Effect of Omega-3 Fatty Acid Supplementation on Plasma Fibroblast Growth Factor 23 Levels in Post-Myocardial Infarction Patients with Chronic Kidney Disease: The Alpha Omega Trial
Abstract
:1. Introduction
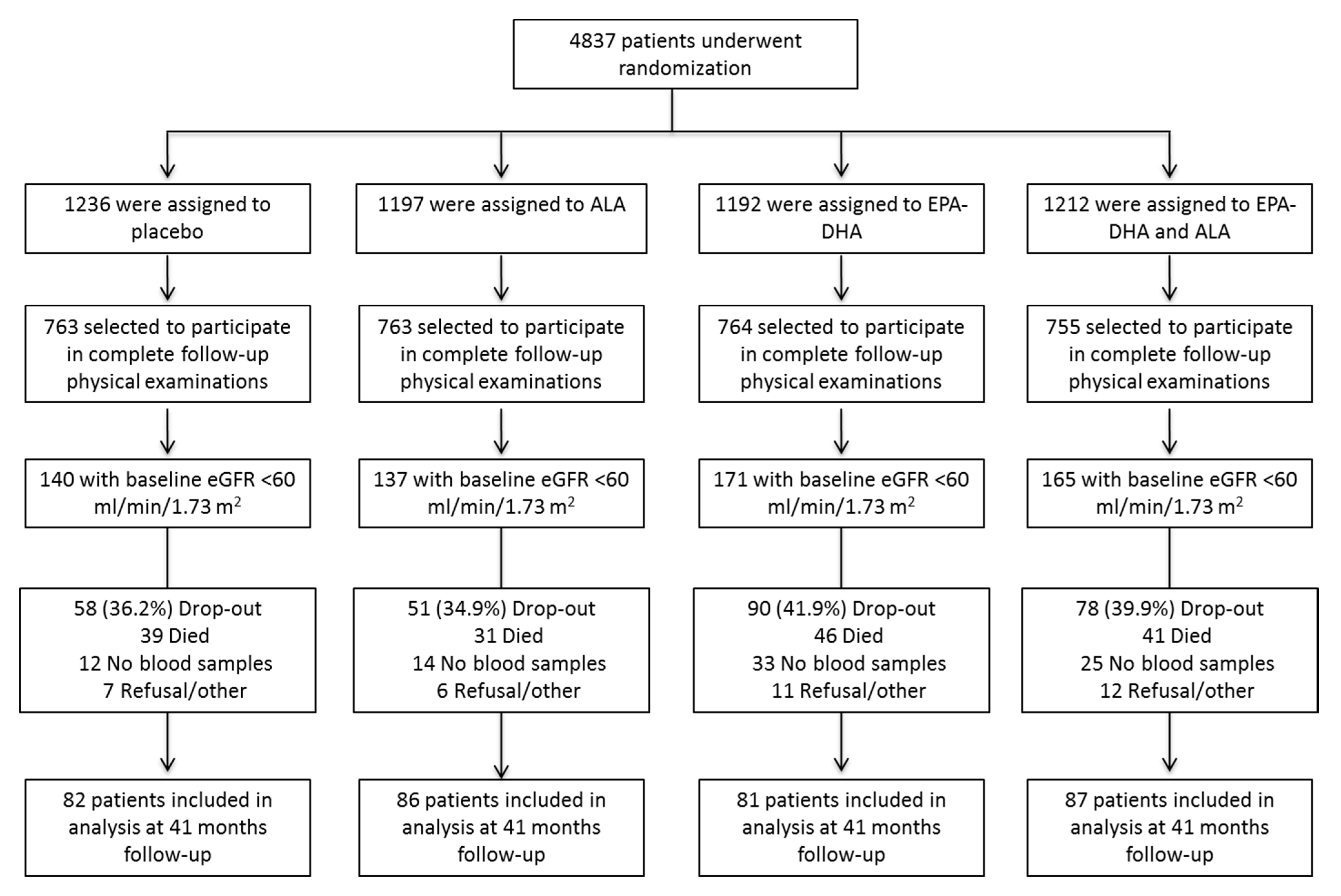
2. Materials and Methods
2.1. Design of the Alpha Omega Trial
2.2. Laboratory Measurements
2.3. Demographic and Clinical Data Collection
3. Statistical Analyses
4. Results
4.1. Characteristics of the Study Population
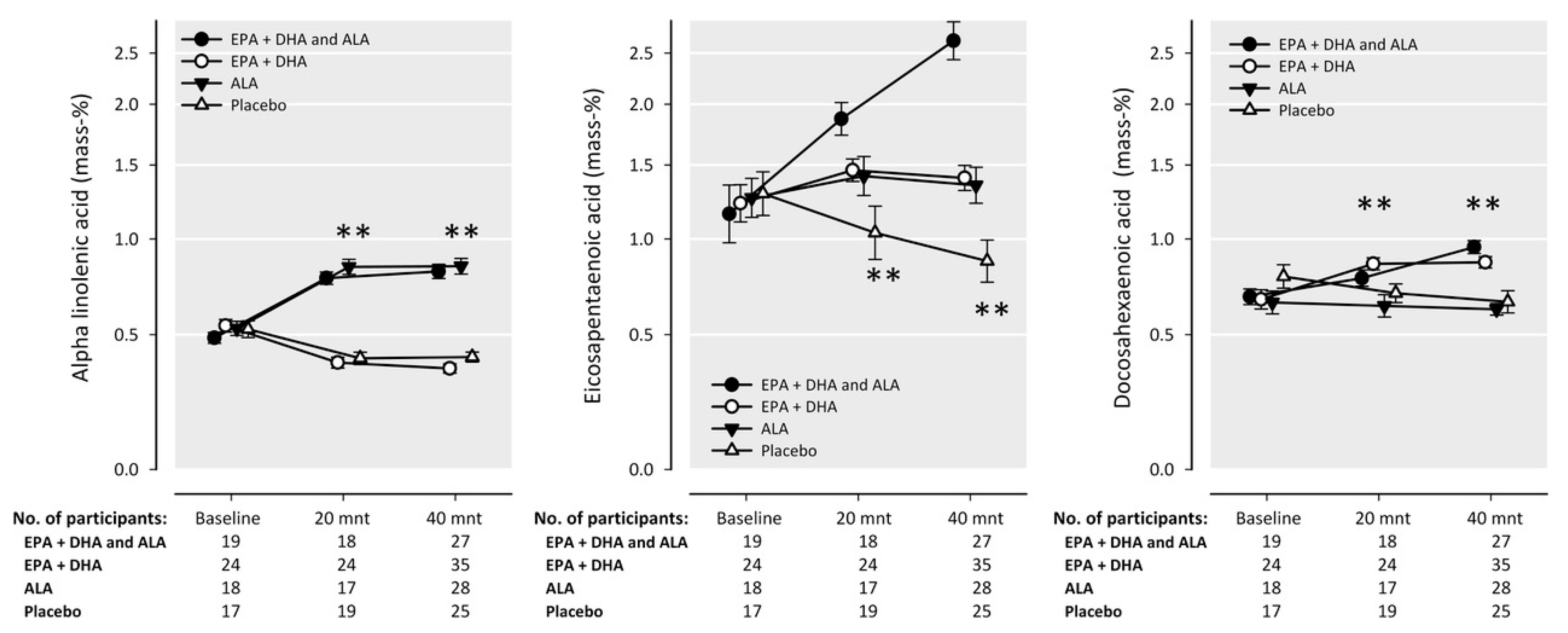
4.2. Intake of n-3 Fatty Acids
4.3. Effect of n-3 Fatty Acids on Plasma FGF23 Levels
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic Kidney Disease: Global Dimension and Perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Freeman, R.V.; Mehta, R.H.; Al Badr, W.; Cooper, J.V.; Kline-Rogers, E.; Eagle, K.A. Influence of Concurrent Renal Dysfunction on Outcomes of Patients with Acute Coronary Syndromes and Implications of the use of Glycoprotein IIb/IIIa Inhibitors. J. Am. Coll. Cardiol. 2003, 41, 718–724. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney Disease as a Risk Factor for Development of Cardiovascular Disease: A Statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef] [PubMed]
- Longenecker, J.C.; Coresh, J.; Powe, N.R.; Levey, A.S.; Fink, N.E.; Martin, A.; Klag, M.J. Traditional Cardiovascular Disease Risk Factors in Dialysis Patients Compared with the General Population: The CHOICE Study. J. Am. Soc. Nephrol. 2002, 13, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.; Sarnak, M.J.; Yan, G.; Dwyer, J.T.; Heyka, R.J.; Rocco, M.V.; Teehan, B.P.; Levey, A.S. Atherosclerotic Cardiovascular Disease Risks in Chronic Hemodialysis Patients. Kidney Int. 2000, 58, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary Calcium as a Predictor of Coronary Events in Four Racial or Ethnic Groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- London, G.M.; Guerin, A.P.; Marchais, S.J.; Metivier, F.; Pannier, B.; Adda, H. Arterial Media Calcification in End-Stage Renal Disease: Impact on all-Cause and Cardiovascular Mortality. Nephrol. Dial. Transplant. 2003, 18, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.; Reichart, B.; Roth, D.; Seibert, E.; Fliser, D.; Heine, G.H. FGF-23 and Future Cardiovascular Events in Patients with Chronic Kidney Disease before Initiation of Dialysis Treatment. Nephrol. Dial. Transplant. 2010, 25, 3983–3989. [Google Scholar] [CrossRef] [PubMed]
