Diet and Asthma: Is It Time to Adapt Our Message?
Abstract
:1. Introduction
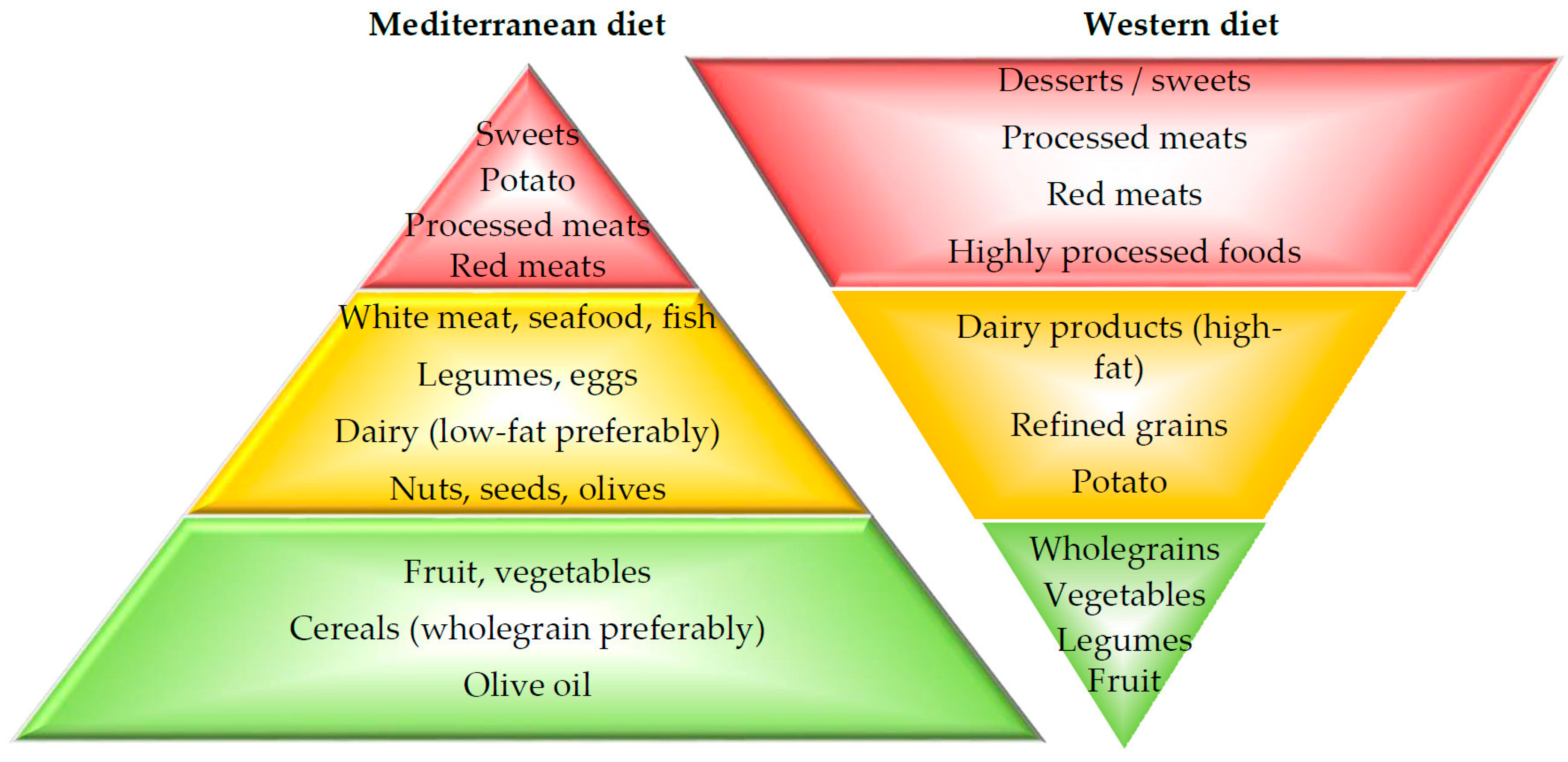
2. Dietary Patterns
3. Diet and Inflammation
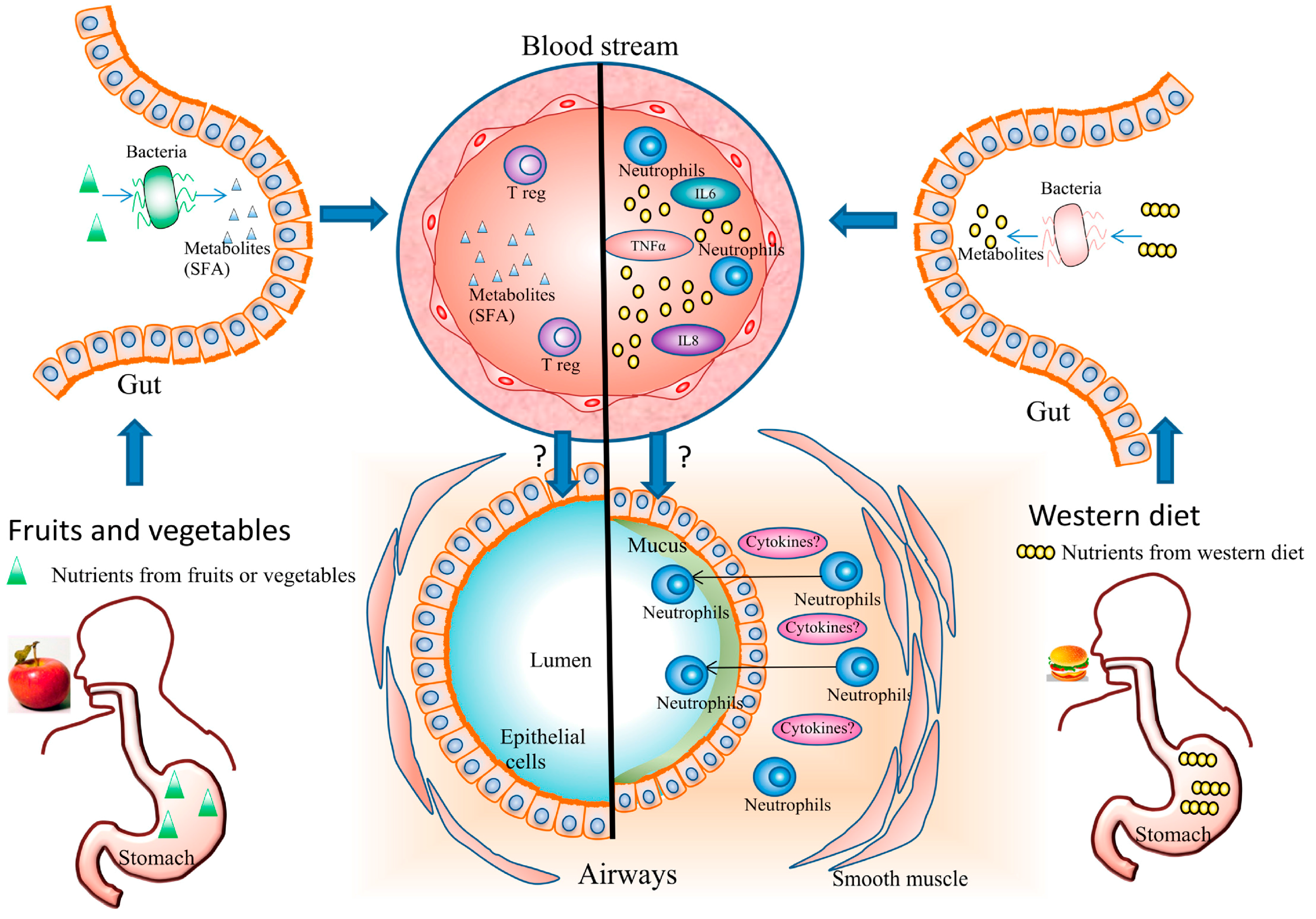
3.1. Diet and Systemic Inflammation
3.2. Diet and Airway Inflammation
3.3. Diet and Gut Microbiota
4. Diet and Risk of Asthma
4.1. Maternal Diet during Pregnancy
4.1.1. Dietary Patterns: No Evident Effect
4.1.2. Vitamin D and E and Fish Oil: Beneficial
4.2. Children
4.2.1. Breastfeeding: Beneficial
4.2.2. Fruit and Vegetables: Beneficial
4.2.3. Dietary Patterns: Med-Diet–No Effect, Western Diet–Detrimental
4.2.4. Vitamins and Fish Oil: Inconclusive Data
4.3. Adults
4.3.1. Breastfeeding: No Effect
4.3.2. Fruits and Vegetables: Beneficial
4.3.3. Vitamins and Fish Oil Supplementation: Inconclusive Data
5. Diet and Asthma Control
5.1. Pregnancy: Studies Are Needed
5.2. Children
5.2.1. Med-Diet, Fruit and Vegetables: Beneficial
5.2.2. PUFA: Conflicting Data
5.2.3. Vitamin C and D: Possible Benefit
5.3. Adults
5.3.1. Fruit and Vegetables: Beneficial
5.3.2. Vitamins or Fish Oil Supplementation: No Effect
5.3.3. Western Diet: Detrimental
6. Diet and Lung Function in Asthma Patients
6.1. Children
6.1.1. The Impact of Breast Feeding on Lung Function Is Low
6.1.2. Med-Diet and Lung Function: Beneficial Effect
6.1.3. Vitamin C and E and Lung Function: A Low Beneficial Effect
6.2. Adults
6.2.1. Fruit and Vegetable Intake: Beneficial
6.2.2. Vitamins and Lung Function: A Low Positive Impact with Vitamin C
6.2.3. Meat and Lung Function: No Impact
7. Diet and Obese-Asthma Phenotype
8. How to Change the Patients’ Behaviour?
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mccollum, E.V. A History of Nutrition; Houghton Mifflin: Boston, MA, USA, 1957. [Google Scholar]
- Roberts, C.K.; Barnard, R.J. Effects of exercise and diet on chronic disease. J. Appl. Physiol. (1985) 2005, 98, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Who, J.; Consultation, F.E. Diet, nutrition and the prevention of chronic diseases—Introduction. WHO Tech. Rep. Ser. 2003, 916, 1–149. [Google Scholar]
- Julia, V.; Macia, L.; Dombrowicz, D. The impact of diet on asthma and allergic diseases. Nat. Rev. Immunol. 2015, 15, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, N.; Abalkhail, B.; Seaton, A. Diet and childhood asthma in a society in transition: A study in urban and rural Saudi Arabia. Thorax 2000, 55, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.I.; Montefort, S.; Bjorksten, B.; Lai, C.K.; Strachan, D.P.; Weiland, S.K.; Williams, H.; Group, I.P.T.S. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Guo, C.H.; Liu, P.J.; Lin, K.P.; Chen, P.C. Nutritional supplement therapy improves oxidative stress, immune response, pulmonary function, and quality of life in allergic asthma patients: An open-label pilot study. Altern. Med. Rev. 2012, 17, 42–56. [Google Scholar] [PubMed]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, D.T. Mechanisms by which obesity impacts upon asthma. Thorax 2017, 72, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; Kermode, J.A.; Downie, S.R.; Brown, N.J.; Hardaker, K.M.; Berend, N.; King, G.G.; Salome, C.M. Obesity is a determinant of asthma control independent of inflammation and lung mechanics. Chest 2011, 140, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Lescher, R.; Strunk, R.; Weiss, S.; Fuhlbrigge, A.; Celedon, J.C. Childhood Asthma Management Program Research Group. Decreased response to inhaled steroids in overweight and obese asthmatic children. J. Allergy Clin. Immunol. 2011, 127, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, nutrients, and dietary patterns: Interconnections and implications for dietary guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Buzina, R.; Suboticanec, K.; Saric, M. Diet patterns and health problems: Diet in southern Europe. Ann. Nutr. Metab. 1991, 35 (Suppl. 1), 32–40. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australia, 2013.
- Health Canada. Eating Well with Canada’s Food Guide—A Resource for Educators and Communicators; Health Canada: Ottawa, ON, Canada, 2011.
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 7 March 2017).
- Public Health England. A Quick Guide to the Government’s Healthy Eating Recommendations; Public Health England: London, UK, 2017.
- Food and Agriculture Organisation of the United Nations. Food-Based Dietary Guidelines. Available online: http://www.fao.org/nutrition/education/food-dietary-guidelines/regions/en/ (accessed on 7 March 2017).
- Micha, R.; Khatibzadeh, S.; Shi, P.; Andrews, K.G.; Engell, R.E.; Mozaffarian, D. Global Burden of Diseases Nutrition; Chronic Diseases Expert Group. Global, regional and national consumption of major food groups in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open 2015, 5, e008705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Very long chain omega-3 (n-3) fatty acids and human health. Eur. J. Lipid Sci. Technol. 2014, 116, 1280–1300. [Google Scholar] [CrossRef]
- National Heart Foundation of Australia. A review of the relationship between dietary fat and cardiovascular disease. Aust. J. Nutr. Diet. 1999, 56, S5–S22. [Google Scholar]
- Carrera-Bastos, P.; Fontes-Villalba, M.; O’Keefe, J.; Lindeberg, S.; Cordain, L. The Western diet and lifestyle and diseases of civilization. Res. Rep. Cin. Cardiol. 2011, 2, 15–35. [Google Scholar] [CrossRef]
- Fu, J.J.; Baines, K.J.; Wood, L.G.; Gibson, P.G. Systemic inflammation is associated with differential gene expression and airway neutrophilia in asthma. OMICS 2013, 17, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Baines, K.J.; Fu, J.; Scott, H.A.; Gibson, P.G. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest 2012, 142, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Ueki, S.; Ito, W.; Chiba, T.; Takeda, M.; Saito, N.; Kayaba, H.; Chihara, J. C-reactive protein levels in the serum of asthmatic patients. Ann. Allergy Asthma Immunol. 2007, 99, 48–53. [Google Scholar] [CrossRef]
- Allam, M.H.; Said, A.F.; El Samie Omran, A.A.; Abd El-Reheim, D.M.; Kasem, A.H. High sensitivity C-reactive protein: Its correlation with sputum cell counts in bronchial asthma. Respir. Med. 2009, 103, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Takemura, M.; Matsumoto, H.; Niimi, A.; Ueda, T.; Matsuoka, H.; Yamaguchi, M.; Jinnai, M.; Muro, S.; Hirai, T.; Ito, Y.; et al. High sensitivity C-reactive protein in asthma. Eur. Respir. J. 2006, 27, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.J.; McDonald, V.M.; Baines, K.J.; Gibson, P.G. Airway IL-1beta and systemic inflammation as predictors of future exacerbation risk in asthma and COPD. Chest 2015, 148, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.C.; McGrath, K.W.; Hawkins, G.A.; Hastie, A.T.; Levy, B.D.; Israel, E.; Phillips, B.R.; Mauger, D.T.; Comhair, S.A.; Erzurum, S.C.; et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: A cross-sectional analysis of two cohorts. Lancet Respir. Med. 2016, 4, 574–584. [Google Scholar] [CrossRef]
