Integrated Evaluation of the Potential Health Benefits of Einkorn-Based Breads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flour Samples
2.3. Lactic Acid Bacteria (LAB) Strains and Sourdough Starter Preparation
2.4. Fermentation and Baking Processes
2.5. In Vitro Digestion of Bread Samples
2.6. HPLC Determination of Phenolic Acid Content
2.6.1. Free Phenolic Acids (FREE Fraction)
2.6.2. Soluble-Conjugated Phenolic Acids (SC Fraction)
2.6.3. Insoluble-Bound Phenolic Acids (IB Fraction)
2.6.4. HPLC Analysis
2.7. HPLC Determination of Carotenoid Content
2.8. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.9. Caco-2 Cell Culture and Supplementation
2.10. Cytokine Quantification in Caco-2 Cells
2.11. Statistical Analysis
3. Results and Discussion
3.1. Carotenoid and Phenolic Acid Profile in Einkorn and Wheat Flours
3.2. Carotenoid and Phenolic Acid Profile in AF and SF Breads Prepared by Different Fermentation Processes
3.3. Carotenoids and Phenolic Acids in In Vitro Digested Samples
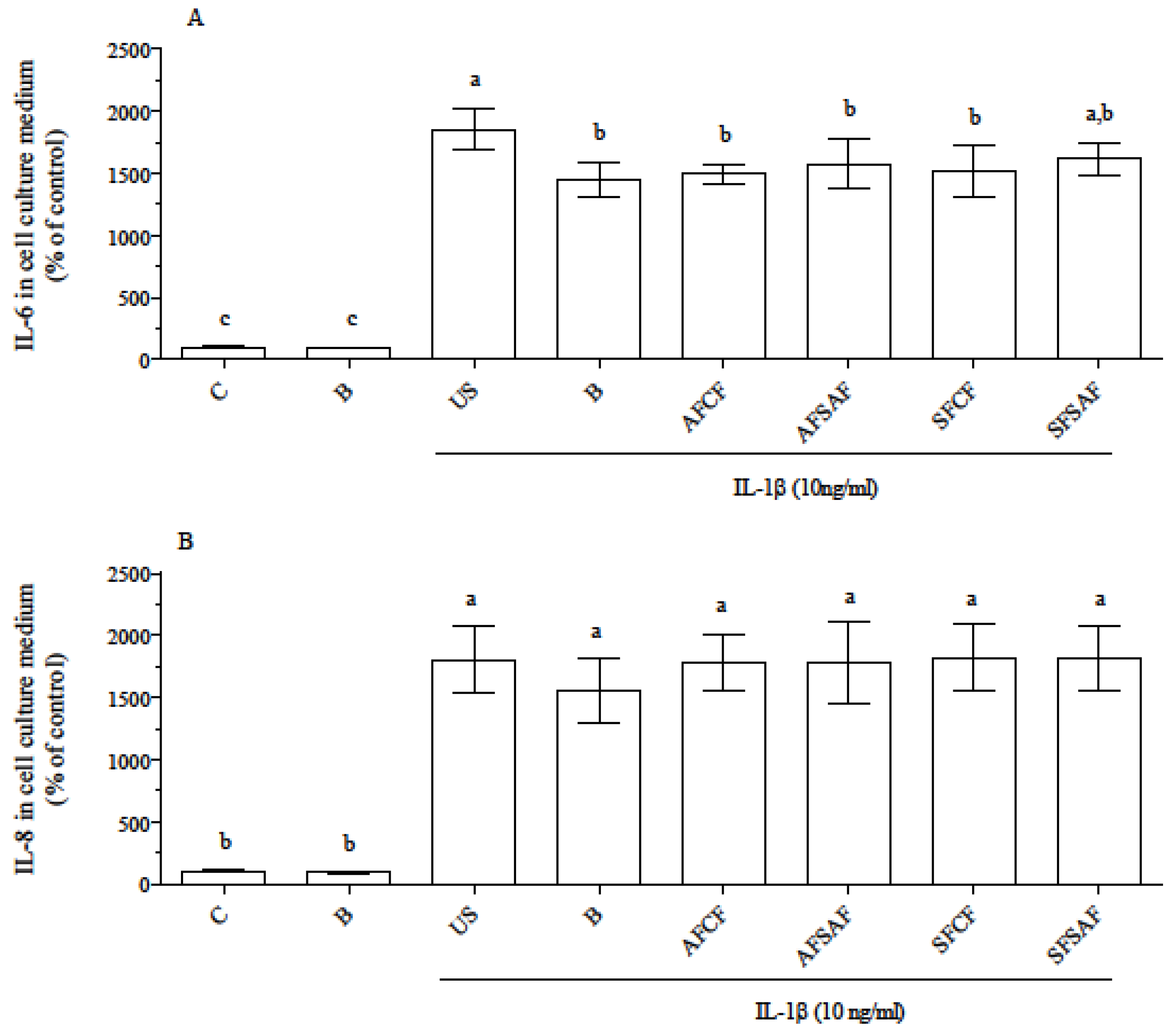
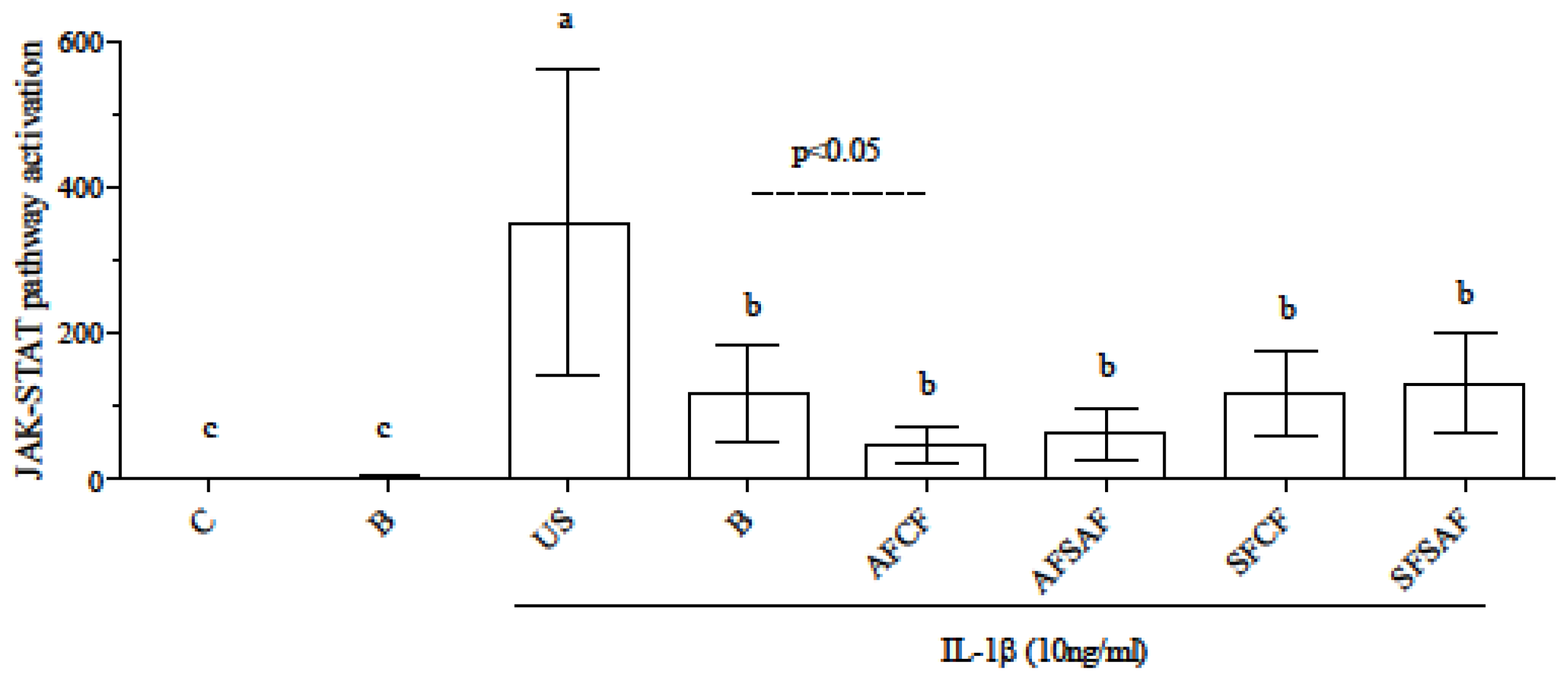
3.4. Anti-Inflammatory Effect of In Vitro Digested Breads
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zong, G.; Gao, A.; Hu, F.B.; Sun, Q. Whole grain intake and mortality from all causes, cardiovascular disease, and cancer: A meta-analysis of prospective cohort studies. Circulation 2016, 133, 2370–2380. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Kyrø, C.; Skeie, G.; Loft, S.; Landberg, R.; Christensen, J.; Lund, E.; Nilsson, L.M.; Palmqvist, R.; Tjønneland, A.; Olsen, A. Intake of whole grains from different cereal and food sources and incidence of colorectal cancer in the Scandinavian HELGA cohort. Cancer Causes Control 2013, 24, 1363–1374. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemical profiles and antioxidant activity of wheat varieties. J. Agric. Food Chem. 2003, 51, 7825–7834. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Young, J.C.; Rabalski, I.; Hucl, P.; Frégeau-Reid, J. Identification and quantification of seed carotenoids in selected wheat species. J. Agric. Food Chem. 2007, 55, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: An application of the Phenol−Explorer database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Young, J.C.; Wood, P.J.; Rabalski, I.; Hucl, P.; Fregeau-Reid, J. Einkorn: A potential candidate for developing high lutein wheat. Cereal Chem. 2002, 79, 455–457. [Google Scholar] [CrossRef]
