Iron Deficiency Anemia, Not Iron Deficiency, Is Associated with Reduced Attention in Healthy Young Women
Abstract
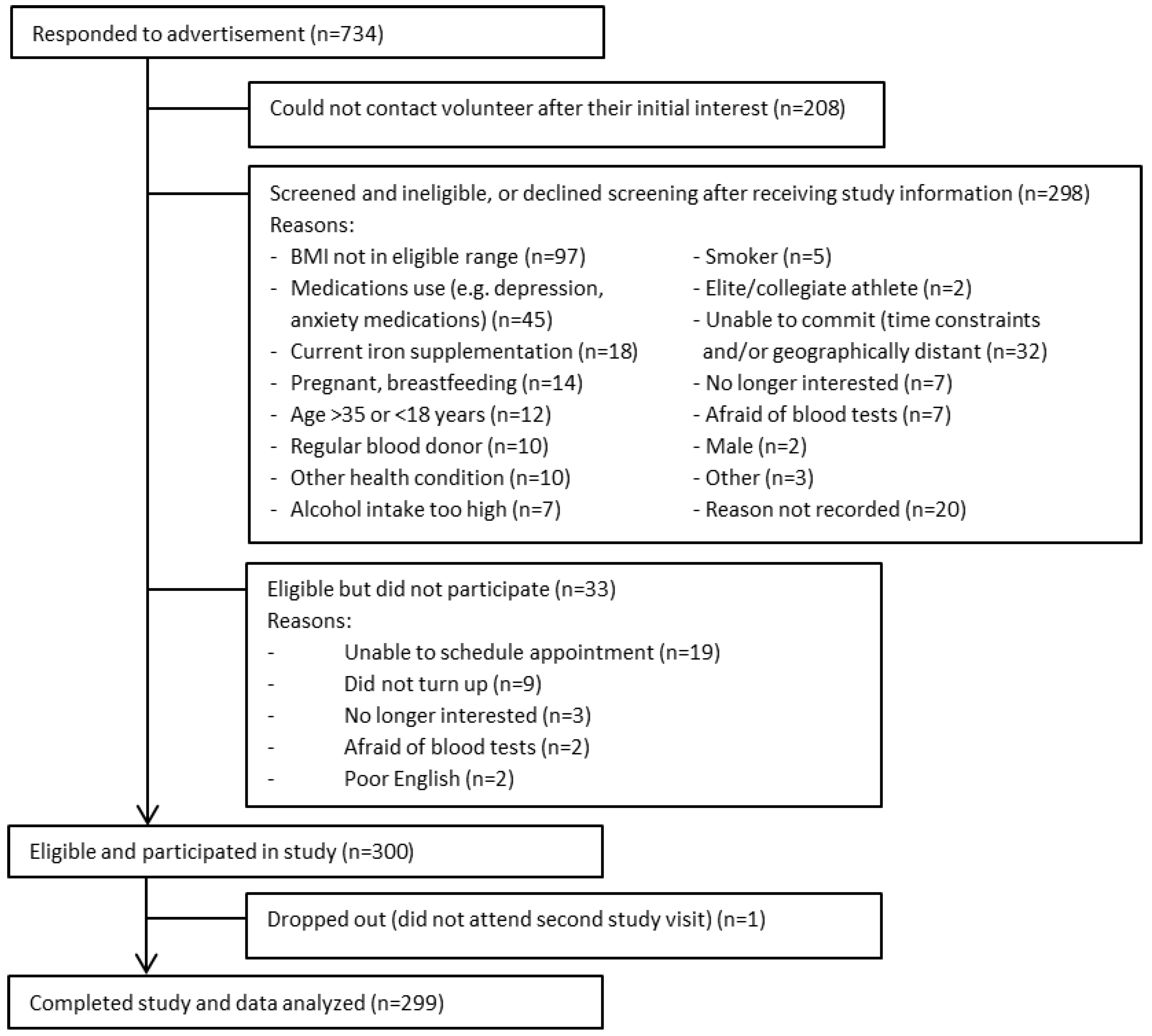
:1. Introduction
2. Materials and Methods
3. Results
3.1. Participant Characteristics
3.2. Iron and Inflammatory Status
3.3. Iron Status and Cognition
3.4. Influence of Covariates on Iron Status and Cognition
3.5. Corrected Ferritin Analysis
3.6. Four-Variable Marker Model of Iron Status
3.7. Participant Location
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beard, J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 2001, 131, 568S–580S. [Google Scholar] [PubMed]
- Murray-Kolb, L.E. Iron and brain functions. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Radlowski, E.C.; Johnson, R.W. Perinatal iron deficiency and neurocognitive development. Front. Hum. Neurosci. 2013, 7, 34–43. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Nutrition: Iron Deficiency Anaemia. Available online: http://www.who.int/nutrition/topics/ida/en/ (accessed on 20 October 2016).
