Pediatric Chronic Intestinal Failure in Italy: Report from the 2016 Survey on Behalf of Italian Society for Gastroenterology, Hepatology and Nutrition (SIGENP)
Abstract
:1. Introduction
2. Materials and Methods
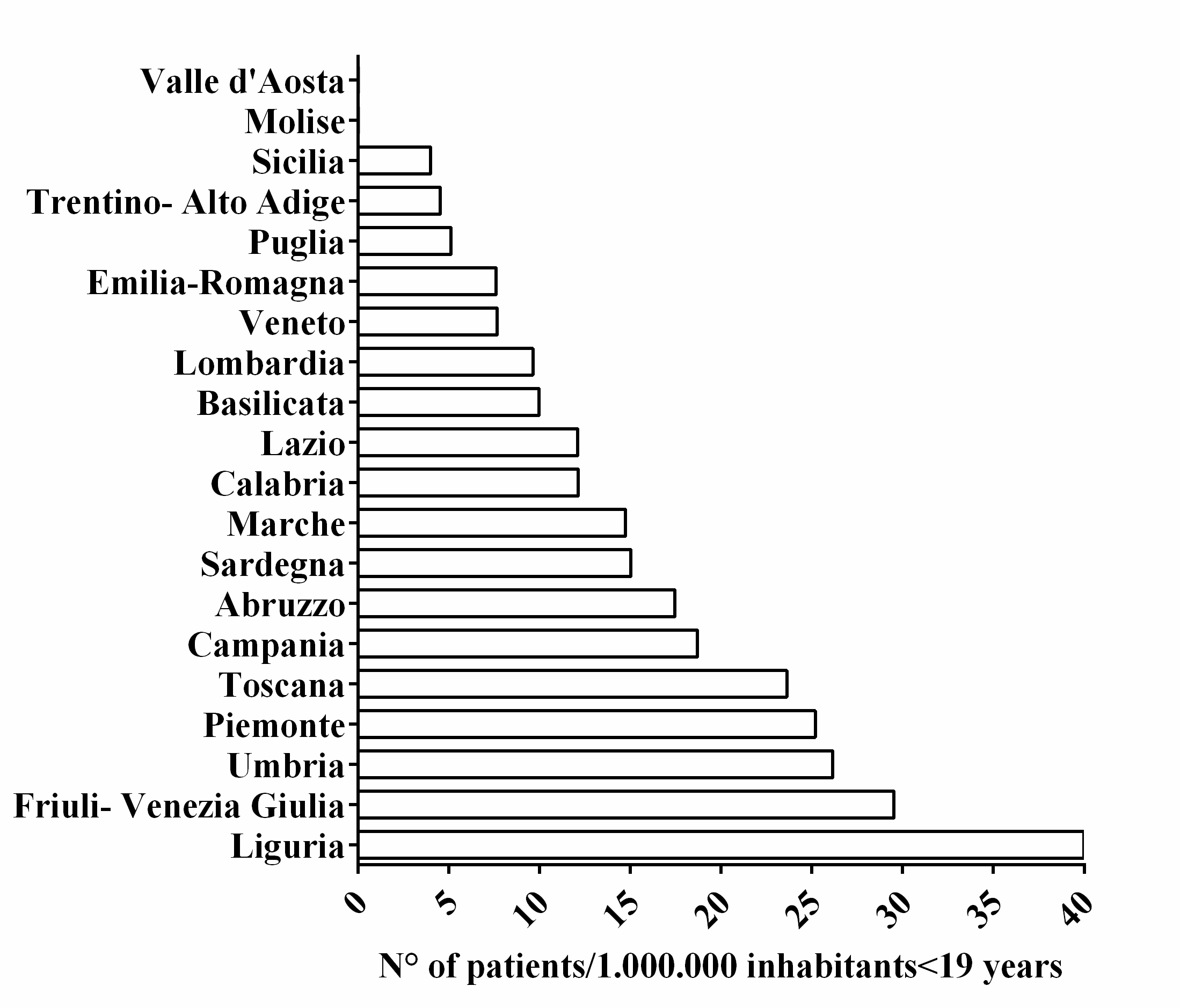
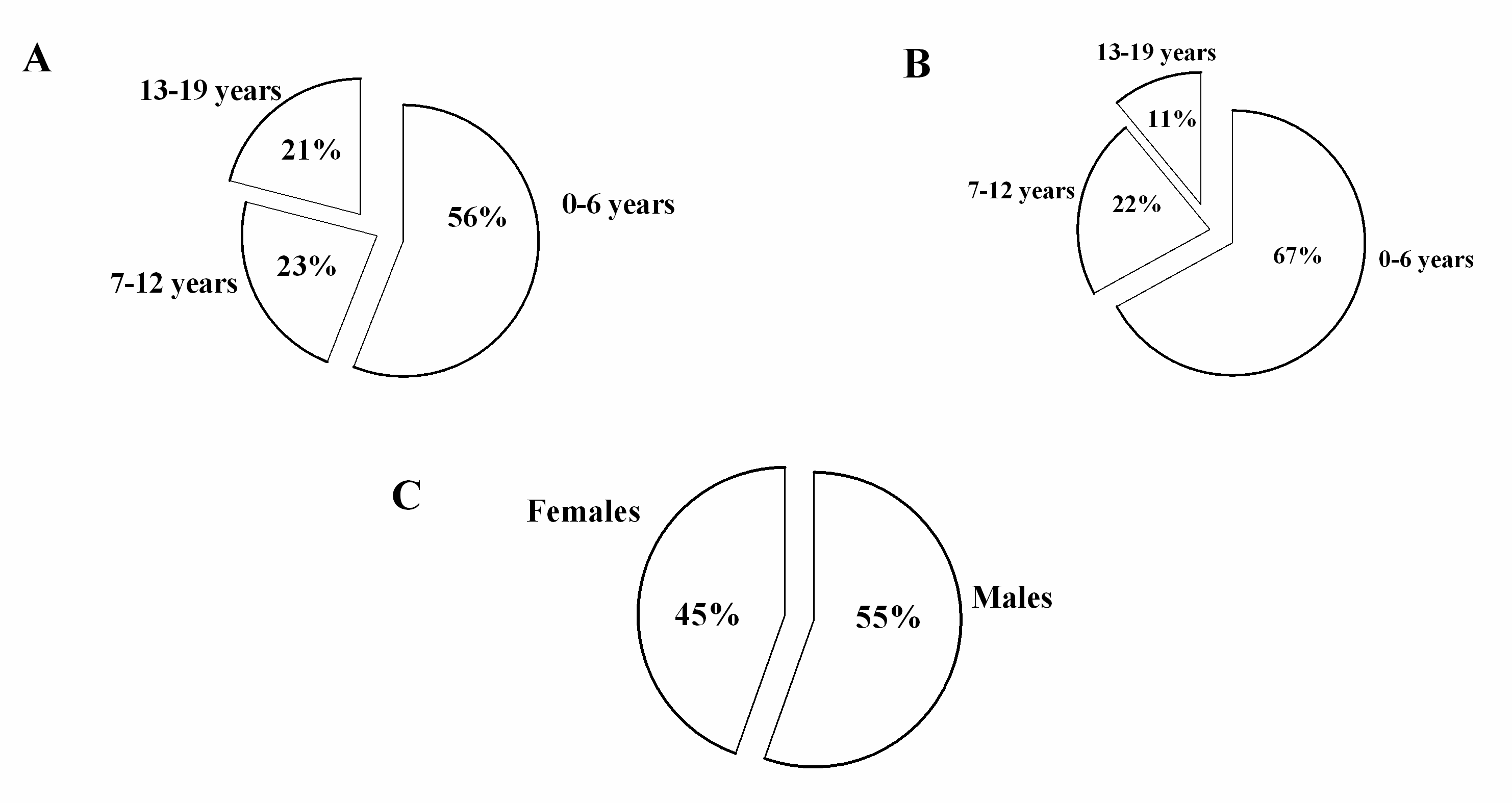
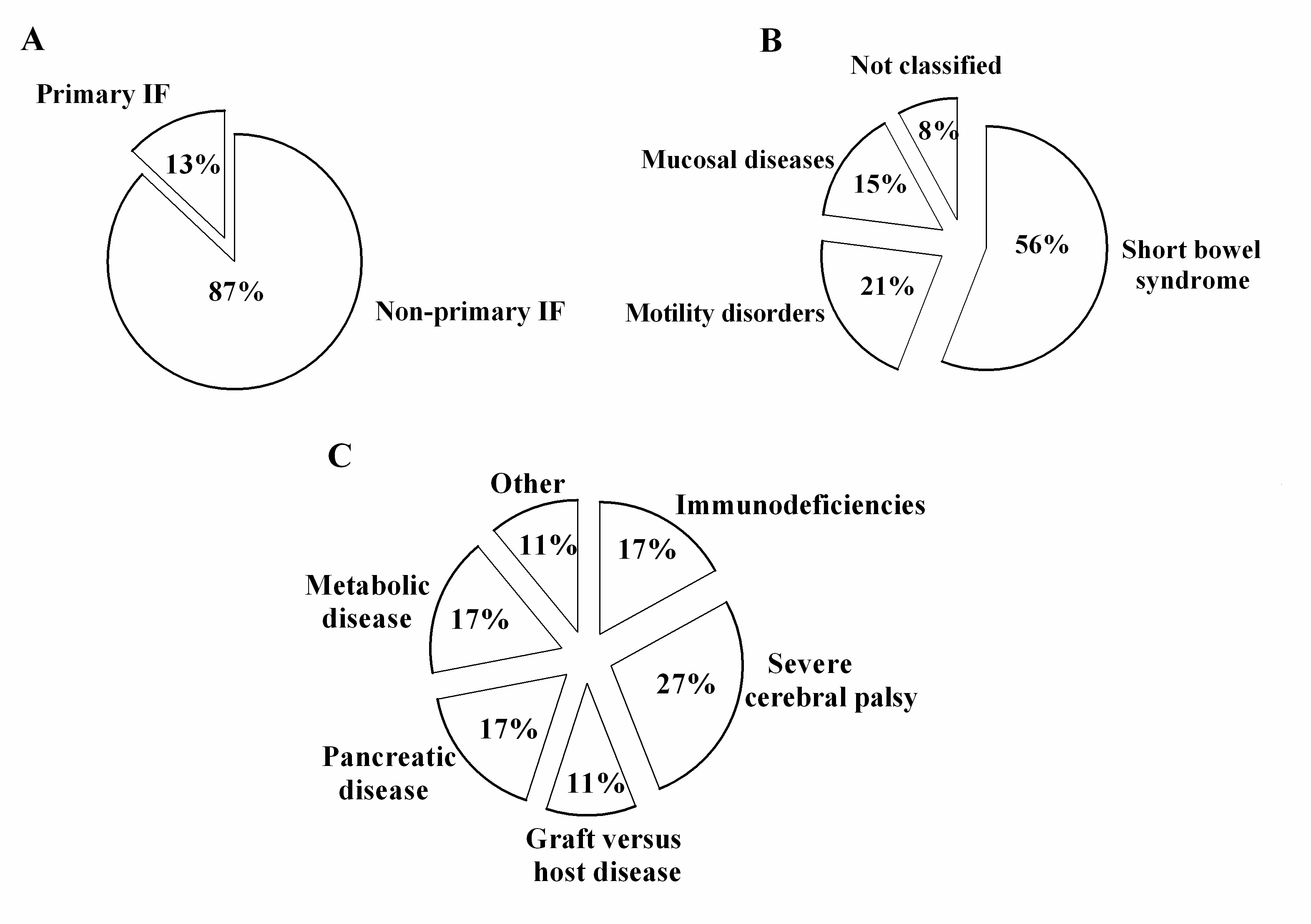
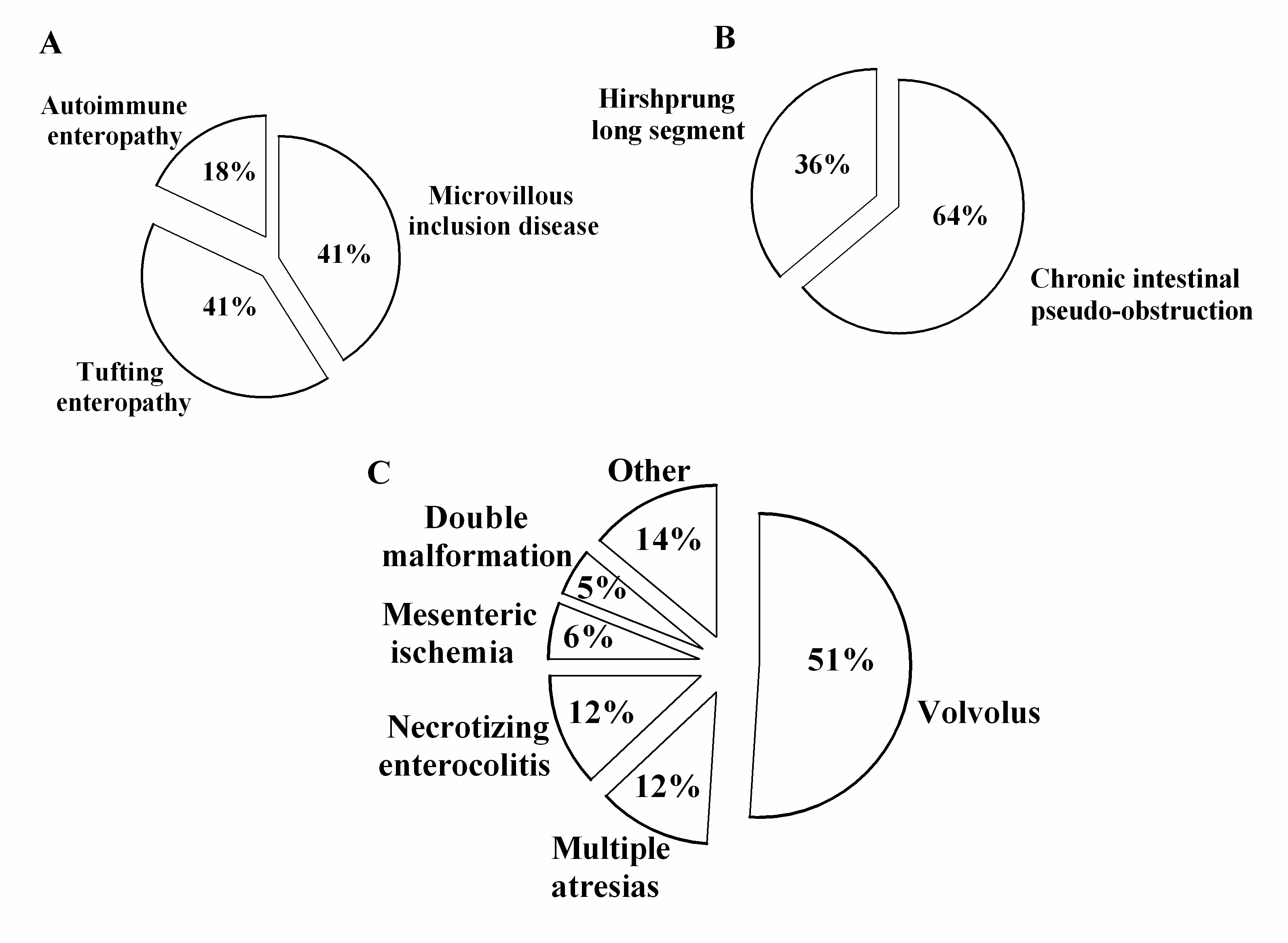
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Goulet, O.; Ruemmele, F. Causes and management of intestinal failure in children. Gastroenterology 2006, 130, S16–S28. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Kedinger, M.; Brousse, N. Intractable diarrhea of infancy with epithelial and basement membrane abnormalities. J. Pediatr. 1995, 127, 212–219. [Google Scholar] [CrossRef]
- Phillips, A.D.; Jenkins, P.; Raafat, F. Congenital microvillous atrophy: Specific diagnostic features. Arch. Dis. Child. 1985, 60, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.J.; Revillon, Y.; Jan, D. Neonatal short bowel syndrome. J. Pediatr. 1991, 119, 18–23. [Google Scholar] [CrossRef]