- Humphries, J.M.; Khachik, F. Distribution of lutein, zeaxanthin and related geometrical isomers in fruit, vegetables, wheat and pasta products. J. Agric. Food Chem. 2003, 51, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Sosulski, F.; Krygier, K.; Hogge, L. Free, esterified, and insoluble-bound phenolic acids. 3. Composition of phenolic acids in cereal and potato flour. J. Agric. Food Chem. 1982, 30, 337–340. [Google Scholar] [CrossRef]
- Poutanen, K.; Shepherd, R.; Shewry, P.R.; Delcour, J.A.; Bjorck, I.; Van Der Kamp, J.W. Beyond whole grain: The European HEALTH GRAIN project aims at healthier cereal foods. Cereal Foods World 2008, 53, 32–35. [Google Scholar] [CrossRef]
- Slavin, J. Why whole grains are protective: Biological mechanisms. Proc. Nutr. Soc. 2003, 62, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, A.; Danesi, F.; Di Nunzio, M.; Taccari, A.; Valli, V. Ancient wheat and health: A legend or the reality? A review on KAMUT khorasan wheat. Int. J. Food Sci. Nutr. 2016, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Brandolini, A. Nutritional properties of einkorn wheat (Triticum monococcum L.). J. Sci. Food Agric. 2014, 94, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Molberg, Ø.; Uhlen, A.K.; Jensen, T.; Flæte, N.S.; Fleckenstein, B.; Arentz-Hansen, H.; Raki, M.; Lundin, K.E.A.; Sollid, L.M. Mapping of gluten T-cell epitopes in the bread wheat ancestors: Implication for celiac disease. Gastroenterology 2005, 128, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pardo, M.E.; Blancas-Nápoles, J.A.; Vázquez-Landaverde, P.A.; Nari, A.; Taglieri, I.; Ortiz-Moreno, A.; Mayorga-Reyes, L.; Sanmartin, C.; Bermúdez-Humarán, L.G.; Torres-Maravilla, E. The use of Mexican xaxtle as leavening agent in Italian straight dough bread making to produce pulque bread. Agrochimica 2016, 60, 329–342. [Google Scholar]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Zinnai, A. Effect of the baking process on artisanal sourdough bread-making: A technological and sensory evaluation. Agrochimica 2016, 60, 222–234. [Google Scholar]
- Ganzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Rizzello, G.C.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Terzuoli, E.; Donnini, S.; Nicolella, C.; Zinnai, A. Development of phenol-enriched olive oil with phenolic compounds extracted from wastewater produced by physical refining. Nutrients 2017, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Turpin, W.; Renaud, C.; Avallone, S.; Hammoumi, A.; Guyot, J.-P.; Humblot, C. PCR of crtNM combined with analytical biochemistry: An efficient way to identify carotenoid producing lactic acid bacteria. Syst. Appl. Microbiol. 2016, 39, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E.; Richelle, M.; Perrot, E.; Smoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef] [PubMed]
- Anson, N.M.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Viadel Crespo, B.; Rivera Patiño, J.D.; Navarro Fayos, M.T.; Tenllado Llavador, I.; Carreres Malonda, J.E.; García Reverter, J.; Blasco Piquer, M.; Subirats Huerta, S. Equipo modular de digestión in vitro. Patent ES2361983B1, 19 Apirl 2012. [Google Scholar]
- Marteau, P.; Flourié, B.; Pochart, P.; Chastang, C.; Desjeux, J.F.; Rambaud, J.C. Role of the microbial lactose EC 3.2.123, activity from yoghurt on the intestinal absorption of lactose: An in vivo study in lactose-deficient humans. Br. J. Nutr. 1990, 64, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Marteau, P.; Havenaar, R.; Huis Veld, J.H.J. A multicompartmental dynamic computer-controlled model simulating the stomach and the small intestine. ATLA 1995, 23, 197–209. [Google Scholar]
- Bengtsson, A.; Larsson, M.; Svanberg, U. In vitro bioaccessibility of β-carotene from heat-processed orange-fleshed sweet potato. J. Agric. Food Chem. 2009, 57, 9693–9698. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Hao, Z.; Zhou, K.; Luther, M.; Costa, J.; Yu, L. Carotenoid, tocopherol, phenolic acid, and antioxidant properties of Maryland-grown soft wheat. J. Agric. Food Chem. 2005, 53, 6649–6657. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Pihlava, J.M.; Hellström, J. Contents of phenolic acids, alkyl- and alkenylresorcinols, and avenanthramides in commercial grain products. J. Agric. Food Sci. 2005, 53, 8290–8295. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Brandolini, A.; Pompei, C.; Piscozzi, R. Carotenoids and tocols of einkorn wheat (Triticum monococcum ssp. monococcum L.). J. Cereal Sci. 2006, 44, 182–193. [Google Scholar] [CrossRef]
- Brandolini, A.; Castoldi, P.; Plizzari, L.; Hidalgo, A. Phenolic acid composition, total polyphenols content and antioxidant activity of Triticum monococcum, Triticum turgidum and Triticum aestivum: A two-years evaluation. J. Cereal Sci. 2013, 58, 123–131. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Rabalski, I. Bioactive compounds and their antioxidant capacity in selected primitive and modern wheat species. Open Agric. J. 2008, 2, 7–14. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A. Protein, ash, lutein and tocols distribution in einkorn (Triticum monococcum L. subsp. Monococcum) seed fractions. Food Chem. 2008, 107, 444–448. [Google Scholar] [CrossRef]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemicals and antioxidant activity of milled fractions of different wheat varieties. J. Agric. Food Chem. 2005, 53, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, F.; Lyan, B.; Rock, E.; Boussard, A.; Potus, J.; Chanliaud, E.; Remesy, C. Wheat lipoxygenase activity induces greater loss of carotenoids than Vitamin E during breadmaking. J. Agric. Food Chem. 2006, 54, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, F.; Lyan, B.; Rock, E.; Boussard, A.; Potus, J.; Chanliaud, E.; Remesy, C. Genetic variability of carotenoid concentration, and lipoxygenase and peroxidase activities among cultivated wheat species and bread wheat varieties. Eur. J. Agron. 2006, 25, 170–176. [Google Scholar] [CrossRef]
- Cevoli, C.; Gianotti, A.; Troncoso, R.; Fabbri, A. Quality evaluation by physical tests of a traditional Italian flat bread Piadina during storage and shelf-life improvement with sourdough and enzymes. Eur. Food Res. Technol. 2015, 240, 1081–1089. [Google Scholar] [CrossRef]