- National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand: Iron. Available online: https://www.nrv.gov.au/nutrients/iron (accessed on 20 October 2016).
- Pasricha, S.-R.; Flecknoe-Brown, S.C.; Allen, K.J.; Gibson, P.R.; McMahon, L.P.; Olynyk, J.K.; Roger, S.D.; Savoia, H.F.; Tampi, R.; Thomson, A.R. Diagnosis and management of iron deficiency anaemia: A clinical update. Med. J. Aust. 2010, 193, 525–532. [Google Scholar] [PubMed]
- Falkingham, M.; Abdelhamid, A.; Curtis, P.; Fairweather-Tait, S.; Dye, L.; Hooper, L. The effects of oral iron supplementation on cognition in older children and adults: A systematic review and meta-analysis. Nutr. J. 2010, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Hermoso, M.; Vucic, V.; Vollhardt, C.; Arsic, A.; Roman-Viñas, B.; Iglesia-Altaba, I.; Gurinovic, M.; Koletzko, B. The effect of iron on cognitive development and function in infants, children and adolescents: A systematic review. Ann. Nutr. Metab. 2011, 59, 154–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qubty, W.; Renaud, D.L. Cognitive impairment associated with low ferritin responsive to iron supplementation. Pediatr. Neurol. 2014, 51, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Greig, A.J.; Patterson, A.J.; Collins, C.E.; Chalmers, K.A. Iron deficiency, cognition, mental health and fatigue in women of childbearing age: A systematic review. J. Nutr. Sci. 2013, 2, e14. [Google Scholar] [CrossRef] [PubMed]
- Sweat, V.; Starr, V.; Bruehl, H.; Arentoft, A.; Tirsi, A.; Javier, E.; Convit, A. C-reactive protein is linked to lower cognitive performance in overweight and obese women. Inflammation 2008, 31, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Bryant, C.; Cook, R.; O’Connor, H.; Rooney, K.; Steinbeck, K. The relationship between obesity and hypoferraemia in adults: A systematic review. Obes. Rev. 2012, 13, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Tussing-Humphreys, L.M.; Nemeth, E.; Fantuzzi, G.; Freels, S.; Guzman, G.; Holterman, A.-X.L.; Braunschweig, C. Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity 2010, 18, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta 2012, 1823, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H.; Strauman, T.A.; Welsh-Bohmer, K.; Browndyke, J.N.; Sherwood, A. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.P.; O’Dwyer, N.; Cook, R.; Vetter, M.; Cheng, H.L.; Rooney, K.; O’Connor, H. Relationship between physical activity and cognitive function in apparently healthy young to middle-aged adults: A systematic review. J. Sci. Med. Sport 2016, 19, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. When does age-related cognitive decline begin? Neurobiol. Aging 2009, 30, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Partridge, S.; Allman-Farinelli, M. Strategies for successful recruitment of young adults to healthy lifestyle programmes for the prevention of weight gain: A systematic review. Obes. Rev. 2016, 17, 178–200. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Lorist, M.M.; Tops, M. Caffeine, fatigue, and cognition. Brain Cognit. 2003, 53, 82–94. [Google Scholar] [CrossRef]