- Wood, L.G.; Shivappa, N.; Berthon, B.S.; Gibson, P.G.; Hebert, J.R. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clin. Exp. Allergy 2015, 45, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Gibson, P.G. Dietary factors lead to innate immune activation in asthma. Pharmacol. Ther. 2009, 123, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Grekas, A.; Christou, A.; Chatzigeorgiou, M.; Skoumas, I.; Tousoulis, D.; et al. Adherence to Mediterranean diet and 10-year incidence (2002–2012) of diabetes: Correlations with inflammatory and oxidative stress biomarkers in the ATTICA cohort study. Diabetes Metab. Res. Rev. 2016, 32, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Crespo, A.; Giner, J.; Torrejon, M.; Belda, A.; Mateus, E.; Granel, C.; Torrego, A.; Ramos-Barbon, D.; Plaza, V. Clinical and inflammatory features of asthma with dissociation between fractional exhaled nitric oxide and eosinophils in induced sputum. J. Asthma 2016, 53, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Gibson, P.G. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J. Allergy Clin. Immunol. 2011, 127, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Powell, H.; Gibson, P.G. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: Proof of concept. Free Radic. Res. 2008, 42, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Berthon, B.S.; Macdonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology 2013, 18, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Barraza-Villarreal, A.; Escamilla-Nunez, C.; Texcalac-Sangrador, J.L.; Hernandez-Cadena, L.; Diaz-Sanchez, D.; De Batlle, J.; Del Rio-Navarro, B.E. Dietary intake, lung function and airway inflammation in Mexico City school children exposed to air pollutants. Respir. Res. 2009, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Baines, K.J.; Gibson, P.G.; Wood, L.G. Changes in expression of genes regulating airway inflammation following a high-fat mixed meal in asthmatics. Nutrients 2016, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Pretto, J.J.; Morgan, P.J.; Callister, R.; Wood, L.G. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: A randomized trial. Clin. Exp. Allergy 2013, 43, 36–49. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.; Tan, J.; Macia, L.; Mackay, C.R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 2017, 278, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011, 12, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef] [PubMed]
- McAleer, J.P.; Kolls, J.K. Contributions of the intestinal microbiome in lung immunity. Eur. J. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shi, L.; Pang, W.; Liu, W.; Li, J.; Wang, H.; Shi, G. Dietary fiber intake regulates intestinal microflora and inhibits ovalbumin-induced allergic airway inflammation in a mouse model. PLoS ONE 2016, 11, e0147778. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Denizot, J.; Thevenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The impact of Western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef] [PubMed]
- Dogaru, C.M.; Nyffenegger, D.; Pescatore, A.M.; Spycher, B.D.; Kuehni, C.E. Breastfeeding and childhood asthma: Systematic review and meta-analysis. Am. J. Epidemiol. 2014, 179, 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- Grabenhenrich, L.B.; Gough, H.; Reich, A.; Eckers, N.; Zepp, F.; Nitsche, O.; Forster, J.; Schuster, A.; Schramm, D.; Bauer, C.P.; et al. Early-life determinants of asthma from birth to age 20 years: A German birth cohort study. J. Allergy Clin. Immunol. 2014, 133, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Apostolaki, G.; Bibakis, I.; Skypala, I.; Bibaki-Liakou, V.; Tzanakis, N.; Kogevinas, M.; Cullinan, P. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax 2007, 62, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Garcia-Marcos, L.; Alfonseda Rojas, J.D.; Valverde-Molina, J.; Sanchez-Solis, M. Mediterranean diet as a protective factor for wheezing in preschool children. J. Pediatr. 2008, 152, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini-Belinchón, J.; Lorente-Toledano, F.; Galindo-Villardón, P.; González-Carvajal, I.; Martín-Martín, J.; Mallol, J.; García-Marcos, L. Factors associated to recurrent wheezing in infants under one year of age in the province of Salamanca, Spain: Is intervention possible? A predictive model. Allergol. Immunopathol. (Madr) 2016, 44, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Torrent, M.; Romieu, I.; Garcia-Esteban, R.; Ferrer, C.; Vioque, J.; Kogevinas, M.; Sunyer, J. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax 2008, 63, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Garcia, R.; Roumeliotaki, T.; Basterrechea, M.; Begiristain, H.; Iniguez, C.; Vioque, J.; Kogevinas, M.; Sunyer, J.; group, I.S.; et al. Mediterranean diet adherence during pregnancy and risk of wheeze and eczema in the first year of life: INMA (Spain) and RHEA (Greece) mother-child cohort studies. Br. J. Nutr. 2013, 110, 2058–2068. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Ramirez-Hernandez, M.; Padilla, O.; Pacheco-Gonzalez, R.M.; Perez-Fernandez, V.; Garcia-Marcos, L. Effect of foods and Mediterranean diet during pregnancy and first years of life on wheezing, rhinitis and dermatitis in preschoolers. Allergol. Immunopathol. (Madr) 2016, 44, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marcos, L.; Castro-Rodriguez, J.A.; Weinmayr, G.; Panagiotakos, D.B.; Priftis, K.N.; Nagel, G. Influence of Mediterranean diet on asthma in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2013, 24, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Grigoropoulou, D.; Priftis, K.N.; Yannakoulia, M.; Papadimitriou, A.; Anthracopoulos, M.B.; Yfanti, K.; Panagiotakos, D.B. Urban environment adherence to the Mediterranean diet and prevalence of asthma symptoms among 10-to 12-year-old children: The Physical Activity, Nutrition, and Allergies in Children Examined in Athens study. Allergy Asthma Proc. 2011, 32, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Barcala, F.J.; Pertega, S.; Bamonde, L.; Garnelo, L.; Perez Castro, T.; Sampedro, M.; Sanchez Lastres, J.; San Jose Gonzalez, M.A.; Lopez Silvarrey, A. Mediterranean diet and asthma in Spanish schoolchildren. Pediatr. Allergy Immunol. 2010, 21, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Weinmayr, G.; Kleiner, A.; Garcia-Marcos, L.; Strachan, D.P. ISAAC Phase Two Study Group. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax 2010, 65, 516–522. [Google Scholar] [CrossRef] [PubMed]
