A Low Geriatric Nutrition Risk Index Is Associated with Progression to Dialysis in Patients with Chronic Kidney Disease
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Patients and Design
2.2. Evaluation of Cardiac Structure and Function
2.3. GNRI Calculation
2.4. Collection of Demographic, Medical, and Laboratory Data
2.5. Definition of Renal End-Point
2.6. Statistical Analysis
3. Results
3.1. Correlation between GNRI and Echocardiographic Parameters
3.2. Determinants of GNRI
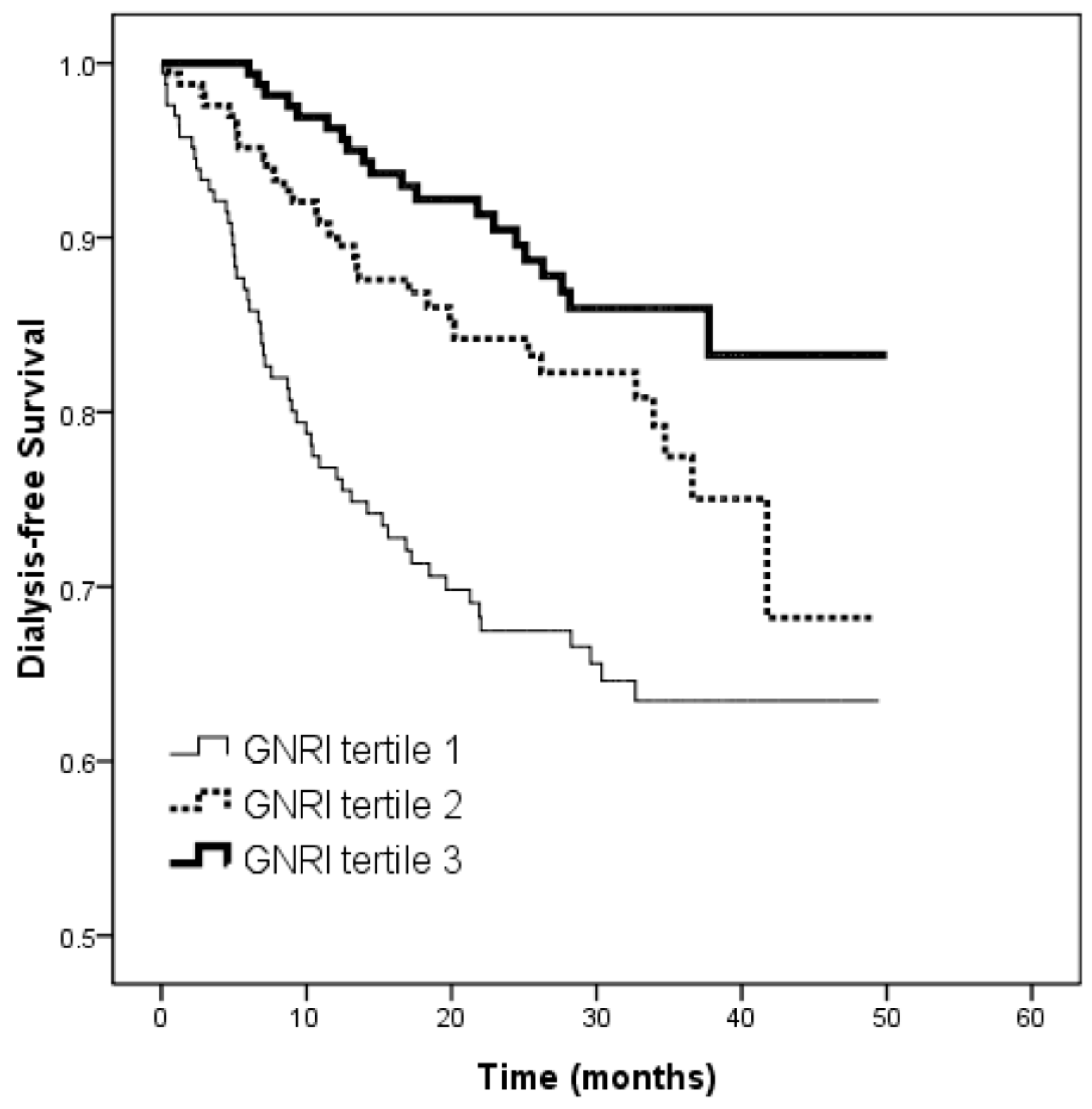
3.3. Risk of Progression to Dialysis
3.4. Comparison of Albumin, BMI and GNRI in Progression to Dialysis
4. Discussion
Author Contributions
Conflicts of Interest
References
- Kalantar-Zadeh, K.; Block, G.; McAllister, C.J.; Humphreys, M.H.; Kopple, J.D. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am. J. Clin. Nutr. 2004, 80, 299–307. [Google Scholar] [PubMed]
- Steiber, A.L.; Kalantar-Zadeh, K.; Secker, D.; McCarthy, M.; Sehgal, A.; McCann, L. Subjective global assessment in chronic kidney disease: A review. J. Ren. Nutr. 2004, 14, 191–200. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Block, G.; Humphreys, M.H. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2001, 38, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; Salva, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef]
- Reilly, H.M.; Martineau, J.K.; Moran, A.; Kennedy, H. Nutritional screening evaluation and implementation of a simple Nutrition Risk Score. Clin. Nutr. 1995, 14, 269–273. [Google Scholar] [CrossRef]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [PubMed]
- Rambod, M.; Bross, R.; Zitterkoph, J.; Benner, D.; Pithia, J.; Colman, S.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of malnutrition-inflammation score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am. J. Kidney Dis. 2009, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Amparo, F.C.; Kamimura, M.A.; Molnar, M.Z.; Cuppari, L.; Lindholm, B.; Amodeo, C.; Carrero, J.J.; Cordeiro, A.C. Diagnostic validation and prognostic significance of the malnutrition-inflammation score in nondialyzed chronic kidney disease patients. Nephrol. Dial. Transplant. 2015, 30, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Furuya, R.; Takita, T.; Maruyama, Y.; Yamaguchi, Y.; Ohkawa, S.; Kumagai, H. Simplified nutritional screening tools for patients on maintenance hemodialysis. Am. J. Clin. Nutr. 2008, 87, 106–113. [Google Scholar] [PubMed]