- Baia, L.C.; Humalda, J.K.; Vervloet, M.G.; Navis, G.; Bakker, S.J.; de Borst, M.H.; NIGRAM Consortium. Fibroblast Growth Factor 23 and Cardiovascular Mortality After Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2013, 8, 1968–1978. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Juppner, H.; et al. Fibroblast Growth Factor 23 and Mortality among Patients Undergoing Hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Xie, H.; Rahman, M.; Anderson, A.H.; Isakova, T.; Ojo, A.; Zhang, X.; Nessel, L.; Hamano, T.; Grunwald, J.E.; et al. Fibroblast Growth Factor-23 and Cardiovascular Events in CKD. J. Am. Soc. Nephrol. 2014, 25, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Humalda, J.K.; Lambers Heerspink, H.J.; Kwakernaak, A.J.; Slagman, M.C.; Waanders, F.; Vervloet, M.G.; Ter Wee, P.M.; Navis, G.; de Borst, M.H.; NIGRAM Consortium. Fibroblast Growth Factor 23 and the Antiproteinuric Response to Dietary Sodium Restriction during Renin-Angiotensin-Aldosterone System Blockade. Am. J. Kidney Dis. 2015, 65, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Baia, L.C.; Van den Berg, E.; Vervloet, M.G.; Heilberg, I.P.; Navis, G.; Bakker, S.J.; Geleijnse, J.M.; Kromhout, D.; Soedamah-Muthu, S.S.; De Borst, M.H.; et al. Fish and Omega-3 Fatty Acid Intake in Relation to Circulating Fibroblast Growth Factor 23 Levels in Renal Transplant Recipients. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, E.K.; Geleijnse, J.M.; Kromhout, D.; Stijnen, T.; Gemen, E.F.; Kusters, R.; Giltay, E.J. Effect of Omega-3 Fatty Acids on Kidney Function after Myocardial Infarction: The Alpha Omega Trial. Clin. J. Am. Soc. Nephrol. 2014, 9, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Schmidt, E.B.; Jorgensen, K.A.; Christensen, J.H.; OPACH Study Group. N-3 Fatty Acids as Secondary Prevention against Cardiovascular Events in Patients Who Undergo Chronic Hemodialysis: A Randomized, Placebo-Controlled Intervention Trial. Clin. J. Am. Soc. Nephrol. 2006, 1, 780–786. [Google Scholar] [CrossRef] [PubMed]