- Paul, R.H.; Lawrence, J.; Williams, L.M.; Clark, R.C.; Cooper, N.; Gordon, E. Preliminary validity of “Integneuro™”: A new computerized battery of neurocognitive tests. Int. J. Neurosci. 2005, 115, 1549–1567. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Simms, E.; Clark, C.; Paul, R.; Rowe, D.; Gordon, E. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “Neuromarker”. Int. J. Neurosci. 2005, 115, 1605–1630. [Google Scholar] [CrossRef] [PubMed]
- Sugarman, R. Integneuro™ User Manual Version 3; Brain Resource Company: Woolloomooloo, NSW, Australia, 2007. [Google Scholar]
- Sherwin, B.B. Estrogen and cognitive functioning in women: Lessons we have learned. Behav. Neurosci. 2012, 126, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Sommer, B. The effect of menstruation on cognitive and perceptual-motor behavior: A review. Psychosom. Med. 1973, 35, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Sundström Poromaa, I.; Gingnell, M. Menstrual cycle influence on cognitive function and emotion processing—From a reproductive perspective. Front. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Northrop-Clewes, C.A.; Thurnham, D.I. Biomarkers for the differentiation of anemia and their clinical usefulness. J. Blood Med. 2013, 4, 11–22. [Google Scholar] [PubMed]
- Cook, J.D.; Flowers, C.H.; Skikne, B.S. The quantitative assessment of body iron. Blood 2003, 101, 3359–3363. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.P.; Murray-Kolb, L.E. Iron status is associated with performance on executive functioning tasks in nonanemic young women. J. Nutr. 2016, 146, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Murray-Kolb, L.E.; Beard, J.L. Iron treatment normalises cognitive function in women. Am. J. Clin. Nutr. 2007, 85, 778–787. [Google Scholar] [PubMed]
- Fayet-Moore, F.; Petocz, P.; Samman, S. Micronutrient status in female university students: Iron, zinc, copper, selenium, vitamin B12 and folate. Nutrients 2014, 6, 5103–5116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Coyne, T.; Dobson, A.; McClintock, C. Iron status among Australian adults: Findings of a population based study in Queensland, Australia. Asia Pac. J. Clin. Nutr. 2008, 17, 40–47. [Google Scholar] [PubMed]
- Lomagno, K.A.; Hu, F.; Riddell, L.J.; Booth, A.O.; Szymlek-Gay, E.A.; Nowson, C.A.; Byrne, L.K. Increasing iron and zinc in pre-menopausal women and its effects on mood and cognition: A systematic review. Nutrients 2014, 6, 5117–5141. [Google Scholar] [CrossRef] [PubMed]
- Murray-Kolb, L.E. Iron status and neuropsychological consequences in women of reproductive age: What do we know and where are we headed? J. Nutr. 2011, 141, 747S–755S. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.P.; De Souza, M.J.; Koehler, K.; Murray-Kolb, L.E. Combined iron deficiency and low aerobic fitness doubly burden academic performance among women attending university. J. Nutr. 2017, 147, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Blanton, C. Improvements in iron status and cognitive function in young women consuming beef or non-beef lunches. Nutrients 2014, 6, 90–110. [Google Scholar] [CrossRef] [PubMed]