- De Batlle, J.; Garcia-Aymerich, J.; Barraza-Villarreal, A.; Antó, J.; Romieu, I. Mediterranean diet is associated with reduced asthma and rhinitis in Mexican children. Allergy 2008, 63, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Uddenfeldt, M.; Janson, C.; Lampa, E.; Leander, M.; Norback, D.; Larsson, L.; Rask-Andersen, A. High BMI is related to higher incidence of asthma, while a fish and fruit diet is related to a lower- Results from a long-term follow-up study of three age groups in Sweden. Respir. Med. 2010, 104, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Bakolis, I.; Hooper, R.; Thompson, R.L.; Shaheen, S.O. Dietary patterns and adult asthma: Population-based case-control study. Allergy 2010, 65, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Hooper, R.; Heinrich, J.; Omenaas, E.; Sausenthaler, S.; Garcia-Larsen, V.; Bakolis, I.; Burney, P. Dietary patterns and risk of asthma: Results from three countries in European Community Respiratory Health Survey-II. Br. J. Nutr. 2010, 103, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Torrent, M.; Romieu, I.; Garcia-Esteban, R.; Ferrer, C.; Vioque, J.; Kogevinas, M.; Sunyer, J. Diet, wheeze, and atopy in school children in Menorca, Spain. Pediatr. Allergy Immunol. 2007, 18, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimon, N.; Fallon, U.; O’Mahony, D.; Loftus, B.; Bury, G.; Murphy, A.; Kelleher, C. Lifeways Cross Generation Cohort Study Steering Group. Mothers’ dietary patterns during pregnancy and risk of asthma symptoms in children at 3 years. Ir. Med. J. 2007, 100, 27–32. [Google Scholar]
- Willers, S.M.; Wijga, A.H.; Brunekreef, B.; Kerkhof, M.; Gerritsen, J.; Hoekstra, M.O.; de Jongste, J.C.; Smit, H.A. Maternal food consumption during pregnancy and the longitudinal development of childhood asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy 2010, 65, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Erkkola, M.; Nwaru, B.I.; Kaila, M.; Kronberg-Kippila, C.; Ilonen, J.; Simell, O.; Veijola, R.; Knip, M.; Virtanen, S.M. Risk of asthma and allergic outcomes in the offspring in relation to maternal food consumption during pregnancy: A Finnish birth cohort study. Pediatr. Allergy Immunol. 2012, 23, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Willers, S.M.; Devereux, G.; Craig, L.C.; McNeill, G.; Wijga, A.H.; Abou El-Magd, W.; Turner, S.W.; Helms, P.J.; Seaton, A. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax 2007, 62, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Seyedrezazadeh, E.; Moghaddam, M.P.; Ansarin, K.; Vafa, M.R.; Sharma, S.; Kolahdooz, F. Fruit and vegetable intake and risk of wheezing and asthma: A systematic review and meta-analysis. Nutr. Rev. 2014, 72, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Willers, S.M.; Wijga, A.H.; Brunekreef, B.; Scholtens, S.; Postma, D.S.; Kerkhof, M.; de Jongste, J.C.; Smit, H.A. Childhood diet and asthma and atopy at 8 years of age: The PIAMA birth cohort study. Eur. Respir. J. 2011, 37, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; Berthon, B.S.; Wark, P.; Wood, L.G. Effects of fruit and vegetable consumption on risk of asthma, wheezing and immune responses: A systematic review and meta-analysis. Nutrients 2017, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Panagiotakos, D.B.; Hatziagorou, E.; Antonogeorgos, G.; Matziou, V.N.; Tsanakas, J.N.; Gratziou, C.; Tsabouri, S.; Priftis, K.N. Antioxidant foods consumption and childhood asthma and other allergic diseases: The Greek cohorts of the ISAAC II survey. Allergol. Immunopathol. (Madr) 2015, 43, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Butland, B.K.; Strachan, D.P.; Anderson, H.R. Fresh fruit intake and asthma symptoms in young British adults: Confounding or effect modification by smoking? Eur. Respir. J. 1999, 13, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [PubMed]
- Romieu, I.; Varraso, R.; Avenel, V.; Leynaert, B.; Kauffmann, F.; Clavel-Chapelon, F. Fruit and vegetable intakes and asthma in the E3N study. Thorax 2006, 61, 209–215. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Decarli, A.; Pagano, R. Vegetable consumption and risk of chronic disease. Epidemiology 1998, 9, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Lin, K.C.; Pan, W.H. Dietary factors associated with physician-diagnosed asthma and allergic rhinitis in teenagers: Analyses of the first Nutrition and Health Survey in Taiwan. Clin. Exp. Allergy 2001, 31, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, O.; Aralis, H.; Flores, M.; Ritz, B. Fast food consumption in pregnancy and subsequent asthma symptoms in young children. Pediatr. Allergy Immunol. 2015, 26, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, I.; Stewart, A.W.; Hancox, R.J.; Beasley, R.; Murphy, R.; Mitchell, E.A. The ISAAC Phase Three Study Group. Fast-food consumption and body mass index in children and adolescents: An international cross-sectional study. BMJ Open 2014, 4, e005813. [Google Scholar] [CrossRef] [PubMed]
- Mai, X.M.; Becker, A.B.; Liem, J.J.; Kozyrskyj, A.L. Fast food consumption counters the protective effect of breastfeeding on asthma in children? Clin. Exp. Allergy 2009, 39, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Brigham, E.P.; Kolahdooz, F.; Hansel, N.; Breysse, P.N.; Davis, M.; Sharma, S.; Matsui, E.C.; Diette, G.; McCormack, M.C. Association between Western diet pattern and adult asthma: A focused review. Ann. Allergy Asthma Immunol. 2015, 114, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.E.; Rifas-Shiman, S.L.; Camargo, C.A.; Gold, D.R.; Gillman, M.W.; Litonjua, A.A. Maternal dietary pattern during pregnancy is not associated with recurrent wheeze in children. J. Allergy Clin. Immunol. 2010, 126, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Okubo, H.; Sasaki, S.; Tanaka, K.; Hirota, Y. Maternal dietary patterns during pregnancy and risk of wheeze and eczema in Japanese infants aged 16–24 months: The Osaka Maternal and Child Health Study. Pediatr. Allergy Immunol. 2011, 22, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Northstone, K.; Newson, R.B.; Emmett, P.M.; Sherriff, A.; Henderson, A.J. Dietary patterns in pregnancy and respiratory and atopic outcomes in childhood. Thorax 2009, 64, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Custovic, A.; Smith, J.A.; Simpson, A.; Kerry, G.; Murray, C.S. Cross-sectional association of dietary patterns with asthma and atopic sensitization in childhood—In a cohort study. Pediatr. Allergy Immunol. 2014, 25, 565–571. [Google Scholar] [PubMed]
- Tromp, I.I.; Kiefte-de Jong, J.C.; de Vries, J.H.; Jaddoe, V.W.; Raat, H.; Hofman, A.; de Jongste, J.C.; Moll, H.A. Dietary patterns and respiratory symptoms in pre-school children: The Generation R Study. Eur. Respir. J. 2012, 40, 681–689. [Google Scholar] [CrossRef] [PubMed]