- Ishii, H.; Takahashi, H.; Ito, Y.; Aoyama, T.; Kamoi, D.; Sakakibara, T.; Umemoto, N.; Kumada, Y.; Suzuki, S.; Murohara, T. The Association of ankle brachial index, protein-energy wasting, and inflammation status with cardiovascular mortality in patients on chronic hemodialysis. Nutrients 2017, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ito, Y.; Ishii, H.; Aoyama, T.; Kamoi, D.; Kasuga, H.; Yasuda, K.; Maruyama, S.; Matsuo, S.; Murohara, T.; et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J. Cardiol. 2014, 64, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Ishimura, E.; Kato, Y.; Okuno, S.; Yamamoto, T.; Yamakawa, T.; Mori, K.; Inaba, M.; Nishizawa, Y. Geriatric Nutritional Risk Index, a simplified nutritional screening index, is a significant predictor of mortality in chronic dialysis patients. Nephrol. Dial. Transplant. 2010, 25, 3361–3365. [Google Scholar] [CrossRef] [PubMed]
- Panichi, V.; Cupisti, A.; Rosati, A.; Di Giorgio, A.; Scatena, A.; Menconi, O.; Bozzoli, L.; Bottai, A. Geriatric nutritional risk index is a strong predictor of mortality in hemodialysis patients: Data from the Riscavid cohort. J. Nephrol. 2014, 27, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Suzuki, S.; Ishii, H.; Hirayama, K.; Aoki, T.; Shibata, Y.; Negishi, Y.; Sumi, T.; Kawashima, K.; Kunimura, A.; et al. Nutrition status predicts severity of vascular calcification in non-dialyzed chronic kidney disease. Circ. J. 2017, 81, 316–321. [Google Scholar] [CrossRef] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Bhatia, R.S.; Tu, J.V.; Lee, D.S.; Austin, P.C.; Fang, J.; Haouzi, A.; Gong, Y.; Liu, P.P. Outcome of heart failure with preserved ejection fraction in a population-based study. N. Engl. J. Med. 2006, 355, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Hsich, E.M.; Grau-Sepulveda, M.V.; Hernandez, A.F.; Peterson, E.D.; Schwamm, L.H.; Bhatt, D.L.; Fonarow, G.C. Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am. Heart J. 2012, 163, 430–437e3. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef]
- Vickery, S.; Stevens, P.E.; Dalton, R.N.; van Lente, F.; Lamb, E.J. Does the ID-MS traceable MDRD equation work and is it suitable for use with compensated Jaffe and enzymatic creatinine assays? Nephrol. Dial. Transplant. 2006, 21, 2439–2445. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Qian, Q. Protein nutrition and malnutrition in CKD and ESRD. Nutrients 2017, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.B.; Shin, H.S.; Jung, Y.S.; Rim, H. Geriatric nutritional risk index may be a significant predictor of mortality in Korean hemodialysis patients: A single center study. Ther. Apher. Dial. 2012, 16, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Cho, K.H.; Park, J.W.; Yoon, K.W.; Do, J.Y. Geriatric nutritional risk index as a prognostic factor in peritoneal dialysis patients. Perit. Dial. Int. 2013, 33, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Kinugasa, Y.; Kato, M.; Sugihara, S.; Hirai, M.; Yamada, K.; Yanagihara, K.; Yamamoto, K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ. J. 2013, 77, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Inoue, K.; Shimizu, K.; Hiraga, K.; Takahashi, E.; Otaki, K.; Yoshikawa, T.; Furuta, K.; Tokunaga, C.; Sakakibara, T.; et al. Comparison of nutritional risk scores for predicting mortality in Japanese chronic hemodialysis patients. J. Ren. Nutr. 2017, 27, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Regolisti, G.; Karupaiah, T.; Sahathevan, S.; Sadu Singh, B.K.; Khor, B.H.; Salhab, N.; Karavetian, M.; Cupisti, A.; Fiaccadori, E. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin. Nutr. 2017, 36, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Su, H.M.; Hung, C.C.; Chang, J.M.; Liu, W.C.; Tsai, J.C.; Lin, M.Y.; Hwang, S.J.; Chen, H.C. Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.T.; Wang, X.J.; Li, J.; Song, G.F.; Huang, Z.Y.; Guo, X.Y.; Guo, J.J.; Lv, Z.Y.; Li, H.W.; Ge, J.B.; et al. Association of left ventricular hypertrophy with a faster rate of renal function decline in elderly patients with non-end-stage renal disease. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; De Nicola, L.; Gabbai, F.B.; Chiodini, P.; Ravera, M.; Pieracci, L.; Marre, S.; Cassottana, P.; Luca, S.; Vettoretti, S.; et al. Associations of left ventricular hypertrophy and geometry with adverse outcomes in patients with CKD and hypertension. Clin. J. Am. Soc. Nephrol. 2016, 11, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.U.; Scherhag, A.; Macdougall, I.C.; Tsakiris, D.; Clyne, N.; Locatelli, F.; Zaug, M.F.; Burger, H.U.; Drueke, T.B. Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. J. Am. Soc. Nephrol. 2009, 20, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Chang, J.M.; Liu, W.C.; Huang, J.C.; Tsai, J.C.; Lin, M.Y.; Su, H.M.; Hwang, S.J.; Chen, H.C. Echocardiographic parameters are independently associated with increased cardiovascular events in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Nakagawa, N.; Saito, E.; Matsuki, M.; Takehara, N.; Akasaka, K.; Sato, N.; Hasebe, N. Malnutrition, renal dysfunction and left ventricular hypertrophy synergistically increase the long-term incidence of cardiovascular events. Hypertens. Res. 2016, 39, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Iimuro, S.; Imai, E.; Matsuo, S.; Makino, H.; Akizawa, T.; Watanabe, T.; Ohashi, Y.; Hishida, A. Risk factors for increased left ventricular hypertrophy in patients with chronic kidney disease. Clin. Exp. Nephrol. 2013, 17, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Huang, J.C.; Tsai, Y.C.; Chen, L.I.; Su, H.M.; Chang, J.M.; Chen, H.C. Body mass index, left ventricular mass index and cardiovascular events in chronic kidney disease. Am. J. Med. Sci. 2016, 351, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Stel, V.S.; Ioannou, K.; Bruck, K.; Dounousi, E.; Pappas, K.; Siamopoulos, K.C.; Zoccali, C.; Jager, K.J.; Tsakiris, D. Longitudinal association of body mass index and waist circumference with left ventricular mass in hypertensive predialysis chronic kidney disease patients. Nephrol. Dial. Transplant. 