- An, W.S.; Lee, S.M.; Son, Y.K.; Kim, S.E.; Kim, K.H.; Han, J.Y.; Bae, H.R.; Rha, S.H.; Park, Y. Omega-3 Fatty Acid Supplementation Increases 1,25-Dihydroxyvitamin D and Fetuin-A Levels in Dialysis Patients. Nutr. Res. 2012, 32, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Giltay, E.J.; Geleijnse, J.M.; Alpha Omega Trial Group. N-3 Fatty Acids and Cardiovascular Events after Myocardial Infarction. N. Engl. J. Med. 2010, 363, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M.; Giltay, E.J.; Schouten, E.G.; de Goede, J.; Oude Griep, L.M.; Teitsma-Jansen, A.M.; Katan, M.B.; Kromhout, D.; Alpha Omega Trial Group. Effect of Low Doses of N-3 Fatty Acids on Cardiovascular Diseases in 4837 Post-Myocardial Infarction Patients: Design and Baseline Characteristics of the Alpha Omega Trial. Am. Heart J. 2010, 159, 539–546.e2. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Mozaffarian, D.; Lefevre, M.; Toner, C.D.; Colombo, J.; Cunnane, S.C.; Holden, J.M.; Klurfeld, D.M.; Morris, M.C.; Whelan, J. Towards Establishing Dietary Reference Intakes for Eicosapentaenoic and Docosahexaenoic Acids. J. Nutr. 2009, 139, 804S–819S. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Innis, S.; Ammerican Dietetic, A.; Dietitians of, C. Position of the American Dietetic Association and Dietitians of Canada: Dietary Fatty Acids. J. Am. Diet. Assoc. 2007, 107, 1599–1611. [Google Scholar] [PubMed]
- Simopoulos, A.P.; Leaf, A.; Salem, N., Jr. Workshop on the Essentiality of and Recommended Dietary Intakes for Omega-6 and Omega-3 Fatty Acids. J. Am. Coll. Nutr. 1999, 18, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Giltay, E.J.; Geleijnse, J.M.; Schouten, E.G.; Katan, M.B.; Kromhout, D. High Stability of Markers of Cardiovascular Risk in Blood Samples. Clin. Chem. 2003, 49, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Greene, T.; Marsh, J.; Stevens, L.A.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology Collaboration. Expressing the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate with Standardized Serum Creatinine Values. Clin. Chem. 2007, 53, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A.; Blirup-Jensen, S.; Lindstrom, V.; Schmidt, C.; Althaus, H.; Zegers, I.; IFCC Working Group on Standardisation of Cystatin C (WG-SCC). First Certified Reference Material for Cystatin C in Human Serum ERM-DA471/IFCC. Clin. Chem. Lab. Med. 2010, 48, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Heijboer, A.C.; Levitus, M.; Vervloet, M.G.; Lips, P.; Ter Wee, P.M.; Dijstelbloem, H.M.; Blankenstein, M.A. Determination of Fibroblast Growth Factor 23. Ann. Clin. Biochem. 