- DeChristopher, L.R.; Uribarri, J.; Tucker, K.L. Intakes of apple juice, fruit drinks and soda are associated with prevalent asthma in US children aged 2–9 years. Public Health Nutr. 2016, 19, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Berentzen, N.E.; van Stokkom, V.L.; Gehring, U.; Koppelman, G.H.; Schaap, L.A.; Smit, H.A.; Wijga, A.H. Associations of sugar-containing beverages with asthma prevalence in 11-year-old children: The PIAMA birth cohort. Eur. J. Clin. Nutr. 2015, 69, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Dal Grande, E.; Taylor, A.W.; Gill, T.K.; Adams, R.; Wittert, G.A. Association between soft drink consumption and asthma and chronic obstructive pulmonary disease among adults in Australia. Respirology 2012, 17, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Blanck, H.M.; Sherry, B.; Jones, S.E.; Pan, L. Regular-soda intake independent of weight status is associated with asthma among US high school students. J. Acad. Nutr. Diet. 2013, 113, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Akinbami, L.J.; McGuire, L.C.; Blanck, H.M. Association of sugar-sweetened beverage intake frequency and asthma among U.S. adults, 2013. Prev. Med. 2016, 91, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Beckhaus, A.A.; Garcia-Marcos, L.; Forno, E.; Pacheco-Gonzalez, R.M.; Celedon, J.C.; Castro-Rodriguez, J.A. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: A systematic review and meta-analysis. Allergy 2015, 70, 1588–1604. [Google Scholar] [CrossRef] [PubMed]
- Pele, F.; Bajeux, E.; Gendron, H.; Monfort, C.; Rouget, F.; Multigner, L.; Viel, J.F.; Cordier, S. Maternal fish and shellfish consumption and wheeze, eczema and food allergy at age two: A prospective cohort study in Brittany, France. Environ. Health-Glob. 2013, 12, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leermakers, E.T.; Sonnenschein-van der Voort, A.M.; Heppe, D.H.; de Jongste, J.C.; Moll, H.A.; Franco, O.H.; Hofman, A.; Jaddoe, V.W.; Duijts, L. Maternal fish consumption during pregnancy and risks of wheezing and eczema in childhood: The Generation R Study. Eur. J. Clin. Nutr. 2013, 67, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Maslova, E.; Strom, M.; Oken, E.; Campos, H.; Lange, C.; Gold, D.; Olsen, S.F. Fish intake during pregnancy and the risk of child asthma and allergic rhinitis—Longitudinal evidence from the Danish National Birth Cohort. Br. J. Nutr. 2013, 110, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Øien, T.; Storrø, O.; Johnsen, R. Do early intake of fish and fish oil protect against eczema and doctor-diagnosed asthma at 2 years of age? A cohort study. J. Epidemiol. Community Health 2010, 64, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Torrent, M.; Garcia-Esteban, R.; Ferrer, C.; Ribas-Fito, N.; Anto, J.M.; Sunyer, J. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin. Exp. Allergy 2007, 37, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.T.; Li, Y.F.; Langholz, B.; Gilliland, F.D. Maternal fish consumption during pregnancy and risk of early childhood asthma. J. Asthma 2005, 42, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Liu, B.; Li, J.; Luo, C.Q.; Zhang, Q.; Chen, J.L.; Sinha, A.; Li, Z.Y. Fish intake during pregnancy or infancy and allergic outcomes in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2017, 28, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Jarvinen, K.M.; Sicherer, S.H. Fish consumption during the first year of life and development of allergic diseases during childhood. Pediatrics 2007, 120, S109. [Google Scholar] [CrossRef]
- Nafstad, P.; Nystad, W.; Magnus, P.; Jaakkola, J.J.K. Asthma and Allergic Rhinitis at 4 Years of Age in Relation to Fish Consumption in Infancy. J. Asthma 2003, 40, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Lumia, M.; Takkinen, H.M.; Luukkainen, P.; Kaila, M.; Lehtinen-Jacks, S.; Nwaru, B.I.; Tuokkola, J.; Niemela, O.; Haapala, A.M.; Ilonen, J.; et al. Food consumption and risk of childhood asthma. Pediatr. Allergy Immunol. 2015, 26, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Goksor, E.; Alm, B.; Thengilsdottir, H.; Pettersson, R.; Aberg, N.; Wennergren, G. Preschool wheeze - impact of early fish introduction and neonatal antibiotics. Acta Paediatr. 2011, 100, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xun, P.; He, K. Fish and fish oil intake in relation to risk of asthma: A systematic review and meta-analysis. PLoS ONE 2013, 8, e80048. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Erkkola, M.; Ahonen, S.; Kaila, M.; Kronberg-Kippila, C.; Ilonen, J.; Simell, O.; Knip, M.; Veijola, R.; Virtanen, S.M. Intake of antioxidants during pregnancy and the risk of allergies and asthma in the offspring. Eur. J. Clin. Nutr. 2011, 65, 937–943. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Dunstan, J.; McCarthy, S.; Metcalfe, J.; D’Vaz, N.; Meldrum, S.; Oddy, W.H.; Tulic, M.K.; Prescott, S.L. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients 2012, 4, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Maslova, E.; Hansen, S.; Strom, M.; Halldorsson, T.I.; Olsen, S.F. Maternal intake of vitamins A, E and K in pregnancy and child allergic disease: A longitudinal study from the Danish National Birth Cohort. Br. J. Nutr. 2014, 111, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Rerksuppaphol, S.; Rerksuppaphol, L. Carotenoids intake and asthma prevalence in Thai children. Pediatr. Rep. 2012, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Checkley, W.; West, K.P., Jr.; Wise, R.A.; Wu, L.; LeClerq, S.C.; Khatry, S.; Katz, J.; Christian, P.; Tielsch, J.M.; Sommer, A. Supplementation with vitamin A early in life and subsequent risk of asthma. Eur. Respir. J. 2011, 38, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Maternal B vitamin intake during pregnancy and wheeze and eczema in Japanese infants aged 16–24 months: The Osaka Maternal and Child Health Study. Pediatr. Allergy Immunol. 2011, 22, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Crider, K.S.; Cordero, A.M.; Qi, Y.P.; Mulinare, J.; Dowling, N.F.; Berry, R.J. Prenatal folic acid and risk of asthma in children: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Martindale, S.; McNeill, G.; Devereux, G.; Campbell, D.; Russell, G.; Seaton, A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am. J. Respir. Crit. Care Med. 2005, 171, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Litonjua, A.A.; Rifas-Shiman, S.L.; Ly, N.P.; Tantisira, K.G.; Rich-Edwards, J.W.; Camargo, C.A.; Weiss, S.T.; Gillman, M.W.; Gold, D.R. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 years of age. Am. J. Clin. Nutr. 2006, 84, 903–911. [Google Scholar] [PubMed]
