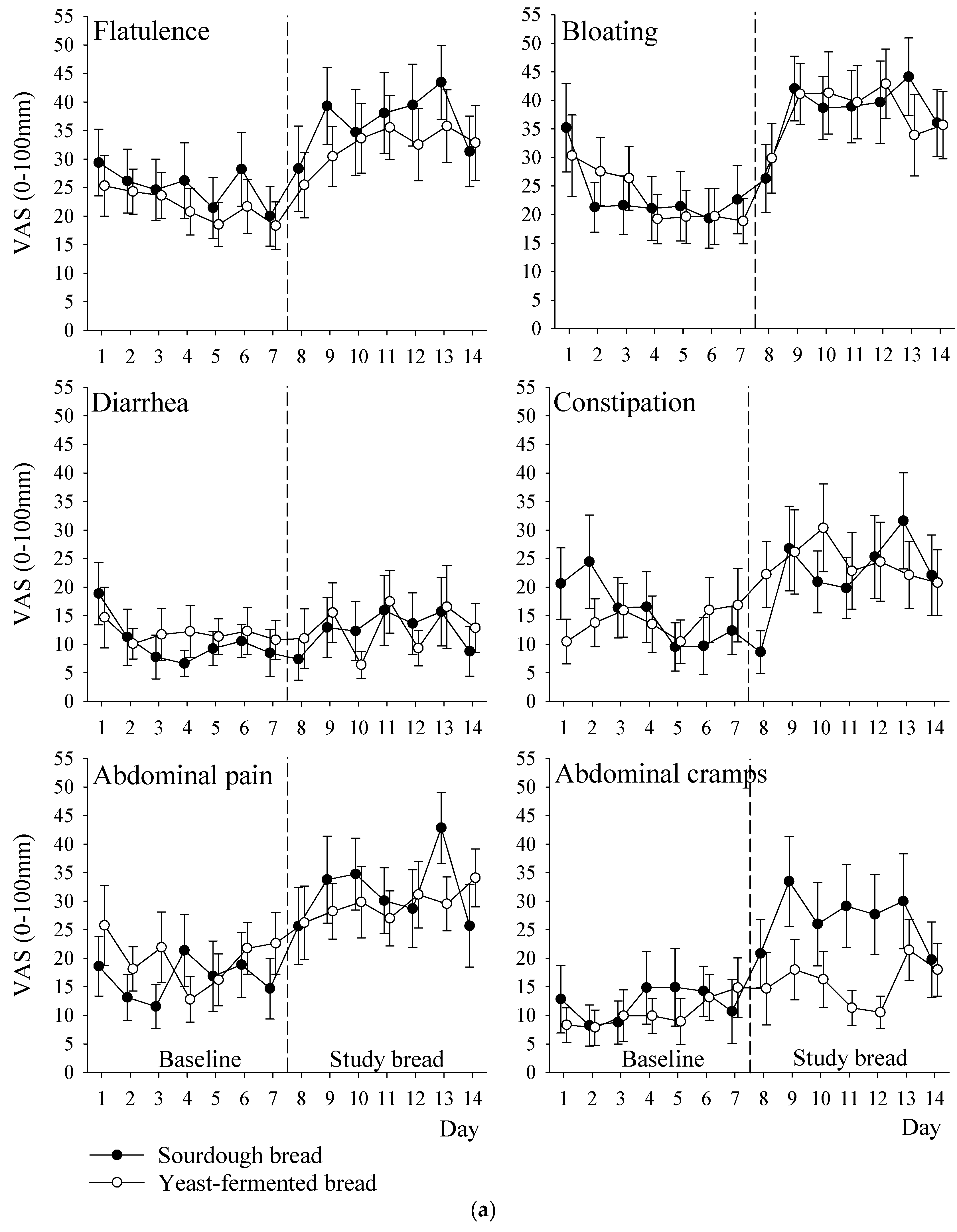
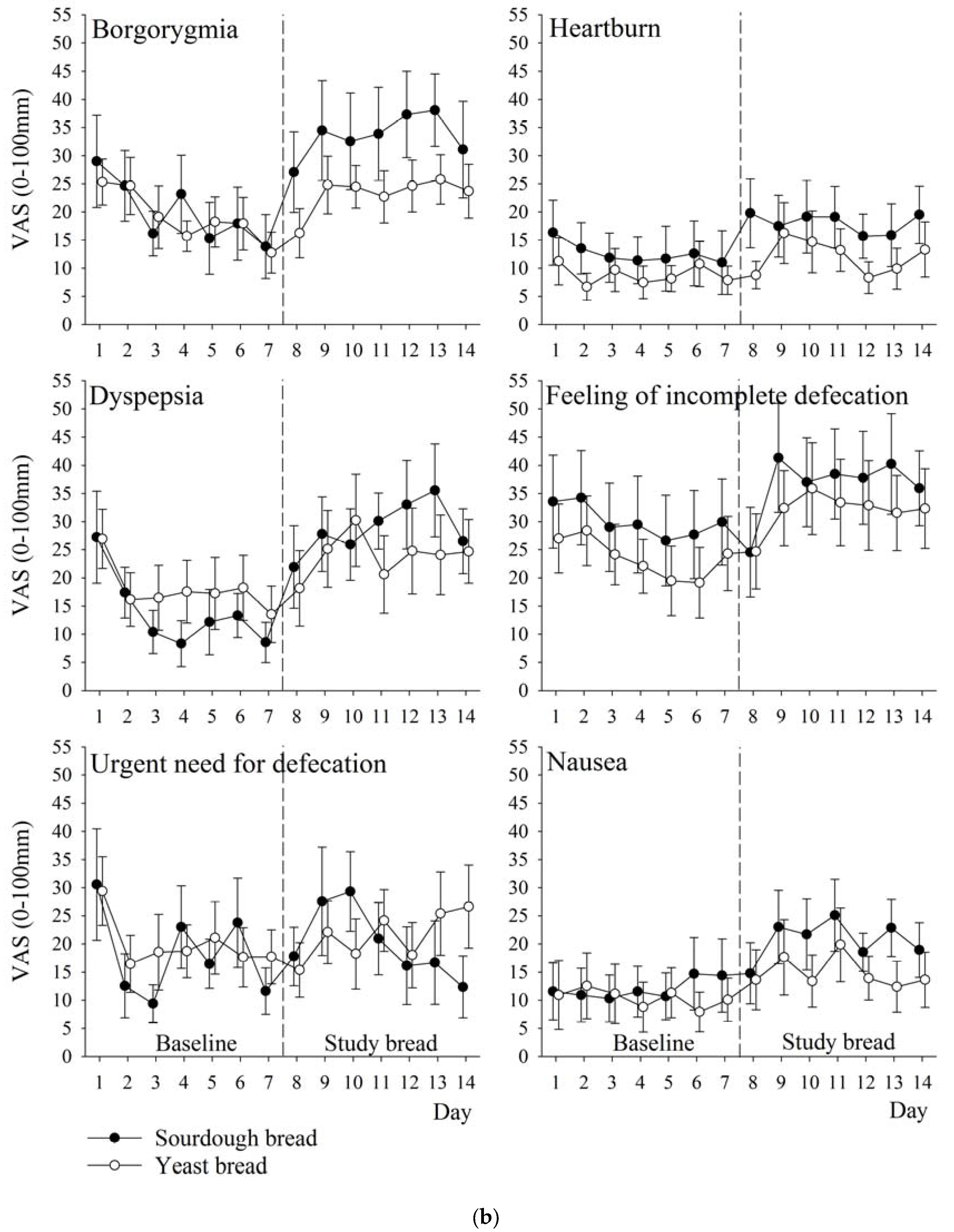
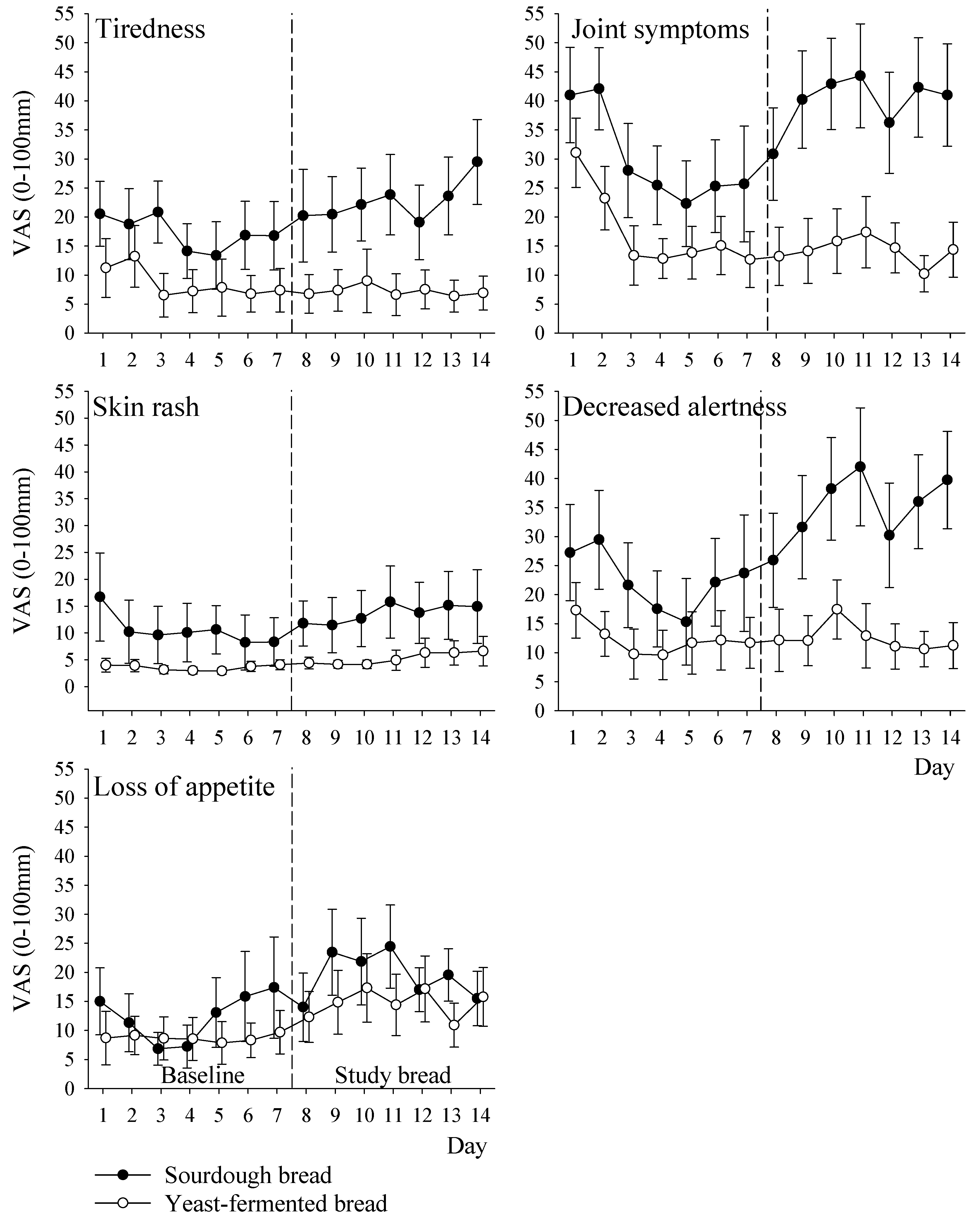
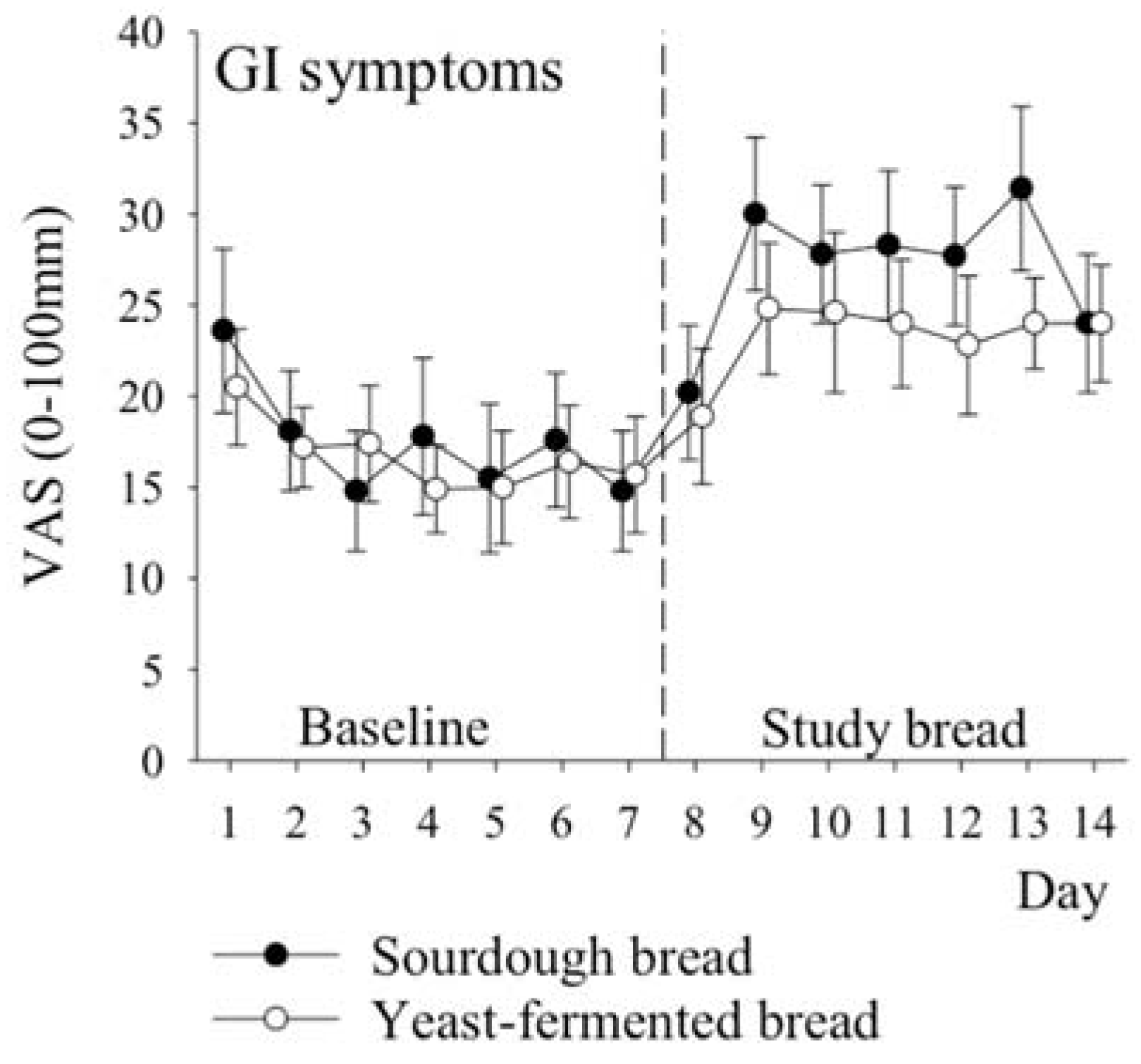
3.5. Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Månsson, A.; Langkilde, A.M.; Svedlund, J.; Abrahamsson, H.; Bengtsson, U.; Björnsson, E.S. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001, 63, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Sanders, D.S.; Grünewald, R.A.; Woodroofe, N.; Boscolo, S.; Aeschlimann, D. Gluten sensitivity: From gut to brain. Lancet Neurol. 2010, 9, 318–330. [Google Scholar] [CrossRef]
- Fasano, A.; Sapone, A.; Zevallos, V.; Schuppan, D. Nonceliac gluten sensitivity. Gastroenterology 2015, 148, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Makharia, A.; Catassi, C.; Makharia, G.K. The Overlap between Irritable Bowel Syndrome and Non-Celiac Gluten Sensitivity: A Clinical Dilemma. Nutrients 2015, 7, 10417–10426. [Google Scholar] [CrossRef] [PubMed]
- Markham, H. You Asked: Do I Have a Gluten Allergy? Time Magazine. 29 April 2015. Available online: http://time.com/3838662/food-allergy-gluten-sensitivity/ (accessed on 30 August 2017).