2013, 28 (Suppl. 4), iv136–iv145. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Sanderson, J.; Sea, M.M.; Wang, M.; Lam, C.W.; Li, P.K.; Lui, S.F.; Woo, J. Important factors other than dialysis adequacy associated with inadequate dietary protein and energy intakes in patients receiving maintenance peritoneal dialysis. Am. J. Clin. Nutr. 2003, 77, 834–841. [Google Scholar] [PubMed]
- Cheng, L.T.; Tang, W.; Wang, T. Strong association between volume status and nutritional status in peritoneal dialysis patients. Am. J. Kidney Dis. 2005, 45, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Goldberg, A.L. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N. Engl. J. Med. 1996, 335, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Tertile 1 (n = 166) | Tertile 2 (n = 166) | Tertile 3 (n = 164) | p | All Patients (n = 496) |
|---|---|---|---|---|---|
| GNRI (score) | 95.4 ± 9.8 | 108.3 ± 2.0 * | 117.4 ± 5.9 *,† | <0.001 | 107.2 ± 11.4 |
| Age (year) | 68.0 ± 11.6 | 67.3 ± 11.8 | 63.6 ± 12.7 *,† | 0.002 | 66.3 ± 12.2 |
| Male gender (%) | 62.7 | 62.7 | 65.2 | 0.854 | 63.5 |
| Smoking history (%) | 34.5 | 28.9 | 29.9 | 0.447 | 31.3 |
| Diabetes mellitus (%) | 54.2 | 54.8 | 59.1 | 0.618 | 56.0 |
| Hypertension (%) | 78.9 | 81.3 | 87.2 | 0.127 | 82.5 |
| Coronary artery disease (%) | 6.6 | 12.7 | 14.6 | 0.057 | 11.3 |
| Cerebrovascular disease (%) | 16.3 | 15.7 | 13.4 | 0.750 | 15.1 |
| Systolic blood pressure (mmHg) | 141.7 ± 25.5 | 140.2 ± 19.5 | 141.9 ± 18.0 | 0.729 | 141.3 ± 21.2 |
| Diastolic blood pressure (mmHg) | 77.9 ± 13.6 | 78.3 ± 11.7 | 81.3 ± 12.8 | 0.031 | 79.2 ± 12.8 |
| Body mass index (kg/m2) | 21.9 ± 2.7 | 25.2 ± 2.3 * | 28.9 ± 3.2 *,† | <0.001 | 25.3 ± 4.0 |
| Laboratory parameters | |||||
| Albumin (g/dL) | 3.73 ± 0.44 | 4.07 ± 0.28 * | 4.24 ± 0.26 *,† | <0.001 | 4.01 ± 0.40 |
| Fasting glucose (mg/dL) | 121.0 ± 52.3 | 126.5 ± 60.2 | 131.6 ± 62.4 | 0.259 | 126.4 ± 58.5 |
| Triglyceride (mg/dL) | 116 (82–177) | 134.5 (103–199.8) | 159 (107–225) * | <0.001 | 137.5 (96–201) |
| Total cholesterol (mg/dL) | 192.0 ± 51.7 | 195.3 ± 45.2 | 194.5 ± 45.6 | 0.812 | 194.0 ± 47.5 |
| Hemoglobin (g/dL) | 10.6 ± 2.2 | 11.7 ± 2.2 * | 12.6 ± 2.1 *,† | <0.001 | 11.6 ± 2.3 |
| eGFR (mL/min/1.73 m2) | 21.9 ± 13.5 | 26.7 ± 14.1 * | 29.7 ± 14.0 * | <0.001 | 26.1 ± 14.2 |
| CaXP product (mg2/dL2) | 39.2 ± 8.5 | 38.0 ± 9.5 | 97.9 ± 8.0 | 0.332 | 38.3 ± 8.7 |
| Uric acid (mg/dL) | 8.0 ± 2.6 | 8.2 ± 2.0 | 8.4 ± 2.1 | 0.161 | 8.2 ± 2.2 |
| Proteinuria (%) | 75.8 | 61.2 * | 61.6 * | 0.006 | 66.2 |
| Echocardiographic data | |||||
| LVEDV (mL) | 112.6 ± 39.9 | 114.7 ± 38.1 | 119.4 ± 40.4 | 0.279 | 115.6 ± 39.5 |