- Vahdaninia, M.; Mackenzie, H.; Helps, S.; Dean, T. Prenatal intake of vitamins and allergic outcomes in the offspring: A systematic review and meta-analysis. J. Allergy Clin. Immunol. Pract. 2017, 5, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Erkkola, M.; Kaila, M.; Nwaru, B.; Kronberg-Kippilä, C.; Ahonen, S.; Nevalainen, J.; Veijola, R.; Pekkanen, J.; Ilonen, J.; Simell, O. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin. Exp. Allergy 2009, 39, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur. Respir. J. 2010, 35, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Chen, Y.; Omand, J.; Birken, C.; Parkin, P.; To, T.; Maguire, J. Vitamin D exposure during pregnancy, but not early childhood, is associated with risk of childhood wheezing. J. Dev. Orig. Health Dis. 2015, 6, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Devereux, G.; Turner, S.W.; Craig, L.C.; McNeill, G.; Martindale, S.; Harbour, P.J.; Helms, P.J.; Seaton, A. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am. J. Respir. Crit. Care Med. 2006, 174, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Larkin, E.K.; Gao, Y.T.; Gebretsadik, T.; Hartman, T.J.; Wu, P.; Wen, W.; Yang, G.; Bai, C.; Jin, M.; Roberts, L.J., 2nd; et al. New risk factors for adult-onset incident asthma. A nested case-control study of host antioxidant defense. Am. J. Respir. Crit. Care Med. 2015, 191, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.J.; Willett, W.C.; Weiss, S.T.; Trichopoulos, D.; Rosner, B.; Speizer, F.E. A prospective study of diet and adult-onset asthma. Am. J. Respir. Crit. Care Med. 1995, 151, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.F.; Osterdal, M.L.; Salvig, J.D.; Mortensen, L.M.; Rytter, D.; Secher, N.J.; Henriksen, T.B. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am. J. Clin. Nutr. 2008, 88, 167–175. [Google Scholar] [PubMed]
- Hansen, S.; Strom, M.; Maslova, E.; Dahl, R.; Hoffmann, H.J.; Rytter, D.; Bech, B.H.; Henriksen, T.B.; Granstrom, C.; Halldorsson, T.I.; et al. Fish oil supplementation during pregnancy and allergic respiratory disease in the adult offspring. J. Allergy Clin. Immunol. 2017, 139, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, H.; Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadottir, E.; Schoos, A.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdottir, S.; et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N. Engl. J. Med. 2016, 375, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Marks, G.B.; Mihrshahi, S.; Kemp, A.S.; Tovey, E.R.; Webb, K.; Almqvist, C.; Ampon, R.D.; Crisafulli, D.; Belousova, E.G.; Mellis, C.M.; et al. Prevention of asthma during the first 5 years of life: A randomized controlled trial. J. Allergy Clin. Immunol. 2006, 118, 53–61. [Google Scholar] [CrossRef] [PubMed]
- D’Vaz, N.; Meldrum, S.J.; Dunstan, J.A.; Martino, D.; McCarthy, S.; Metcalfe, J.; Tulic, M.K.; Mori, T.A.; Prescott, S.L. Postnatal fish oil supplementation in high-risk infants to prevent allergy: Randomized controlled trial. Pediatrics 2012, 130, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Schindler, T.; Sinn, J.K.; Osborn, D.A. Polyunsaturated fatty acid supplementation in infancy for the prevention of allergy. Cochrane Database Syst Rev. 2016, 10, CD010112. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Linseisen, J. Dietary intake of fatty acids, antioxidants and selected food groups and asthma in adults. Eur. J. Clin. Nutr. 2005, 59, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xun, P.; Zamora, D.; Sood, A.; Liu, K.; Daviglus, M.; Iribarren, C.; Jacobs, D.; Shikany, J.M.; He, K. Intakes of long-chain omega-3 (n-3) PUFAs and fish in relation to incidence of asthma among American young adults: The CARDIA study. Am. J. Clin. Nutr. 2013, 97, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Saglani, S.; Bush, A. The early-life origins of asthma. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Prenatal factors and the development of asthma. Curr. Opin. Pediatr. 2008, 20, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Young, O.M.; Kumar, R.; Simhan, H.; Celedon, J.C. Maternal obesity in pregnancy, gestational weight gain, and risk of childhood asthma. Pediatrics 2014, 134, e535–e546. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.C.; Elsas, P.X.; Maximiano, E.S.; Elsas, M.I. Impact of diet on the immunological microenvironment of the pregnant uterus and its relationship to allergic disease in the offspring—A review of the recent literature. Sao Paulo Med. J. 2006, 124, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Symonds, M.E.; Sebert, S.P.; Budge, H. The impact of diet during early life and its contribution to later disease: Critical checkpoints in development and their long-term consequences for metabolic health. Proc. Nutr. Soc. 2009, 68, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Sewell, D.A.; Hammersley, V.S.; Devereux, G.; Robertson, A.; Stoddart, A.; Weir, C.; Worth, A.; Sheikh, A. Investigating the effectiveness of the Mediterranean diet in pregnant women for the primary prevention of asthma and allergy in high-risk infants: Protocol for a pilot randomised controlled trial. Trials 2013, 14, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Guideline: Vitamin D Supplementation in Pregnant Women; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Emmanouil, E.; Manios, Y.; Grammatikaki, E.; Kondaki, K.; Oikonomou, E.; Papadopoulos, N.; Vassilopoulou, E. Association of nutrient intake and wheeze or asthma in a Greek pre-school population. Pediatr. Allergy Immunol. 2010, 21, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, F.; Pistelli, R.; Sestini, P.; Fortes, C.; Renzoni, E.; Rusconi, F.; Dell'Orco, V.; Ciccone, G.; Bisanti, L. Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. SIDRIA Collaborative Group, Italy (Italian Studies on Respiratory Disorders in Children and the Environment). Thorax 2000, 55, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kesse-Guyot, E.; Dumas, O.; Garcia-Aymerich, J.; Leynaert, B.; Pison, C.; Le Moual, N.; Romieu, I.; Siroux, V.; Camargo, C.A.; et al. Longitudinal study of diet quality and change in asthma symptoms in adults, according to smoking status. Br. J. Nutr. 2017, 117, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.C.; Tunnicliffe, W.S.; Duncanson, R.C.; Ayres, J.G. Dietary antioxidants and magnesium in type 1 brittle asthma: A case control study. Thorax 1999, 54, 115–118. [Google Scholar] [CrossRef] [PubMed]
- De Luis, D.A.; Armentia, A.; Aller, R.; Asensio, A.; Sedano, E.; Izaola, O.; Cuellar, L. Dietary intake in patients with asthma: A case control study. Nutrition 2005, 21, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Riccioni, G.; Bucciarelli, T.; Mancini, B.; Di Ilio, C.; Della Vecchia, R.; D’Orazio, N. Plasma lycopene and antioxidant vitamins in asthma: The PLAVA study. J. Asthma 2007, 44, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention 2012 (Update). Available online: http://wwwginasthmaorg (accessed on 30 July 2017).