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Buscarini, E. Evidence for the presence of non-celiac gluten sensitivity in patients with functional gastrointestinal symptoms: Results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Peters, S.L.; Newnham, E.D.; Rosella, O.; Muir, J.G.; Gibson, P.R. Effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013, 145, 320–328. [Google Scholar] [CrossRef] [PubMed]
- De Giorgio, R.; Volta, U.; Gibson, P.R. Sensitivity to wheat, gluten and FODMAPs in IBS: Facts or fiction? Gut 2016, 65, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Schuppan, D. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerii, M.C.; Ricci, C.; Spisni, E.; Di Silverstro, R.; Cavazza, E.; Dinelli, G. Responses of peripheral blood mononucleated cells from non-celiac gluten sensitive patients to various cereal sources. Food Chem. 2015, 176, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Schuppan, D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.; Chavan, S. Sourdough technology—A traditional way for wholesome foods: A review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 169–182. [Google Scholar] [CrossRef]
- Loponen, J.; Sontag-Strohm, T.; Venäläinen, J.; Salovaara, H. Prolamin hydrolysis in wheat sourdoughs with differing proteolytic activities. J. Agric. Food Chem. 2007, 55, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, J.; Steiner, D.; Longin, C.; Friedrich, H.; Würschum, T.; Schweiggert, R.; Carle, R. Wheat and the irritable bowel syndrome—FODMAP levels of modern and ancient species and their retention during bread making. J. Funct. Foods 2016, 25, 257–266. [Google Scholar] [CrossRef]
- Laatikainen, R.; Koskenpato, J.; Hongisto, S.M.; Loponen, J.; Poussa, T.; Korpela, R. Randomised clinical trial: Low-FODMAP rye bread vs. regular rye bread to relieve the symptoms of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 44, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstadt, K.; Hattlebak, J.G.; Lied, G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Ajamian, M.; Caio, G.; DiGiorgio, R.; Indart, A.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Whittaker, A.; Gori, A.M.; Cesari, F.; Surrenti, E.; Casini, A. Effect of Triticum turgidum subsp. turanicum wheat on irritable bowel syndrome: A double-blinded randomised dietary intervention trial. Br. J. Nutr. 2014, 111, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; DeVries, J.W.; Rader, J.I.; Cohen, G.; Prosky, L.; Mugford, D.C.; Okuma, K.J. Determination of insoluble, soluble, and total dietary fiber (CODEX definition) by enzymatic-gravimetric method and liquid chromatography: Collaborative study. AOAC Int. 2012, 95, 824–844. [Google Scholar] [CrossRef]
- McCleary, B.V.; Monaghan, D.A. Measurement of resistant starch. J. AOAC Int. 2002, 85, 665–675. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Murphy, A.; Mugford, D.C. Measurement of total fructan in foods by enzymatic/spectrophotometric method: Collaborative study. J AOAC Int. 2000, 83, 356–364. [Google Scholar] [PubMed]