| LVESV (mL) | 39.2 ± 26.5 | 38.1 ± 20.9 | 37.9 ± 27.8 | 0.875 | 38.4 ± 25.2 |
| LAD > 4.7 cm (%) | 6.0 | 7.2 | 6.1 | 0.883 | 6.5 |
| LVMI (g/m2) | 150.2 ± 56.1 | 135.3 ± 45.7 * | 140.2 ± 47.3 | 0.022 | 141.9 ± 50.2 |
| LVH (%) | 65.7 | 55.4 | 56.7 | 0.118 | 59.3 |
| LVEF (%) | 67.0 ± 12.8 | 68.3 ± 9.7 | 69.7 ± 9.5 * | 0.081 | 68.3 ± 10.8 |
| LVEF < 50% (%) | 10.2 | 4.2 | 2.4 * | 0.005 | 5.6 |
| Echocardiographic Parameters | Pearson’s r | p |
|---|---|---|
| LVEDV (mL) | 0.033 | 0.463 |
| LVESV (mL) | −0.049 | 0.275 |
| LAD > 4.7 cm (%) | −0.007 | 0.874 |
| LVMI (g/m2) | −0.116 | 0.001 |
| LVH (%) | −0.095 | 0.035 |
| LVEF (%) | 0.111 | 0.014 |
| LVEF < 50% (%) | −0.138 | 0.002 |
| Parameter | Univariable | Multivariable | ||
|---|---|---|---|---|
| Unstandardized Coefficient β (95% CI) | p | Unstandardized Coefficient β | p | |
| Age (per 1 year) | −0.137 (−0.219, −0.055) | 0.001 | −0.097 (−0.176, −0.018) | 0.017 |
| Male versus female | −0.508 (−2.600, 1.583) | 0.633 | – | – |
| Smoking history | −1.174 (−3.344, 0.997) | 0.289 | – | – |
| Diabetes mellitus | 0.599 (−1.430, 2.628) | 0.562 | – | – |
| Hypertension | 2.666 (0.028, 5.304) | 0.048 | 1.898 (−0.689, 4.486) | 0.150 |
| Coronary artery disease | 4.066 (0.904, 7.229) | 0.012 | 3.912 (0.919, 6.905) | 0.011 |
| Cerebrovascular disease | −1.318 (−4.127, 1.491) | 0.357 | – | – |
| Systolic blood pressure (per 1 mmHg) | −0.013 (−0.062, 0.037) | 0.502 | – | – |
| Diastolic blood pressure (per 1 mmHg) | 0.076 (−0.005, 0.157) | 0.065 | – | – |
| Laboratory parameters | ||||
| Fasting glucose (per 1 mg/dL) | 0.007 (−0.011, 0.024) | 0.435 | – | – |
| Triglyceride (per log 1 mg/dL) | 9.154 (5.061, 13.248) | <0.001 | 6.672 (2.687, 10.657) | 0.001 |
| Total cholesterol (per 1 mg/dL) | 0.013 (−0.009, 0.034) | 0.242 | – | – |
| Hemoglobin (per 1 g/dL) | 1.548 (1.141, 1.956) | <0.001 | 1.343 (0.775, 1.910) | <0.001 |
| eGFR (per 1 mL/min/1.73 m2) | 0.165 (0.096, 0.235) | <0.001 | 0.003 (−0.101, 0.107) | 0.954 |
| CaXP product (per 1 mg2/dL2) | −0.089 (−0.207, 0.208) | 0.136 | – | – |
| Uric acid (per 1 mg/dL) | 0.197 (−0.253, 0.648) | 0.390 | – | – |
| Proteinuria | −2.578 (−4.702, −0.455) | 0.017 | −1.037 (−3.506, 1.432) | 0.409 |
| Echocardiographic data | ||||
| LVEDV (per 1 mL) | 0.010 (−0.016, 0.035) | 0.463 | – | – |
| LVESV (per 1 mL) | −0.022 (−0.062, 0.018) | 0.275 | – | – |
| LAD > 4.7 cm | −0.332 (−4.432, 3.768) | 0.874 | – | – |
| LVH | −2.199 (−4.239, −0.158) | 0.035 | −0.345 (−2.392, 1.703) | 0.741 |
| LVEF < 50% | −0.832 (−11.154, −2.509) | 0.002 | −5.261 (−9.531, −0.991) | 0.016 |
| Parameter | Univariable | |
|---|---|---|
| HR (95% CI) | p | |
| GNRI (per 1 score) | 0.966 (0.958–0.977) | <0.001 |
| Age (per 1 year) | 0.988 (0.973–1.004) | 0.137 |
| Male versus female | 0.520 (0.354–0.762) | 0.001 |