- Kretsch, M.J.; Fong, A.K.; Green, M.W.; Johnson, H.L. Cognitive function, iron status, and hemoglobin concentration in obese dieting women. Eur. J. Clin. Nutr. 1998, 52, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.J.; Chalmers, K.A.; Collins, C.E.; Patterson, A.J. A study of the effects of latent iron deficiency on measures of cognition: A pilot randomised controlled trial of iron supplementation in young women. Nutrients 2014, 6, 2419–2435. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.L.; Connor, J.R. Iron status and neural functioning. Ann. Rev. Nutr. 2003, 23, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Lozoff, B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J. Nutr. 2011, 141, 740S–746S. [Google Scholar] [CrossRef] [PubMed]
- Nieoullon, A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002, 67, 53–83. [Google Scholar] [CrossRef]
- Youdim, M.B.; Ben-Shachar, D.; Yehuda, S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. Am. J. Clin. Nutr. 1989, 50, 607–615. [Google Scholar] [PubMed]
- Erikson, K.M.; Jones, B.C.; Hess, E.J.; Zhang, Q.; Beard, J.L. Iron deficiency decreases dopamine d 1 and d 2 receptors in rat brain. Pharmacol. Biochem. Behav. 2001, 69, 409–418. [Google Scholar] [CrossRef]
- Unger, E.L.; Wiesinger, J.A.; Hao, L.; Beard, J.L. Dopamine D2 receptor expression is altered by changes in cellular iron levels in PC12 cells and rat brain tissue. J. Nutr. 2008, 138, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Hager, F.; Volz, H.P.; Gaser, C.; Mentzel, H.J.; Kaiser, W.A.; Sauer, H. Challenging the anterior attentional system with a continuous performance task: A functional magnetic resonance imaging approach. Eur. Arch. Psychiatry Clin. Neurosci. 1998, 248, 161–170. [Google Scholar] [PubMed]
- Prickett, C.; Brennan, L.; Stolwyk, R. Examining the relationship between obesity and cognitive function: A systematic literature review. Obes. Res. Clin. Pract. 2015, 9, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.J.; Brown, W.J.; Powers, J.R.; Roberts, D.C.K. Iron deficiency, general health and fatigue: Results from the australian longitudinal study on women’s health. Qual. Life Res. 2000, 9, 491–497. [Google Scholar] [CrossRef] [PubMed]

| All Participants (n = 299) | NW Group (n = 157) | OB Group (n = 142) | p-Value * | |
|---|---|---|---|---|
| Age (years) | 25.8 ± 5.1 | 24.9 ± 4.6 | 26.9 ± 5.4 | <0.001 |
| Education (years) | 16.2 ± 2.2 | 16.5 ± 2.2 | 15.9 ± 2.2 | 0.022 |
| Highest qualification: (n, %) | 0.018 | |||
| Secondary school | 82 (28%) | 43 (28%) | 39 (27%) | |
| Technical college | 47 (16%) | 16 (10%) | 31 (22%) | |
| University | 169 (57%) | 97 (62%) | 72 (51%) | |
| Height (cm) | 165.4 ± 6.9 | 165.5 ± 7.3 | 165.3 ± 6.6 | 0.805 |
| Weight (kg) | 78.1 ± 23.5 | 59.7 ± 7.0 | 98.5 ± 17.9 | <0.001 |
| BMI (kg/m2) | 28.6 ± 8.6 | 21.8 ± 1.7 | 36.1 ± 6.8 | <0.001 |
| Obese Class I | 79 (26%) | N/A | 79 (56%) | |