- Gomez, L.; Sanchez-Monge, R.; Garcia-Olmedo, F.; Salcedo, G. Wheat tetrameric inhibitors of insect α-amylases: Alloploid heterosis at the molecular level. Proc. Natl. Acad. Sci. USA 1989, 86, 3242–3246. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Loponen, J.; König, K.; Wu, J.; Gänzle, M.G. Influence of thiol metabolism of lactobacilli on egg white proteins in wheat sourdoughs. J. Agric. Food Chem. 2008, 56, 3357–3362. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Maeda, K.; Murayama, R.; DiMagno, E.P. Character of a wheat amylase inhibitor preparation and effects on fasting human pancreaticobiliary secretions and hormones. Gastroenterology 1996, 111, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Marciani, L.; Pritchard, S.E.; Hellier-Woods, C.; Costigan, C.; Spiller, R.C. Delayed gastric emptying and reduced postprandial small bowel water content of equicaloric whole meal bread versus rice meals in healthy subjects: Novel MRI insights. Eur. J. Clin. Nutr. 2013, 67, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Fritscher-Ravens, A.; Schuppan, D.; Ellrichmann, M.; Schoch, S.; Milla, P.J. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014, 147, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Rosella, O.; Rose, R.; Liels, K.; Barrett, J.S.; Muir, J.G. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J. Hum. Nutr. Diet. 2011, 24, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Carroccio, A. Suspected Nonceliac Gluten Sensitivity Confirmed in Few Patients after Gluten Challenge in Double-Blind, Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2017, 15, 339–348. [Google Scholar] [CrossRef] [PubMed]

| Sourdough Bread */100 g | Yeast-Fermented Bread */100 g | |
|---|---|---|
| Energy, kJ (kcal) | 972 (229) | 1116 (264) |
| Protein, g | 9.3 | 9.9 |
| Gluten, g | 4.8 | 6.0 |
| Fat, g | 1.2 | 2.4 |
| Carbohydrates, g | 43.4 | 48.4 |
| Dietary fibre, g | 3.9 | 4.5 |
| Salt, g | 1.2 | 1.1 |
| Fructans, g | 0.06 | 0.23 |
| LMWDF, g | 0.9 | 1.0 |
| Insoluble HMWDF, g | 1.8 | 2 |
| Soluble HMWDF, g | 1.2 | 1.4 |
| Resistant starch, g | 0.7 | 0.8 |
| Dominant ATI form | Monomeric | Dimeric |
| Additives/Improvers | Not present | Present |
| Characteristics | Sourdough Bread (n = 13) | Yeast-Fermented Bread (n = 13) | Total (n = 26) |
|---|---|---|---|
| Females | 12 (92) | 13 (100) | 25 (96) |
| Age (years) | 39 (21–56) | 47 (24–64) | 43 (21–64) |
| Body mass index, BMI (kg/m2) | 25.1 (20.2–37.7) | 24.9 (19.6–31.8) | 25.0 (19.6–37.7) |
| Weight (kg) | 70 (59–109) | 65 (54–92) | 68 (54–109) |
| Self-reported daily consumption of any bread (number of slices) | 3.0 (1–4) | 2.5 (1–7.5) | 3.0 (1–7.5) |
| Occasional use of some wheat bread | 0 (0) | 4 (31) | 4 (15) |
| Sourdough Bread (n = 13) | Yeast-Fermented Bread (n = 13) | p-Value * | ||
|---|---|---|---|---|
| Energy, kJ/day | Baseline | 8422 (7521–9530) | 8177 (7062–8425) | 0.80 |
| Study bread | 8214 (7826–8558) | 7609 (6498–9899) | ||
| Energy, kcal/day | Baseline | 2012 (1797–2276) | 1953 (1687–2012) | 0.84 |
| Study bread | 1962 (1869–2044) | 1818 (1553–2365) | ||
| Carbohydrates, g/day | Baseline | 196 (147–236) | 182 (161–215) | 0.88 |
| Study bread | 198 (169–221) | 181 (166–281) | ||
| Protein, g/day | Baseline | 88 (64–103) | 78 (64–102) | 0.36 |
| Study bread | 89 (85–107) | 85 (64–107) | ||
| Fat, g/day | Baseline | 89 (66–103) | 73 (69–88) | 0.34 |
| Study bread | 79 (78–86) | 70 (53–83) | ||
| Dietary fibre, g/day | Baseline | 20 (15–22) | 16 (14–22) | 0.36 |
| Study bread | 20 (17–22) | 16 (15–24) |
| Sourdough Bread (n = 12) | Yeast-Fermented Bread (n = 12) | Sourdough Bread vs. Yeast-Fermented Bread | ||||
|---|---|---|---|---|---|---|
| Baseline | Study Bread | Baseline | Study Bread | Baseline-Adjusted Difference | ||
| Ratio (95% CI) | p-Value * | |||||
| IL-8 (pg/mL) | 11.6 (9.8–13.8) | 11.2 (10.1–12.4) | 13.7 (11.0–17.0) | 13.3 (10.5–16.9) | 0.91 (0.73 to 1.14) | 0.38 |