- Grieger, J.A.; Grzeskowiak, L.E.; Wood, L.G.; Clifton, V.L. Asthma control in pregnancy is associated with pre-conception dietary patterns. Public Health Nutr. 2016, 19, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Calatayud-Saez, F.M.; Calatayud Moscoso Del Prado, B.; Gallego Fernandez-Pacheco, J.G.; Gonzalez-Martin, C.; Alguacil Merino, L.F. Mediterranean diet and childhood asthma. Allergol. Immunopathol. (Madr) 2016, 44, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Antonogeorgos, G.; Panagiotakos, D.B.; Grigoropoulou, D.; Yfanti, K.; Papoutsakis, C.; Papadimitriou, A.; Anthracopoulos, M.B.; Bakoula, C.; Priftis, K.N. Investigating the associations between Mediterranean diet, physical activity and living environment with childhood asthma using path analysis. Endocr. Metab. Immune Disord Drug Targets 2014, 14, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, A.W.; Antoniak, M.; Venn, A.J.; Davies, L.; Goodwin, A.; Salfield, N.; Britton, J.R.; Lewis, S.A. A natural experiment on the impact of fruit supplementation on asthma symptoms in children. Eur. Respir. J. 2009, 33, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, P.; Asher, M.I.; García-Marcos, L.; Williams, H.; Keil, U.; Robertson, C.; Nagel, G.; Aït-Khaled, N.; Anderson, H.; Asher, M. Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Thorax 2013. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Yang, Y.H.; Chuang, S.Y.; Huang, S.Y.; Pan, W.H. Reduced medication use and improved pulmonary function with supplements containing vegetable and fruit concentrate, fish oil and probiotics in asthmatic school children: A randomised controlled trial. Br. J. Nutr. 2013, 110, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marcos, L.; Canflanca, I.M.; Garrido, J.B.; Varela, A.L.; Garcia-Hernandez, G.; Guillen Grima, F.; Gonzalez-Diaz, C.; Carvajal-Uruena, I.; Arnedo-Pena, A.; Busquets-Monge, R.M.; et al. Relationship of asthma and rhinoconjunctivitis with obesity, exercise and Mediterranean diet in Spanish schoolchildren. Thorax 2007, 62, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, F.; Priftis, K.N.; Papadimitriou, A.; Yiallouros, P.; Kapsokefalou, M.; Anthracopoulos, M.B.; Panagiotakos, D.B. Salty-snack eating, television or video-game viewing, and asthma symptoms among 10-to 12-year-old children: The PANACEA study. J. Am. Diet. Assoc. 2011, 111, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mihrshahi, S.; Peat, J.K.; Webb, K.; Oddy, W.; Marks, G.B.; Mellis, C.M. Effect of omega-3 fatty acid concentrations in plasma on symptoms of asthma at 18 months of age. Pediatr. Allergy Immunol. 2004, 15, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Thien, F.C.; De Luca, S.; Woods, R.K.; Abramson, M.J. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Libr. 2002, 3, CD001283. [Google Scholar]
- Peat, J.K.; Mihrshahi, S.; Kemp, A.S.; Marks, G.B.; Tovey, E.R.; Webb, K.; Mellis, C.M.; Leeder, S.R. Three-year outcomes of dietary fatty acid modification and house dust mite reduction in the Childhood Asthma Prevention Study. J. Allergy Clin. Immunol. 2004, 114, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Covar, R.; Gleason, M.; Macomber, B.; Stewart, L.; Szefler, P.; Engelhardt, K.; Murphy, J.; Liu, A.; Wood, S.; DeMichele, S.; et al. Impact of a novel nutritional formula on asthma control and biomarkers of allergic airway inflammation in children. Clin. Exp. Allergy 2010, 40, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Blatter, J.; Brehm, J.M.; Sordillo, J.; Forno, E.; Boutaoui, N.; Acosta-Perez, E.; Alvarez, M.; Colon-Semidey, A.; Weiss, S.T.; Litonjua, A.A.; et al. Folate deficiency, atopy, and severe asthma exacerbations in puerto rican children. Ann. Am. Thorac. Soc. 2016, 13, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Blatter, J.; Han, Y.Y.; Forno, E.; Brehm, J.; Bodnar, L.; Celedon, J.C. Folate and asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Hemila, H.; Al-Biltagi, M.; Baset, A.A. Vitamin C and asthma in children: Modification of the effect by age, exposure to dampness and the severity of asthma. Clin. Transl. Allergy 2011, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Mittal, K. Effect of vitamin D supplementation on moderate to severe bronchial asthma. Indian J. Pediatr. 2014, 81, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Kerley, C.P.; Hutchinson, K.; Cormican, L.; Faul, J.; Greally, P.; Coghlan, D.; Elnazir, B. Vitamin D3 for uncontrolled childhood asthma: A pilot study. Pediatr. Allergy Immunol. 2016, 27, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.E.; Mailhot, G.; Alos, N.; Rousseau, E.; White, J.H.; Khamessan, A.; Ducharme, F.M. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): A pilot randomised controlled trial. Trials 2016, 17, 353. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Moreira, A.; Fonseca, J.; de Oliveira, J.F.; Delgado, L.; Castel-Branco, M.G.; Haahtela, T.; Lopes, C.; Moreira, P. Adherence to the Mediterranean diet and fresh fruit intake are associated with improved asthma control. Allergy 2008, 63, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Sexton, P.; Black, P.; Metcalf, P.; Wall, C.R.; Ley, S.; Wu, L.; Sommerville, F.; Brodie, S.; Kolbe, J. Influence of mediterranean diet on asthma symptoms, lung function, and systemic inflammation: A randomized controlled trial. J. Asthma 2013, 50, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Iikura, M.; Yi, S.; Ichimura, Y.; Hori, A.; Izumi, S.; Sugiyama, H.; Kudo, K.; Mizoue, T.; Kobayashi, N. Effect of lifestyle on asthma control in Japanese patients: Importance of periodical exercise and raw vegetable diet. PLoS ONE 2013, 8, e68290. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.D.; Welch, A.A.; Bingham, S.A.; Luben, R.N.; Day, N.E.; Khaw, K.T.; Lomas, D.A.; Wareham, N.J. Dietary antioxidants and asthma in adults. Thorax 2006, 61, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Smart, J.M.; Scott, H.A.; Barker, D.; Gibson, P.G. Manipulating antioxidant intake in asthma: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Thuesen, B.; Husemoen, L.; Ovesen, L.; Jørgensen, T.; Fenger, M.; Gilderson, G.; Linneberg, A. Atopy, asthma, and lung function in relation to folate and vitamin B12 in adults. Allergy 2010, 65, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, A.; Lewis, S.A.; Scrivener, S.L.; Antoniak, M.; Pacey, S.; Pringle, M.; Britton, J. Oral magnesium and vitamin C supplements in asthma: A parallel group randomized placebo-controlled trial. Clin. Exp. Allergy 2003, 33, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Pearson, P.J.; Lewis, S.A.; Britton, J.; Fogarty, A. Vitamin E supplements in asthma: A parallel group randomised placebo controlled trial. Thorax 2004, 59, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; King, T.S.; Kunselman, S.J.; Cabana, M.D.; Denlinger, L.; Holguin, F.; Kazani, S.D.; Moore, W.C.; Moy, J.; Sorkness, C.A.; et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: The VIDA randomized clinical trial. JAMA 2014, 311, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Surette, M.E.; Stull, D.; Lindemann, J. The impact of a medical food containing gammalinolenic and eicosapentaenoic acids on asthma management and the quality of life of adult asthma patients. Curr. Med. Res. Opin. 2008, 24, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, J.; David Pampe, E.; Peterkin, J.J.; Orozco-Cronin, P.; Belofsky, G.; Stull, D. Clinical study of the effects on asthma-related QOL and asthma management of a medical food in adult asthma patients. Curr. Med. Res. Opin. 2009, 25, 2865–2875. [Google Scholar] [CrossRef] [PubMed]