- Ferri, M.; Serrazanetti, D.I.; Tassoni, A.; Baldissarri, M.; Gianotti, A. Improving functional and technological profile of cereal fermented foods by Lactobacillus plantarum strains selected via a metabolomics approach. Food Res. Int. 2016, 89, 1095–1105. [Google Scholar] [CrossRef]
- Garrido-Fernandez, J.; Maldonado-Barragan, A.; Caballero-Guerrero, B.; Hornero-Mendez, D.; Ruiz-Barba, J.L. Carotenoid production in Lactobacillus plantarum. Int. J. Food Microbiol. 2010, 140, 34–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breithaupt, D.E.; Schwack, W.; Wolf, G.; Hammes, W.P. Characterization of the triterpenoid diaponeurosporene and its isomers in food-associated bacteria. Eur. Food Res. Technol. 2001, 213, 231–233. [Google Scholar] [CrossRef]
- Katina, K.; Laitila, A.; Jovonen, R.; Liukkonen, K.-H.; Kariluoto, S.; Piironen, V.; Landgerg, R.; Åman, P.; Poutanen, K. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007, 24, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Boskov Hansen, H.; Andreasen, M.G.; Nielsen, M.M.; Larsen, L.M.; Bach Knudsen, K.E.; Meyer, A.S.; Christensen, L.P.; Hansen, Å. Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread-making. Eur. Food Res. Technol. 2002, 214, 33–42. [Google Scholar] [CrossRef]
- Hole, A.S.; Rud, I.; Grimmer, S.; Sigl, S.; Narvhus, J.; Sahlstrøm, S. Improved bioavailability of dietary phenolics in whole grain barley and oat groat following fermentation with probiotic Lactobacillus acidophilus, Lactobacillus johnsonii, and Lactobacillus reuteri. J. Agric. Food Chem. 2012, 60, 6369–6375. [Google Scholar] [CrossRef] [PubMed]
- Svensson, L.; Sekwati-Monang, B.; Lutz, D.L.; Schieber, A.; Ganzle, M.G. Phenolic acids and flavonoids in nonfermented and fermented red sorghum Sorghum bicolor L., Moench. J. Agric. Food Chem. 2010, 58, 9214–9220. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; De Angelis, M.; Corsetti, A.; Di Cagno, R. Biochemistry and physiology of sourdough lactic acid bacteria. Trends Food Sci. Technol. 2005, 16, 57–69. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Read, A.; Wright, A.; Abdel-Aal, E.S.M. In vitro bioaccessibility and monolayer uptake of lutein from wholegrain baked foods. Food Chem. 2015, 174, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Courraud, J.; Berger, J.; Cristol, J.P.; Avallone, S. Stability and bioaccessibility of different forms of carotenoids and vitamin A during in vitro digestion. Food Chem. 2013, 136, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Juvonen, R.; Laitila, A.; Flander, L.; Nordlund, E.; Kariluoto, S.; Piironen, V.; Poutanen, K. Fermented wheat bran as a functional ingredient in baking. Cereal Chem. 2012, 89, 126–134. [Google Scholar] [CrossRef]
- O’Connell, O.; Ryan, L.; O’Sullivan, L.; Aherne-Bruce, S.A.; O’Brien, N.M. Carotenoid micellarization varies greatly between individual and mixed vegetables with or without the addition of fat or fiber. Int. J. Vitam. Nutr. Res. 2008, 78, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Egashira, Y.; Sanada, H. Digestion and absorption of ferulic acid sugar esters in rat gastrointestinal tract. J. Agric. Food Chem. 2003, 51, 5034–5039. [Google Scholar] [CrossRef] [PubMed]
- Kroon, P.A.; Faulds, C.B.; Ryden, P.; Robertson, J.A.; Williamson, G. Release of covalently bound ferulic acid from fiber in the human colon. J. Agric. Food Chem. 1997, 45, 661–667. [Google Scholar] [CrossRef]
- Laveti, D.; Kumar, M.; Hemalatha, R.; Sistla, R.; Naidu, V.G.; Talla, V.; Verma, V.; Kaur, N.; Nagpal, R. Anti-inflammatory treatments for chronic diseases: A review. Inflamm. All. Drug Targets 2013, 12, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Katoh, T.; Ogawa, A.; Bamba, S.; Andoh, A.; Koyama, S.; Fujiyama, Y.; Bamba, T. Bile acid modulates transepithelial permeability via the generation of reactive oxygen species in the Caco-2 cell line. Free Radic. Biol. Med. 2005, 39, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Di Toro, R.; Campana, G.; Murari, G.; Spampinato, S. Effects of specific bile acids on c-fos messenger RNA levels in human colon carcinoma Caco-2 cells. Eur. J. Pharm. Sci. 2000, 11, 291–298. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Grootaert, C.; Capanoglu, E.; Ozkan, C.; Smagghe, G.; Raes, K.; Van Camp, J. Anti-inflammatory potential of black carrot (Daucus carota L.) polyphenols in a co-culture model of intestinal Caco-2 and endothelial EA.hy926 cells. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Marmet, C.; Actis-Goretta, L.; Renouf, M.; Giuffrida, F. Quantification of phenolic acids and their methylates, glucuronides, sulfates and lactones metabolites in human plasma by LC-MS/MS after oral ingestion of soluble coffee. J. Pharm. Biomed. Anal. 2014, 88, 617–625. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Chen, C.Y.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.B.J.; Lajczak, N.K.; Kelly, O.B.; O’Dwyer, A.M.; Giddam, A.K.; Ní Gabhann, J.; Franco, P.; Tambuwala, M.M.; Jefferies, C.A.; Keely, S.; et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G550–G558. [Google Scholar] [CrossRef] [PubMed]
- Fantini, M.C.; Pallone, F. Cytokines: From gut inflammation to colorectal cancer. Curr. Drug Targets. 2008, 9, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Sereni, A.; Cesari, F.; Gori, A.M.; Maggini, N.; Marcucci, R.; Casini, A.; Sofi, F. Cardiovascular benefits from ancient grain bread consumption: Findings from a double-blinded randomized crossover intervention trial. Int. J. Food Sci. Nutr. 2017, 68, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, S.; Primiterra, M.; Tagliamonte, M.C.; Carnevali, A.; Gianotti, A.; Bordoni, A.; Canestrari, F. Counteraction of oxidative damage in the rat liver by an ancient grain (Kamut brand khorasan wheat). Nutrition 2012, 28, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Whittaker, A.; Gori, A.M.; Cesari, F.; Surrenti, E.; Abbate, R.; Gensini, G.F.; Benedettelli, S.; Casini, A. Effect of Triticum turgidum subsp. turanicum wheat on irritable bowel syndrome: A double-blinded randomised dietary intervention trial. Br. J. Nutr. 2014, 111, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
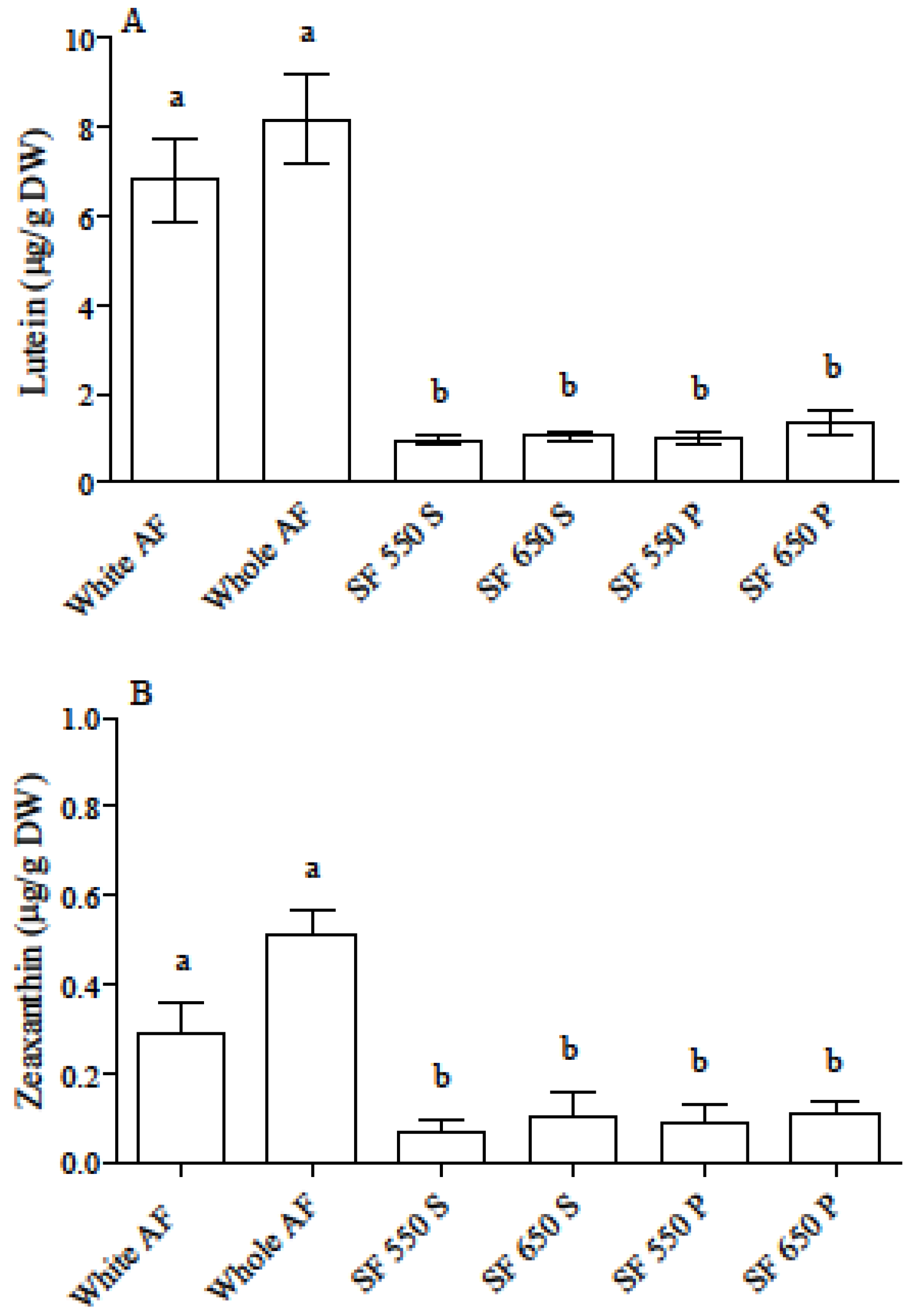
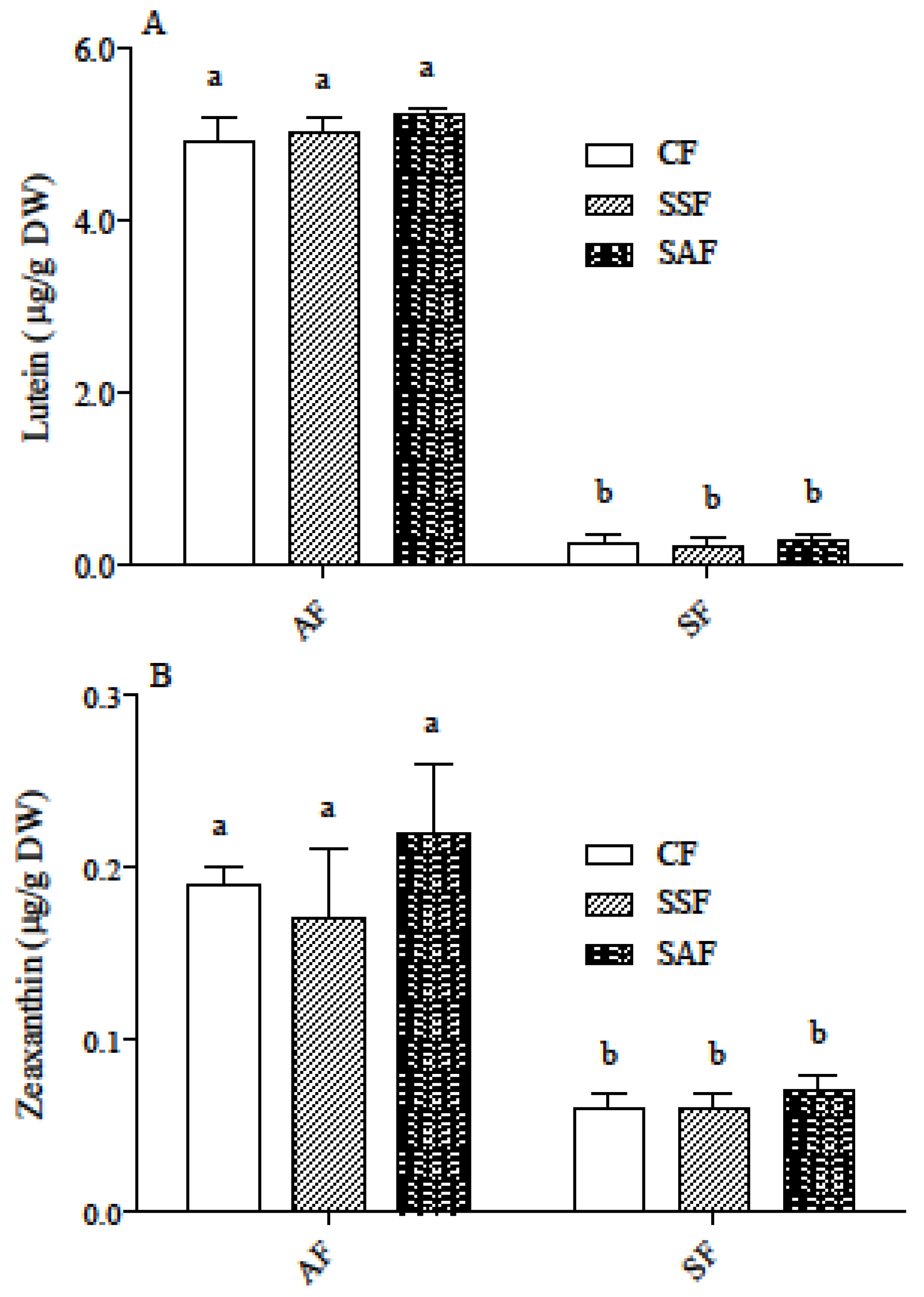
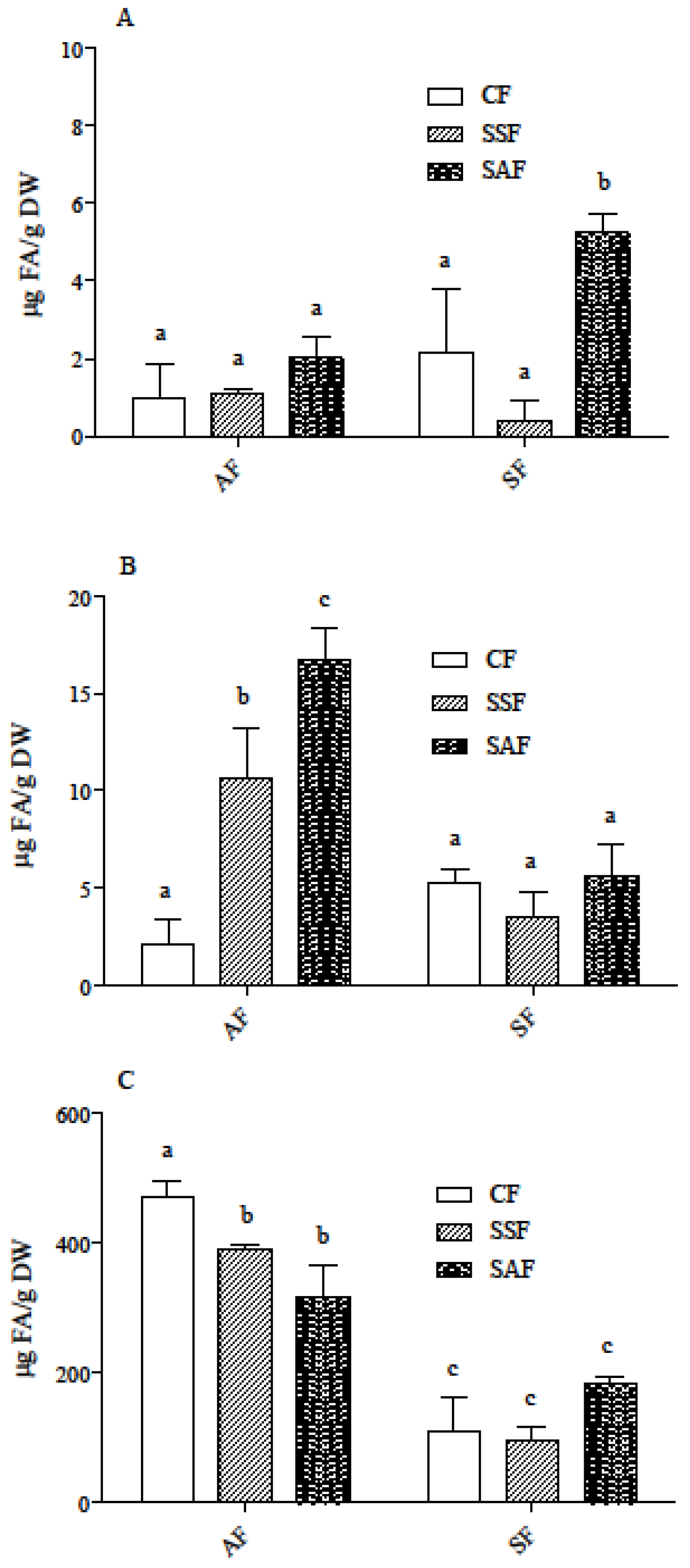
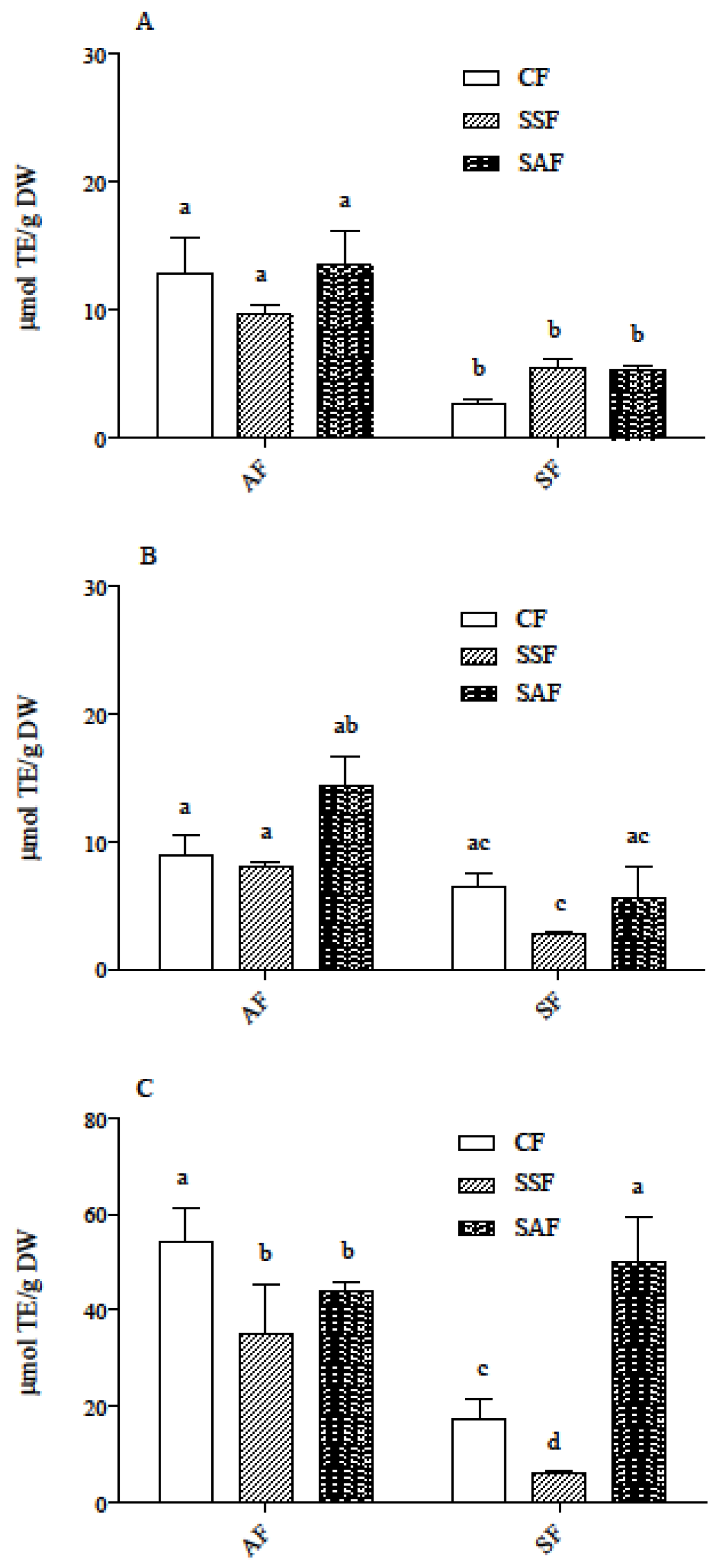
| Sample | Type of Flour | Type of Fermentation |
|---|---|---|
| AFCF | AF | CF |
| AFSAF | AF | SAF |
| AFSSF | AF | SSF |
| SFCF | SF | CF |
| SFSAF | SF | SAF |
| SFSSF | SF | SSF |
| Flour Type | FREE | SC | IB | Total Soluble | Total |
|---|---|---|---|---|---|
| Ferulic Acid Content (µg/g DW) | |||||