- Pironi, L.; Arends, J.; Bozzetti, F. ESPEN guidelines on chronic intestinal failure in adults. Clin. Nutr. 2016, 37, 247–307. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, J. Intestinal failure: Definition and service development. Clin. Nutr. 2002, 21 (Suppl. S1), 144–145. [Google Scholar] [CrossRef]
- Van Gossum, J.; Colomb, V.; Hebuteme, X. Home parenteral nutrition (HPN) in children: A multicentre survey in Europe in 1997. Clin. Nutr. 1998, 17, 42–43. [Google Scholar] [CrossRef]
- Pironi, L.; Candusso, M.; Biondo, A. Prevalence of home artificial nutrition in Italy in 2005: A survey by the Italian Society for Parenteral and Enteral Nutrition (SINPE). Clin. Nutr. 2007, 26, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Beath, S.V.; Gowen, H.; Puntis, J.W.L. Trends in pediatric home parenteral nutrition and implications for service development. Clin. Nutr. 2011, 30, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Neelis, E.G.; Roskott, A.M.; Dijkstra, G. Presentation of a nationwide multicenter registry of intestinal failure and intestinal transplantation. Clin. Nutr. 2016, 35, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Italian Institute for National Statitistics (ISTAT) Home Page. Available online: http://www.istat.it/en/ (accessed on 30 December 2016).
- Colomb, V.; Dabbas-Tyan, M.; Taupin, P. Long-term outcome of children receiving home parenteral nutrition: A 20-year single-center experience in 302 Patients. J. Pediatr. Gastroenterol. Nutr. 2007, 44, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Van Gossum, A.; Vahedi, K.; Abdel, M. Clinical, social and rehabilitation status of long-term home parenteral nutrition patients: Results of a European multicenter survey. Clin. Nutr. 2001, 20, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.R.; Paxton, C.E.; Gillett, P. Regionally