2009, 46, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, E.K.; Geleijnse, J.M.; Kromhout, D.; Giltay, E.J. No Effect of N-3 Fatty Acids on High-Sensitivity C-Reactive Protein after Myocardial Infarction: The Alpha Omega Trial. Eur. J. Prev. Cardiol. 2014, 21, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Glatz, J.F.; Soffers, A.E.; Katan, M.B. Fatty Acid Composition of Serum Cholesteryl Esters and Erythrocyte Membranes as Indicators of Linoleic Acid Intake in Man. Am. J. Clin. Nutr. 1989, 49, 269–276. [Google Scholar] [PubMed]
- Soedamah-Muthu, S.S.; Geleijnse, J.M.; Giltay, E.J.; Kromhout, D.; Alpha Omega Trial Group. Cardiovascular Risk Factor Management of Myocardial Infarction Patients with and without Diabetes in The Netherlands between 2002 and 2006: A Cross-Sectional Analysis of Baseline Data. BMJ Open 2012, 2, e001360. [Google Scholar] [CrossRef] [PubMed]
- Eisenga, M.F.; van Londen, M.; Leaf, D.E.; Nolte, I.M.; Navis, G.; Bakker, S.J.L.; de Borst, M.H.; Gaillard, C.A.J.M. C-Terminal Fibroblast Growth Factor 23, Iron Deficiency, and Mortality in Renal Transplant Recipients. J. Am. Soc. Nephrol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Altman, D.G. Statistics Notes: Analysing Controlled Trials with Baseline and Follow up Measurements. BMJ 2001, 323, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.; Di Micco, L.; Torraca, S.; Sirico, M.L.; Russo, L.; Pota, A.; Mirenghi, F.; Russo, D. Acute Effects of very-Low-Protein Diet on FGF23 Levels: A Randomized Study. Clin. J. Am. Soc. Nephrol. 2012, 7, 581–587. [Google Scholar] [CrossRef] [PubMed]
- De Borst, M.H.; Vervloet, M.G.; Ter Wee, P.M.; Navis, G. Cross Talk between the Renin-Angiotensin-Aldosterone System and Vitamin D-FGF-23-Klotho in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Izquierdo, M.C.; Sanchez-Nino, M.D.; Suarez-Alvarez, B.; Lopez-Larrea, C.; Jakubowski, A.; Blanco, J.; Ramirez, R.; Selgas, R.; Ruiz-Ortega, M.; et al. The Inflammatory Cytokines TWEAK and TNFalpha Reduce Renal Klotho Expression through NFkappaB. J. Am. Soc. Nephrol. 2011, 22, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Munoz Mendoza, J.; Isakova, T.; Ricardo, A.C.; Xie, H.; Navaneethan, S.D.; Anderson, A.H.; Bazzano, L.A.; Xie, D.; Kretzler, M.; Nessel, L.; et al. Fibroblast Growth Factor 23 and Inflammation in CKD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, M.M.; El-Shehaby, A.R.; Osman, N.A.; Fayad, T.; Nassef, A.; Salem, M.M.; Sharaf El Din, U.A. The Association between Fibroblast Growth Factor-23 