- Varraso, R.; Kauffmann, F.; Leynaert, B.; Le Moual, N.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Romieu, I. Dietary patterns and asthma in the E3N study. Eur. Respir. J. 2009, 33, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rava, M.; Bédard, A.; Dumas, O.; Garcia-Aymerich, J.; Leynaert, B.; Pison, C.; Le Moual, N.; Romieu, I.; Siroux, V. Cured meat intake is associated with worsening asthma symptoms. Thorax 2017. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, M.; Norback, D. Diet among Japanese female university students and asthmatic symptoms, infections, pollen and furry pet allergy. Respir. Med. 2008, 102, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Waidyatillake, N.T.; Allen, K.J.; Lodge, C.J.; Dharmage, S.C.; Abramson, M.J.; Simpson, J.A.; Lowe, A.J. The impact of breastfeeding on lung development and function: A systematic review. Expert Rev. Clin. Immunol. 2013, 9, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Buchele, G.; Weinmayr, G.; Bjorksten, B.; Chen, Y.Z.; Wang, H.; Nystad, W.; Saraclar, Y.; Braback, L.; Batlles-Garrido, J.; et al. Effect of breastfeeding on asthma, lung function and bronchial hyperreactivity in ISAAC Phase II. Eur. Respir. J. 2009, 33, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Elliott, L.; Henderson, J.; Northstone, K.; Chiu, G.Y.; Dunson, D.; London, S.J. Prospective study of breast-feeding in relation to wheeze, atopy, and bronchial hyperresponsiveness in the Avon Longitudinal Study of Parents and Children (ALSPAC). J. Allergy Clin. Immunol. 2008, 122, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, Y.H.; Kim, M.J.; Lee, H.S.; Han, Y.K.; Kim, K.W.; Sohn, M.H.; Kim, K.-E. Effect of breastfeeding on lung function in asthmatic children. Allergy Asthma Proc. 2015, 36, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.L.; Romero, K.M.; Davila, R.M.G.; Meza, C.T.; Bilderback, A.; D’Ann, L.W.; Breysse, P.N.; Bose, S.; Checkley, W.; Hansel, N.N. Association between adherence to the Mediterranean diet and asthma in Peruvian children. Lung 2015, 193, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Milan, S.J.; Hart, A.; Wilkinson, M. Vitamin C for asthma and exercise-induced bronchoconstriction. Cochrane Database Syst. Rev. 2013, CD010391. [Google Scholar] [CrossRef]
- Han, Y.Y.; Forno, E.; Celedon, J.C. Vitamin D insufficiency and asthma in a US nationwide study. J. Allergy Clin. Immunol. Pract. 2017, 5, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, D.; Liu, C. Can vitamin D supplementation in addition to asthma controllers decrease asthmatic exacerbations in patients with asthma? A meta-analysis. Chest 2016, 149, A12. [Google Scholar] [CrossRef]
- Riverin, B.D.; Maguire, J.L.; Li, P. Vitamin D supplementation for childhood asthma: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0136841. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, J.; Farid Hossiani, R.; Khalilian, A.; Nahanmoghadam, N.; Salehifar, E.; Rafatpanah, H. Vitamin E supplementation, lung functions and clinical manifestations in children with moderate asthma: A randomized double blind placebo- controlled trial. Iran. J. Allergy Asthma Immunol. 2014, 13, 98–103. [Google Scholar] [PubMed]
- Willemsen, L.E.M. Dietary n-3 long chain polyunsaturated fatty acids in allergy prevention and asthma treatment. Eur. J. Pharmacol. 2016, 785, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Holbrook, J.T.; Wise, R.; Blumenthal, M.; Dozor, A.J.; Mastronarde, J.; Williams, L. American Lung Association Asthma Clinical Research Centers. Dietary intake of soy genistein is associated with lung function in patients with asthma. J. Asthma 2004, 41, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Goldsobel, A. Effect of a soy isoflavone supplement on lung function and clinical outcomes in patients with poorly controlled asthma: A randomized clinical trial. Pediatrics 2015, 136, S270–S271. [Google Scholar] [CrossRef]
- Gao, J.; Gao, X.; Li, W.; Zhu, Y.; Thompson, P.J. Observational studies on the effect of dietary antioxidants on asthma: A meta-analysis. Respirology 2008, 13, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Thuesen, B.H.; Skaaby, T.; Husemoen, L.L.; Fenger, M.; Jorgensen, T.; Linneberg, A. The association of serum 25-OH vitamin D with atopy, asthma, and lung function in a prospective study of Danish adults. Clin. Exp. Allergy 2015, 45, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Peters, J.I.; McKinney, J.M.; Smith, B.; Wood, P.; Forkner, E.; Galbreath, A.D. Impact of obesity in asthma: Evidence from a large prospective disease management study. Ann. Allergy Asthma Immunol. 2011, 106, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.E.; Gibson, P.G.; Collins, C.E.; Wood, L.G. Airway and systemic inflammation in obese children with asthma. Eur. Respir. J. 2013, 42, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Celedon, J.C. The effect of obesity, weight gain, and weight loss on asthma inception and control. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Jessri, M.; Wolfinger, R.D.; Lou, W.Y.; L’Abbe, M.R. Identification of dietary patterns associated with obesity in a nationally representative survey of Canadian adults: Application of a priori, hybrid, and simplified dietary pattern techniques. Am. J. Clin. Nutr. 2017, 105, 669–684. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Strub, P.; Xiao, L.; Lavori, P.W.; Camargo, C.A., Jr.; Wilson, S.R.; Gardner, C.D.; Buist, A.S.; Haskell, W.L.; Lv, N. Behavioral weight loss and physical activity intervention in obese adults with asthma. A randomized trial. Ann. Am. Thorac. Soc. 2015, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dias-Junior, S.A.; Reis, M.; de Carvalho-Pinto, R.M.; Stelmach, R.; Halpern, A.; Cukier, A. Effects of weight loss on asthma control in obese patients with severe asthma. Eur. Respir. J. 2014, 43, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Luna-Pech, J.A.; Torres-Mendoza, B.M.; Luna-Pech, J.A.; Garcia-Cobas, C.Y.; Navarrete-Navarro, S.; Elizalde-Lozano, A.M. Normocaloric diet improves asthma-related quality of life in obese pubertal adolescents. Int. Arch. Allergy Immunol. 2014, 163, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.E.; Gibson, P.G.; Collins, C.E.; Hilton, J.M.; Wood, L.G. Diet-induced weight loss in obese children with asthma: A randomized controlled trial. Clin. Exp. Allergy 2013, 43, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Stenius-Aarniala, B.; Poussa, T.; Kvarnstrom, J.; Gronlund, E.L.; Ylikahri, M.; Mustajoki, P. Immediate and long term effects of weight reduction in obese people with asthma: Randomised controlled study. BMJ 2000, 320, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Rifai, L.; Silver, M.A. A review of the DASH diet as an optimal dietary plan for symptomatic heart failure. Prog. Cardiovasc. Dis. 2016, 58, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Blonstein, A.C.; Lv, N.; Camargo, C.A.; Wilson, S.R.; Buist, A.S.; Rosas, L.G.; Strub, P.; Ma, J. Acceptability and feasibility of the ‘DASH for Asthma’ intervention in a randomized controlled trial pilot study. Public Health Nutr. 2016, 19, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Strub, P.; Lv, N.; Xiao, L.; Camargo, C.A., Jr.; Buist, A.S.; Lavori, P.W.; Wilson, S.R.; Nadeau, K.C.; Rosas, L.G. Pilot randomised trial of a healthy eating behavioural intervention in uncontrolled asthma. Eur. Respir. J. 2016, 47, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Kouba, J.; Velsor-Friedrich, B.; Militello, L.; Harrison, P.R.; Becklenberg, A.; White, B.; Surya, S.; Ahmed, A. Efficacy of the I Can Control Asthma and Nutrition Now (ICAN) pilot program on health outcomes in high school students with asthma. J. Sch. Nurs. 2013, 29, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Prelip, M.; Slusser, W.; Thai, C.L.; Kinsler, J.; Erausquin, J.T. Effects of a school-based nutrition program diffused throughout a large urban community on attitudes, beliefs, and behaviors related to fruit and vegetable consumption. J. Sch. Health 2011, 81, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Larsen, V.; Del Giacco, S.R.; Moreira, A.; Bonini, M.; Charles, D.; Reeves, T.; Carlsen, K.H.; Haahtela, T.; Bonini, S.; Fonseca, J.; et al. Asthma and dietary intake: An overview of systematic reviews. Allergy 2016, 71, 433–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 Beneficial effect;
Beneficial effect;  negative effect;
negative effect;  No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of randomized controlled trials (RCTs); Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on asthma risk. * One Meta-analysis found a negative association with asthma or wheeze and one found no association; ** conflicting results in cross-sectional studies; *** from diet but not from supplementation.