acquired intestinal failure data suggest an underestimate in national service requirements. Arch. Dis. Child. 2009, 94, 938–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarino, A.; De Marco, G. For the Italian National Network for Pediatric Intestinal Failure. Natural history of intestinal failure, investigated through a network-based approach. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Howard, L.; Ament, M.; Fleming, C.R. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology 1995, 109, 355–365. [Google Scholar] [CrossRef]
- Howard, L.; Heaphey, L.; Fleming, C.R. Four years of North American registry home parenteral nutrition outcome data and their implications for patient management. J. Parent. Enter. Nutr. 1991, 15, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Gandullia, P.; Lugani, F.; Costabelloa, L. Long-term home parenteral nutrition in children with chronic intestinal failure: A 15-year experience at a single Italian center. Dig. Liver Dis. 2011, 43, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Dalzell, A.M. Management of intestinal failure in children. Arch. Dis. Child. 2015, 100, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Capriati, T.; Giorgio, D.; Fusaro, F. Pediatric short bowel syndrome: Predicting four-year outcome after massive neonatal resection. Eur. J. Pediatr. Surg. 2017. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, A.; Conforti, A.; Panetta, F. Long-term outcome of home parenteral nutrition in patients with ultra-short bowel syndrome. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Ahle, M.; Drott, P.; Andersson, R.E. Epidemiology and trends of necrotizing enterocolitis in Sweden: 1987–2009. Pediatrics 2013, 132, e443–e451. [Google Scholar] [CrossRef] [PubMed]
- Italian Ministry for Health. Linee Guida sulla Nutrizione Artificiale Domiciliare; Marchesini Franco: Roma, Italy, 2006.