and Vascular Calcification is Mitigated by Inflammation Markers. Nephron Extra 2013, 3, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA Reduce LPS-Induced Inflammation Responses in HK-2 Cells: Evidence for a PPAR-Gamma-Dependent Mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Taccone-Gallucci, M.; Manca-di-Villahermosa, S.; Battistini, L.; Stuffler, R.G.; Tedesco, M.; Maccarrone, M. N-3 PUFAs Reduce Oxidative Stress in ESRD Patients on Maintenance HD by Inhibiting 5-Lipoxygenase Activity. Kidney Int. 2006, 69, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Takeshita, Y.; Murohara, T.; Sasaki, K.; Egami, K.; Shintani, S.; Katsuda, Y.; Ikeda, H.; Nabeshima, Y.; Imaizumi, T. Angiogenesis and Vasculogenesis are Impaired in the Precocious-Aging Klotho Mouse. Circulation 2004, 110, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, H.; Sasaki, S.; Kuwahara, N.; Irie, H.; Kusaba, T.; Kameyama, H.; Tamagaki, K.; Hatta, T.; Takeda, K.; Matsubara, H. HMG-CoA Reductase Inhibitors up-Regulate Anti-Aging Klotho mRNA via RhoA Inactivation in IMCD3 Cells. Cardiovasc. Res. 2004, 64, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.; Christensen, J.H.; Blom, M.; Schmidt, E.B. The Effect of Dietary N-3 Fatty Acids on Serum Concentrations of C-Reactive Protein: A Dose-Response Study. Br. J. Nutr. 2003, 89, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Geelen, A.; Brouwer, I.A.; Schouten, E.G.; Kluft, C.; Katan, M.B.; Zock, P.L. Intake of N-3 Fatty Acids from Fish does not Lower Serum Concentrations of C-Reactive Protein in Healthy Subjects. Eur. J. Clin. Nutr. 2004, 58, 1440–1442. [Google Scholar] [CrossRef] [PubMed]
- Madsen, T.; Christensen, J.H.; Schmidt, E.B. C-Reactive Protein and N-3 Fatty Acids in Patients with a Previous Myocardial Infarction: A Placebo-Controlled Randomized Study. Eur. J. Nutr. 2007, 46, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Zock, P.L.; Mensink, R.P.; Harryvan, J.; de Vries, J.H.; Katan, M.B. Fatty Acids in Serum Cholesteryl Esters as Quantitative Biomarkers of Dietary Intake in Humans. Am. J. Epidemiol. 1997, 145, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.R.; Grams, M.E. Serum Phosphorus and Mortality in the Third National Health and Nutrition Examination Survey (NHANES III): Effect Modification by Fasting. Am. J. Kidney Dis. 2014, 64, 567–573. [Google Scholar] [CrossRef] [PubMed]