| IL-6 (pg/mL) | 0.67 (0.34–1.32) | 0.72 (0.42–1.21) | 1.22 (0.78–1.90) | 1.09 (0.73–1.64) | 1.01 (0.74 to 1.39) | 0.38 |
| Mean (95% CI) | p-Value | |||||
| LBP (µg/mL) | 8.39 (5.16–11.62) | 8.48 (5.09–11.88) | 8.15 (6.45–9.85) | 9.42 (7.15–11.70) | −1.19 (−2.55 to 0.17) | 0.08 |
| Symptom | Sourdough Bread (n = 13) | Yeast-Fermented Bread (n = 13) | Sourdough Bread vs. Yeast-Fermented Bread | |||
|---|---|---|---|---|---|---|
| Baseline | Study Bread | Baseline | Study Bread | Baseline-Adjusted Difference | p-Value * | |
| Flatulence | 25 (16) | 37 (20) | 22 (12) | 32 (17) | 3 (−11 to 17) | 0.66 |
| Bloating | 23 (17) | 38 (16) | 23 (15) | 38 (20) | 1 (−13 to 15) | 0.93 |
| Diarrhoea | 10 (8) | 13 (15) | 12 (10) | 13 (10) | 1 (−9 to 10) | 0.90 |
| Constipation | 16 (13) | 22 (17) | 14 (13) | 24 (20) | −4 (−16 to 8) | 0.54 |
| Abdominal pain | 16 (14) | 32 (15) | 20 (15) | 29 (15) | 4 (−7 to 15) | 0.44 |
| Abdominal cramps | 12 (17) | 27 (19) | 10 (10) | 16 (13) | 10 (−1 to 21) | 0.06 |
| Borgorygmia | 20 (20) | 34 (24) | 19 (12) | 23 (13) | 10 (−1 to 21) | 0.07 |
| Heartburn | 13 (16) | 18 (16) | 9 (7) | 12 (10) | 3 (−5 to 12) | 0.43 |
| Dyspepsia | 14 (14) | 29 (17) | 18 (17) | 24 (23) | 8 (−6 to 22) | 0.25 |
| Feeling of incomplete defecation | 30 (25) | 37 (25) | 24 (16) | 32 (24) | −1 (−14 to 13) | 0.93 |
| Urgent need for defecation | 18 (14) | 21 (19) | 20 (15) | 21 (16) | 1 (−10 to 12) | 0.90 |
| Nausea | 12 (17) | 21 (15) | 10 (16) | 15 (16) | 5 (−4 to 13) | 0.29 |
| Gastrointestinal total score | 17 (12) | 27 (12) | 17 (9) | 23 (11) | 4 (−4 to 11) | 0.33 |
| Symptom | Sourdough Bread (n = 13) | Yeast-Fermented Bread (n = 13) | Sourdough Bread vs. Yeast-Fermented Bread | |||
|---|---|---|---|---|---|---|
| Baseline | Study Bread | Baseline | Study Bread | Baseline-Adjusted Difference | p-Value * | |
| Tiredness | 30 (24) | 40 (25) | 17 (15) | 14 (15) | 16 (4 to 27) | 0.01 |
| Joint symptoms | 17 (19) | 23 (21) | 9 (14) | 7 (13) | 7 (1 to 13) | 0.03 |
| Skin rash | 11 (18) | 14 (18) | 3.5 (3) | 5 (5) | 3 (−5 to 11) | 0.45 |
| Decreased alertness | 22 (25) | 35 (26) | 12 (15) | 13 (12) | 14 (5 to 22) | 0.003 |
| Loss of appetite | 12 (18) | 19 (15) | 9 (12) | 15 (15) | 2 (−6 to 9) | 0.62 |
| Other symptoms total score | 19 (17) | 26 (18) | 10 (11) | 11 (10) | 8 (2 to 14) | 0.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laatikainen, R.; Koskenpato, J.; Hongisto, S.-M.; Loponen, J.; Poussa, T.; Huang, X.; Sontag-Strohm, T.; Salmenkari, H.; Korpela, R. Pilot Study: Comparison of Sourdough Wheat Bread and Yeast-Fermented Wheat Bread in Individuals with Wheat Sensitivity and Irritable Bowel Syndrome. Nutrients 2017, 9, 1215. https://doi.org/10.3390/nu9111215
Laatikainen R, Koskenpato J, Hongisto S-M, Loponen J, Poussa T, Huang X, Sontag-Strohm T, Salmenkari H, Korpela R. Pilot Study: Comparison of Sourdough Wheat Bread and Yeast-Fermented Wheat Bread in Individuals with Wheat Sensitivity and Irritable Bowel Syndrome. Nutrients. 2017; 9(11):1215. https://doi.org/10.3390/nu9111215
Chicago/Turabian StyleLaatikainen, Reijo, Jari Koskenpato, Sanna-Maria Hongisto, Jussi Loponen, Tuija Poussa, Xin Huang, Tuula Sontag-Strohm, Hanne Salmenkari, and Riitta Korpela. 2017. "Pilot Study: Comparison of Sourdough Wheat Bread and Yeast-Fermented Wheat Bread in Individuals with Wheat Sensitivity and Irritable Bowel Syndrome" Nutrients 9, no. 11: 1215. https://doi.org/10.3390/nu9111215