| Obese Class II | 41 (14%) | N/A | 41 (29%) | |
| Obese Class III | 22 (7%) | N/A | 22 (15%) | |
| Waist circumference (cm) | 84.5 ± 18.7 | 69.7 ± 4.2 | 101.2 ± 14.1 | <0.001 |
| Below 80 cm (n, %) | 156 (52%) | 154 (98%) | 2 (1%) | <0.001 |
| 80–88 cm inclusive (n, %) | 20 (7%) | 3 (2%) | 17 (12%) | |
| Above 88 cm (n, %) | 120 (40%) | 0 (0%) | 120 (85%) | |
| Physical activity (MET-min/week) | 2603 ± 2141 | 3076 ± 2302 | 2080 ± 1815 | <0.001 |
| All Participants (n = 299) | NW Group (n = 157) | OB group (n = 142) | p-Value * | |
|---|---|---|---|---|
| Iron Studies | ||||
| Serum ferritin (µg/L) | 49.9 ± 38.3 | 47.3 ± 31.4 | 52.7 ± 44.7 | 0.22 |
| Hb (g/L) | 134.1 ± 9.9 | 132.9 ± 9.7 | 135.4 ± 10.1 | 0.035 |
| sTfR (nmol/L) | 1.2 ± 0.4 | 1.1 ± 0.3 | 1.3 ± 0.4 | <0.001 |
| Transferrin saturation (%) | 24.5 ± 10.4 | 27.1 ± 10.8 | 21.6 ± 9.2 | 0.103 |
| RCDW (%) | 12.5 ± 1.1 | 12.3 ± 1.1 | 12.7 ± 1.1 | 0.461 |
| IDA (n, %) | 18 (6%) | 13 (8%) | 5 (4%) | 0.21 |
| ID (n, %) | 41 (14%) | 21 (14%) | 20 (14%) | |
| Replete (n, %) | 235 (80%) | 119 (78%) | 116 (82%) | |
| Total body iron (mg/kg) | 10.7 (3.1) | 10.6 (3.0) | 10.8 (3.3) | 0.489 |
| Corrected serum ferritin ** (µg/L) | 42.4 ± 32.0 | 45.3 ± 30.6 | 39.4 ± 33.4 | 0.11 |
| ID (n, %) | 60 (20%) | 22 (14%) | 38 (27%) | 0.010 |
| Replete (n, %) | 216 (74%) | 118 (77%) | 98 (70%) | |
| Total body iron (mg/kg) | 10.4 (3.1) | 10.6 (3.0) | 10.2 (3.3) | 0.256 |
| CRP (mg/L) | 3.4 ± 4.3 | 1.4 ± 2.1 | 5.5 ± 5.0 | <0.001 |
| CRP < 5 mg/L | 233 (78%) | 149 (95%) | 84 (59%) | <0.001 |
| CRP ≥ 5 mg/L | 63 (21%) | 6 (4%) | 58 (41%) | |
| α1GP (mg/L) | 0.75 ± 0.22 | 0.60 ± 0.14 | 0.90 ± 0.19 | <0.001 |
| α1GP < 1 mg/L | 257 (86%) | 153 (98%) | 104 (73%) | <0.001 |
| α1GP ≥ 1 mg/L | 37 (12%) | 0 (0%) | 37 (26%) | |
| Summary of inflammatory status: | ||||
| Normal CRP + normal α1GP | 218 (73%) | 147 (96%) | 71 (50%) | <0.001 |
| Raised CRP only | 39 (13%) | 6 (4%) | 33 (23%) | |
| Raised CRP + α1GP | 24 (8%) | 0 (0%) | 24 (17%) | |
| Raised α1GP only | 13 (5%) | 0 (0%) | 13 (9%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, R.L.; O’Dwyer, N.J.; Parker, H.M.; Donges, C.E.; Cheng, H.L.; Steinbeck, K.S.; Cox, E.P.; Franklin, J.L.; Garg, M.L.; Rooney, K.B.; et al. Iron Deficiency Anemia, Not Iron Deficiency, Is Associated with Reduced Attention in Healthy Young Women. Nutrients 2017, 9, 1216. https://doi.org/10.3390/nu9111216
Cook RL, O’Dwyer NJ, Parker HM, Donges CE, Cheng HL, Steinbeck KS, Cox EP, Franklin JL, Garg ML, Rooney KB, et al. Iron Deficiency Anemia, Not Iron Deficiency, Is Associated with Reduced Attention in Healthy Young Women. Nutrients. 2017; 9(11):1216. https://doi.org/10.3390/nu9111216
Chicago/Turabian StyleCook, Rebecca L., Nicholas J. O’Dwyer, Helen M. Parker, Cheyne E. Donges, Hoi Lun Cheng, Katharine S. Steinbeck, Eka P. Cox, Janet L. Franklin, Manohar L. Garg, Kieron B. Rooney, and et al. 2017. "Iron Deficiency Anemia, Not Iron Deficiency, Is Associated with Reduced Attention in Healthy Young Women" Nutrients 9, no. 11: 1216. https://doi.org/10.3390/nu9111216