| EPA-DHA and ALA (n = 87) | EPA-DHA (n =8 1) | ALA (n = 86) | Placebo (n = 82) | |
|---|---|---|---|---|
| Age (y) | 73.0 ± 5.0 | 73.5 ± 4.7 | 73.1 ± 4.8 | 72.2 ± 4.8 |
| BMI (kg/m2) | 28.2 ± 4.6 | 27.7 ± 4.3 | 28.7 ± 4.1 | 28.1 ± 4.1 |
| Time since MI (y) | 4.7 ± 3.0 | 4.4 ± 3.6 | 4.7 ± 2.8 | 4.2 ± 3.0 |
| Systolic BP (mmHg) | 144.3 ± 25.6 | 141.5 ± 22.7 | 145.6 ± 20.7 | 143.6 ± 24.0 |
| Diastolic BP (mmHg) | 79.1 ± 10.8 | 78.7 ± 11.1 | 77.5 ± 11.3 | 78.1 ± 11.3 |
| Glucose (mmol/L) | 5.8 ± 1.4 | 5.9 ± 1.7 | 6.6 ± 2.6 | 6.3 ± 2.0 |
| Total serum cholesterol (mmol/L) | 4.9 ± 1.1 | 4.9 ± 1.2 | 5.0 ± 1.1 | 5.1 ± 0.9 |
| LDL-cholesterol (mmol/L) | 2.7 ± 0.8 | 2.8 ± 1.0 | 2.8 ± 1.0 | 2.9 ± 0.8 |
| HDL-cholesterol (mmol/L) | 1.2 ± 0.3 | 1.3 ± 0.4 | 1.2 ± 0.3 | 1.2 ± 0.3 |
| Triglycerides (mmol/L) | 1.9 (1.3, 2.5) | 1.8 (1.4, 2.2) | 2.0 (1.5, 2.6) | 1.9 (1.5, 2.6) |
| Protein intake (g/kg body weight) | 0.80 ± 0.23 | 0.81 ± 0.27 | 0.79 ± 0.22 | 0.81 ± 0.24 |
| Fish intake (g/day) | 9.9 (1.4, 17.5) | 9.7 (4.2, 18.3) | 11.0 (4.6, 18.2) | 15.0 (5.0, 22.7) |
| EPA + DHA (mg/day) | 76.0 (33.9, 154.1) | 69.1 (25.5, 150.8) | 98.8 (42.6, 166.6) | 114.9 (51.7, 194.2) |
| Serum cystatin C (mg/L) | 1.4 ± 0.3 | 1.4 ± 0.2 | 1.4 ± 0.3 | 1.4 ± 0.4 |
| Serum creatinine (µmol/L) | 132.5 ± 53.4 | 125.7 ± 33.4 | 127.5 ± 32.9 | 130.0 ± 45.6 |
| hsCRP (mg/L) | 2.8 (1.5, 5.7) | 3.1 (1.4, 6.4) | 3.1 (1.3, 5.2) | 2.9 (1.5, 5.9) |
| Sex (men) | 71 (62) | 68 (55) | 65 (56) | 71 (58) |
| Ethnicity, white | 99 (86) | 99 (80) | 99 (85) | 100 (82) |
| Current Smokers | 14 (12) | 16 (13) | 12 (10) | 13 (11) |
| Alcohol use | ||||
| none | 14 (11) | 12 (9) | 4 (3) | 7 (5) |
| <10 g/day | 55 (44) | 61 (46) | 65 (52) | 57 (43) |
| ≥10–20 g/day | 11 (9) | 15 (11) | 18 (14) | 18 (14) |
| ≥20 g/day | 20 (16) | 13 (10) | 14 (11) | 18 (14) |
| Education | ||||
| Low | 16 (14) | 30 (24) | 23 (19) | 32 (26) |
| Middle | 70 (61) | 62 (50) | 69 (58) | 60 (49) |
| High | 14 (12) | 9 (7) | 8 (7) | 9 (7) |
| Diabetes * | 24 (21) | 22 (18) | 37 (32) | 27 (22) |
| Obesity | 29 (25) | 31 (25) | 33 (28) | 28 (23) |
| Antihypertensive medication | 98 (85) | 95 (77) | 95 (82) | 93 (76) |
| ACE-inhibitor and/or ARB | 68 (59) | 52 (42) | 71 (61) | 66 (54) |
| Statins | 81 (70) | 78 (63) | 79 (68) | 81 (66) |
| Physically active | ||||
| No | 8 (7) | 10 (8) | 11 (9) | 11 (9) |
| Light active (<3 MET) | 47 (40) | 45 (36) | 38 (32) | 48 (39) |