| White AF | 2.2 ± 0.3a | 10.0 ± 0.2a | 367.8 ± 2.6a | 12.2 ± 0.3a | 380.0 ± 2.7a |
| Whole AF | 1.0± 0.3b | 18.0 ± 0.4b | 475.8 ± 18.8b | 19.0 ± 0.5b | 494.8± 18.8b |
| SF 550 S | n.d. | 4.8 ± 0.4c | 126.8 ± 7.9c | 4.8 ± 0.4c | 131.6 ± 8.1c |
| SF 650 S | n.d. | 3.1 ± 0.1d | 117.2 ± 6.6c | 3.1 ± 0.1d | 120.3 ± 6.6c |
| SF 550 P | n.d. | 4.5 ± 0.2c | 124.4 ± 8.1c | 4.5 ± 0.2c | 128.9 ± 8.1c |
| SF 650 P | n.d. | 5.0 ± 0.1c | 131.2 ± 7.4c | 5.0 ± 0.1c | 136.2 ± 7.4c |
| p-Coumaric acid content (µg/g DW) | |||||
| White AF | 4.7 ± 0.9a | 1.5 ± 0.3a | 6.7 ± 0.9a | 6.2 ± 1.0a | 12.9 ± 1.3a |
| Whole AF | 6.9 ± 0.9a | 1.9 ± 0.9a | 5.3 ± 0.9ab | 8.8 ± 1.2a | 14.1 ± 1.6a |
| SF 550 S | 25.1 ± 1.9b | 2.2 ± 0.8a | 11.7 ± 1.9c | 27.3 ± 2.1b | 39.0 ± 2.8b |
| SF 650 S | 26.3 ± 2.5b | 2.2 ± 0.8a | 8.7 ± 0.7b | 28.5 ± 2.7b | 37.2 ± 2.7b |
| SF 550 P | 24.1 ± 1.3b | 1.6 ± 0.6a | 8.4 ± 0.9b | 25.7 ± 1.4b | 34.1 ± 1.7b |
| SF 650 P | 23.9 ± 1.3b | 0.3 ± 0.04a | 7.7 ± 1.1b | 24.2 ± 1.3b | 31.9 ± 1.7b |
| Sample | Lutein | Zeaxanthin | Ferulic Acid |
|---|---|---|---|
| AFCF | 44.1 | 64 | 0.7 |
| AFSSF | 16 | 13 | 0.6 |
| AFSAF | 8 | 38 | 0.7 |
| SFCF | 100 | 64 | n.d. |
| SFSSF | 52 | 42 | n.d. |
| SFSAF | 43 | 60 | n.d. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antognoni, F.; Mandrioli, R.; Bordoni, A.; Di Nunzio, M.; Viadel, B.; Gallego, E.; Villalba, M.P.; Tomás-Cobos, L.; Taneyo Saa, D.L.; Gianotti, A. Integrated Evaluation of the Potential Health Benefits of Einkorn-Based Breads. Nutrients 2017, 9, 1232. https://doi.org/10.3390/nu9111232
Antognoni F, Mandrioli R, Bordoni A, Di Nunzio M, Viadel B, Gallego E, Villalba MP, Tomás-Cobos L, Taneyo Saa DL, Gianotti A. Integrated Evaluation of the Potential Health Benefits of Einkorn-Based Breads. Nutrients. 2017; 9(11):1232. https://doi.org/10.3390/nu9111232
Chicago/Turabian StyleAntognoni, Fabiana, Roberto Mandrioli, Alessandra Bordoni, Mattia Di Nunzio, Blanca Viadel, Elisa Gallego, María Paz Villalba, Lidia Tomás-Cobos, Danielle Laure Taneyo Saa, and Andrea Gianotti. 2017. "Integrated Evaluation of the Potential Health Benefits of Einkorn-Based Breads" Nutrients 9, no. 11: 1232. https://doi.org/10.3390/nu9111232