- D’Antiga, L.; Goulet, O. Intestinal failure in children: The European view. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 118–126. [Google Scholar] [CrossRef] [PubMed]

| Hospital | Administrative Region |
|---|---|
| Burlo Garofalo Children’s Hospital, Trieste | Friuli Venezia Giulia |
| University Hospital, Padova | Veneto |
| Cà Granda Ospedale Maggiore University Hospital, Milan | Lombardia |
| Papa Giovanni XXIII Hospital, Bergamo | Lombardia |
| Spedali Civili Children’s Hospital, Brescia | Lombardia |
| Regina Margherita Children’s Hospital, Turin | Piemonte |
| Giannina Gaslini Children’s Hospital, Genoa | Liguria |
| Salesi Children’s Hospital, Ancona | Marche |
| Sant’Orsola-Malpighi, Bologna | Emilia-Romagna |
| Arcispedale Santa Maria Nuova Hospital, Reggio Emilia | Emilia-Romagna |
| Meyer Children’s Hospital, Florence | Toscana |
| Bambino Gesù Children’s Hospital, Rome | Lazio |
| University Hospital Polyclinic Umberto I, Rome | Lazio |
| University Hospital Policlinic Federico II, Naples | Campania |
| Santobono-Pausillipon Children’s Hospital, Naples | Campania |
| University Hospital Policlinic, Bari | Puglia |
| Ospedali Riuniti University Hospital, Foggia | Puglia |
| University Hospital, Messina | Sicilia |
| Mediterranean Institute for Transplantations (ISMETT), Palermo | Sicilia |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diamanti, A.; Capriati, T.; Gandullia, P.; Di Leo, G.; Lezo, A.; Lacitignola, L.; Spagnuolo, M.I.; Gatti, S.; D’Antiga, L.; Verlato, G.; et al. Pediatric Chronic Intestinal Failure in Italy: Report from the 2016 Survey on Behalf of Italian Society for Gastroenterology, Hepatology and Nutrition (SIGENP). Nutrients 2017, 9, 1217. https://doi.org/10.3390/nu9111217
Diamanti A, Capriati T, Gandullia P, Di Leo G, Lezo A, Lacitignola L, Spagnuolo MI, Gatti S, D’Antiga L, Verlato G, et al. Pediatric Chronic Intestinal Failure in Italy: Report from the 2016 Survey on Behalf of Italian Society for Gastroenterology, Hepatology and Nutrition (SIGENP). Nutrients. 2017; 9(11):1217. https://doi.org/10.3390/nu9111217
Chicago/Turabian StyleDiamanti, Antonella, Teresa Capriati, Paolo Gandullia, Grazia Di Leo, Antonella Lezo, Laura Lacitignola, Maria Immacolata Spagnuolo, Simona Gatti, Lorenzo D’Antiga, Giovanna Verlato, and et al. 2017. "Pediatric Chronic Intestinal Failure in Italy: Report from the 2016 Survey on Behalf of Italian Society for Gastroenterology, Hepatology and Nutrition (SIGENP)" Nutrients 9, no. 11: 1217. https://doi.org/10.3390/nu9111217