No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of randomized controlled trials (RCTs); Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on asthma risk. * One Meta-analysis found a negative association with asthma or wheeze and one found no association; ** conflicting results in cross-sectional studies; *** from diet but not from supplementation.
 Beneficial effect;
Beneficial effect;  negative effect;
negative effect;  No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of randomized controlled trials (RCTs); Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on asthma risk. * One Meta-analysis found a negative association with asthma or wheeze and one found no association; ** conflicting results in cross-sectional studies; *** from diet but not from supplementation.
No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of randomized controlled trials (RCTs); Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on asthma risk. * One Meta-analysis found a negative association with asthma or wheeze and one found no association; ** conflicting results in cross-sectional studies; *** from diet but not from supplementation.| Diet | Diet During Life Stages | |||||
|---|---|---|---|---|---|---|
| Pregnancy | Childhood | Adulthood | ||||
| Effect | Evidence | Effect | Evidence | Effect | Evidence | |
| Post-natal breast feeding |  [56] [56] | Very strong |  [57] [57] | Low | ||
| Mediterranean diet |  [58,59,60,61,62,63] [58,59,60,61,62,63] | Low |  [59,63,64,65,66,67,68] [59,63,64,65,66,67,68] | Low |  [69,70,71] [69,70,71] | Low |
| Fruit |  [58,61,63,72,73,74,75,76,77] [58,61,63,72,73,74,75,76,77] | Low |  [78,79,80,81] [78,79,80,81] | Low |  [69,82,83] [69,82,83] | Low |
| Vegetables |  [58,61,63,72,73,74,75,76,77] [58,61,63,72,73,74,75,76,77] | Low |  [78,79,80,81] [78,79,80,81] | Low |  ** [70,83,84,85] ** [70,83,84,85] | Very low |
| Fast food |  [60,63,86,87] [60,63,86,87] | Low |  [63,88,89] [63,88,89] | Low |  [90] [90] | Low |
| “Western” diet |  [91,92,93] [91,92,93] | Low |  [88,94,95,96,97] [88,94,95,96,97] | Very low |  [90,98,99,100] [90,98,99,100] | Low |
| Meat |  [62,101] [62,101] | Low |  [63] [63] | Low |  [90] [90] | Low |
| Fish |  [74,77,92,102,103,104,105,106,107,108] [74,77,92,102,103,104,105,106,107,108] | Low |  * [95,105,108,109,110,111,112,113] * [95,105,108,109,110,111,112,113] | Low |  [69,70,71] [69,70,71] | Low |
| Vitamin A |  [114,115,116] [114,115,116] | Low |  [117,118] [117,118] | Low | ? | ? |
| Vitamin B |  [114,119,120] [114,119,120] | Low | ? | ? | ? | ? |
| Vitamin C |  [75,114,115,121,122] [75,114,115,121,122] | Low | ? | ? | ? | ? |
| Vitamin D |  [101,123,124,125] [101,123,124,125] | Very Strong |  [126] [126] | Low | ? | ? |
| Vitamin E |  [75,101,115,121,122,127] [75,101,115,121,122,127] | Low | ? | ? |  *** [128,129] *** [128,129] | Low |
| LC n-3 PUFA (Fish oil) |  [130,131,132] [130,131,132] | Strong |  [133,134,135] [133,134,135] | Very Strong |  [113,136,137] [113,136,137] | Low |
 Beneficial effect;
Beneficial effect;  negative effect;
negative effect;  No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of RCTs; Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on asthma control.
No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of RCTs; Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on asthma control.
 Beneficial effect;
Beneficial effect;  negative effect;
negative effect;  No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of RCTs; Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on asthma control.
No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of RCTs; Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on asthma control.| Diet | Childhood | Adulthood | ||
|---|---|---|---|---|
| Effect | Evidence | Effect | Evidence | |
| Mediterranean diet |  [153,154] [153,154] | Low |  [170,171] [170,171] | Strong |
| Fruit |  [155,156] [155,156] | Very low |  [174] [174] | Strong |
| Vegetables |  [156] [156] | Very low |  [174] [174] | Strong |
| Fast food |  [156,158,159] [156,158,159] | Very low |  [183] [183] | Very low |
| “Western” diet | ? | ? |  [181] [181] | Very low |
| Meat | ? | ? |  [182] [182] | Low |
| Fish | ? | ? | ? | ? |
| Vitamin A | ? | ? | ? | ? |
| Vitamin B | ? | ? | ? | ? |
| Vitamin C |  [166] [166] | Low |  [176] [176] | Strong |
| Vitamin D |  [167] [167] | Strong |  [178] [178] | Strong |
| Vitamin E | ? | ? |  [177] [177] | Strong |
| LC n-3 PUFA (Fish oil) |  [160,161,162,163] [160,161,162,163] | Strong |  [161] [161] | Very strong |
 Beneficial effect;
Beneficial effect;  No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of RCTs, Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on lung function.
No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of RCTs, Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on lung function.
 Beneficial effect;
Beneficial effect;  No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of RCTs, Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on lung function.
No effect; ? = no data. Very strong evidence is defined as data obtained in meta-analysis of RCTs, Strong evidence is defined as data obtained in individual RCT; Low evidence is defined as data obtained in individual prospective studies or meta-analysis of prospective studies; Very low evidence is defined as data obtained in individual cross-sectional or case-control studies, or meta-analysis of cross-sectional or case-control studies. In case of conflicting results between studies, data from the studies with the most robust methodology are used to define the effect of diet on lung function.| Childhood | Adulthood | |||
|---|---|---|---|---|
| Effect | Evidence | Effect | Evidence | |
| New born Breast feeding |  [185,186,187] [185,186,187] | Low | ? | ? |
| Mediterranean diet |  [43,188] [43,188] | Very low |  [43,170,171,188] [43,170,171,188] | Strong |
| Fruit |  [43] [43] | Very low |  [172,173,174] [172,173,174] | Strong |
| Vegetables |  [43] [43] | Very low |  [174] [174] | Strong |
| Fast food | ? | ? | ? | ? |
| “Western” diet | ? | ? | ? | ? |
| Meat | ? | ? |  [71] [71] | Low |
| Fish | ? | ? | ? | ? |
| Vitamin A |  [118] [118] | Low | ? | ? |
| Vitamin B | ? |  [175] [175] | Very low | |
| Vitamin C |  [166,189] [166,189] | Strong |  [197] [197] | Strong |
| Vitamin D |  [190,191,192] [190,191,192] | Very strong |  [190,191,198] [190,191,198] | Very strong |
| Vitamin E |  [193] [193] | Strong |  [197] [197] | Strong |
| LC n-3 PUFA (Fish oil) |  [194] [194] | Strong |  [194] [194] | Strong |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients 2017, 9, 1227. https://doi.org/10.3390/nu9111227
Guilleminault L, Williams EJ, Scott HA, Berthon BS, Jensen M, Wood LG. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients. 2017; 9(11):1227. https://doi.org/10.3390/nu9111227
Chicago/Turabian StyleGuilleminault, Laurent, Evan J. Williams, Hayley A. Scott, Bronwyn S. Berthon, Megan Jensen, and Lisa G. Wood. 2017. "Diet and Asthma: Is It Time to Adapt Our Message?" Nutrients 9, no. 11: 1227. https://doi.org/10.3390/nu9111227