| Smoking (ever versus never) | 1.049 (0.695–1.585) | 0.819 |
| Diabetes mellitus | 1.796 (1.193–2.704) | 0.005 |
| Hypertension | 2.863 (1.392–5.888) | 0.004 |
| Coronary artery disease | 1.413 (0.818–2.441) | 0.215 |
| Cerebrovascular disease | 1.255 (0.755–2.086) | 0.381 |
| Systolic blood pressure (per 1 mmHg) | 1.025 (1.017–1.033) | <0.001 |
| Diastolic blood pressure (per 1 mmHg) | 0.998 (0.983–1.013) | 0.793 |
| Laboratory parameters | ||
| Fasting glucose (per 1 mg/dL) | 1.003 (1.001–1.006) | 0.017 |
| Triglyceride (per log 1 mg/dL) | 1.017 (0.453–2.284) | 0.968 |
| Cholesterol (per 1 mg/dL) | 1.001 (0.997–1.006) | 0.512 |
| Hemoglobin (per 1 g/dL) | 0.595 (0.539–0.657) | <0.001 |
| eGFR (per 1 mL/min/1.73 m2) | 0.844 (0.817–0.872) | <0.001 |
| CaXP product (per 1 mg2/dL2) | 1.070 (1.056–1.085) | <0.001 |
| Uric acid (per 1 mg/dL) | 1.106 (1.011–1.213) | 0.028 |
| Proteinuria | 15.015 (5.527–40.787) | <0.001 |
| LAD > 4.7 cm | 2.298 (1.285–4.109) | 0.005 |
| LVH | 4.234 (2.486–7.210) | <0.001 |
| LVEF < 50% | 1.532 (0.744–3.157) | 0.247 |
| Parameter | Multivariable: Model 1 | Multivariable: Model 2 | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| GNRI (per 1 score) | 0.965 (0.955–0.976) | <0.001 | 0.975 (0.963–0.987) | <0.001 |
| Male versus female | 0.565 (0.383–0.832) | 0.004 | ||
| Diabetes mellitus | 1.508 (0.988–2.304) | 0.057 | ||
| Hypertension | 1.986 (0.951–4.149) | 0.068 | ||
| Systolic blood pressure (per 1 mmHg) | 1.021 (1.012–1.029) | <0.001 | ||
| Laboratory parameters | ||||
| Fasting glucose (per 1 mg/dL) | 1.003 (1.001–1.006) | 0.017 | ||
| Hemoglobin (per 1 g/dL) | 0.885 (0.770–1.018) | 0.087 | ||
| eGFR (per 1 mL/min/1.73 m2) | 0.875 (0.838–0.913) | <0.001 | ||
| CaXP product (per 1 mg2/dL2) | 1.018 (0.996–1.040) | 0.111 | ||
| Uric acid (per 1 mg/dL) | 1.046 (0.934–1.171) | 0.436 | ||
| Proteinuria | 2.991 (0.702–12.750) | 0.139 | ||
| LAD > 4.7 cm | 1.412 (0.741–2.691) | 0.294 | ||
| LVH | 1.583 (0.914–2.743) | 0.101 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, I.-C.; Huang, J.-C.; Wu, P.-Y.; Chen, S.-C.; Chang, J.-M.; Chen, H.-C. A Low Geriatric Nutrition Risk Index Is Associated with Progression to Dialysis in Patients with Chronic Kidney Disease. Nutrients 2017, 9, 1228. https://doi.org/10.3390/nu9111228
Kuo I-C, Huang J-C, Wu P-Y, Chen S-C, Chang J-M, Chen H-C. A Low Geriatric Nutrition Risk Index Is Associated with Progression to Dialysis in Patients with Chronic Kidney Disease. Nutrients. 2017; 9(11):1228. https://doi.org/10.3390/nu9111228
Chicago/Turabian StyleKuo, I-Ching, Jiun-Chi Huang, Pei-Yu Wu, Szu-Chia Chen, Jer-Ming Chang, and Hung-Chun Chen. 2017. "A Low Geriatric Nutrition Risk Index Is Associated with Progression to Dialysis in Patients with Chronic Kidney Disease" Nutrients 9, no. 11: 1228. https://doi.org/10.3390/nu9111228