| 0–5 days moderate/vigorously active (≥3 MET) | 31 (26) | 30 (24) | 29 (24) | 33 (27) |
| ≥5 days moderate/vigorously active (≥3 MET) | 14 (12) | 15 (12) | 23 (19) | 9 (7) |
| Pre-Treatment (95% CI) a | Post-Treatment (95% CI) a | Post-Treatment Adjusted for Pre-Treatment (95% CI) a | Treatment Effect (95% CI) b | p-Value c | |
|---|---|---|---|---|---|
| Placebo (n = 82) | 159.0 (111.1, 206.9) | 201.1 (146.8, 255.3) **,d | 197.7 (141.5, 254.0) | ||
| Active intervention groups | |||||
| EPA-DHA (n = 81) | 179.1 (108.7, 249.5) | 191.0 (152.0, 230.0) ** | 180.3 (123.6, 237.0) | −17 (−97, 62) | 0.7 |
| ALA (n = 86) | 136.5 (115.9, 157.0) | 229.2 (157.8, 300.7) *** | 234.1 (179.2, 289.1) | 36 (−42, 115) | 0.4 |
| EPA-DHA plus ALA (n = 87) | 127.1 (103.2, 151.1) | 223.6 (162.0, 285.2) *** | 231.9 (177.2, 286.6) | 34 (−44, 113) | 0.4 |
| Pre-Treatment (95% CI) a | Post-Treatment (95% CI) a | Post-Treatment Adjusted for Pre-Treatment (95% CI) a | Treatment Effect (95% CI) b | p-Value c | |
|---|---|---|---|---|---|
| n-3 fatty acids group combined vs. placebo (n = 254) | 146.9 (122.2, 171.6) | 215.1 (181.1, 249.1) | 216.2 (184.2, 248.1) | 18 (−46, 83) | 0.6 |
| ALA or combination of EPA-DHA and ALA vs. placebo or EPA-DHA only (n = 173 vs. 163) d | 131.8 (116.1, 147.4) | 226.4 (179.7, 273.0) | 233.0 (194.3, 271.7) | 44 (−12, 100) | 0.1 |
| EPA-DHA or combination of EPA-DHA and ALA vs. placebo or ALA only (n = 168 vs. 168) d | 152.2 (116.2, 188.1) | 207.9 (171.2, 244.6) | 207.1 (167.7, 246.4) | −9 (−64, 46) | 0.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Borst, M.H.; Baia, L.C.; Hoogeveen, E.K.; Giltay, E.J.; Navis, G.; Bakker, S.J.L.; Geleijnse, J.M.; Kromhout, D.; Soedamah-Muthu, S.S. Effect of Omega-3 Fatty Acid Supplementation on Plasma Fibroblast Growth Factor 23 Levels in Post-Myocardial Infarction Patients with Chronic Kidney Disease: The Alpha Omega Trial. Nutrients 2017, 9, 1233. https://doi.org/10.3390/nu9111233
De Borst MH, Baia LC, Hoogeveen EK, Giltay EJ, Navis G, Bakker SJL, Geleijnse JM, Kromhout D, Soedamah-Muthu SS. Effect of Omega-3 Fatty Acid Supplementation on Plasma Fibroblast Growth Factor 23 Levels in Post-Myocardial Infarction Patients with Chronic Kidney Disease: The Alpha Omega Trial. Nutrients. 2017; 9(11):1233. https://doi.org/10.3390/nu9111233
Chicago/Turabian StyleDe Borst, Martin H., Leandro C. Baia, Ellen K. Hoogeveen, Erik J. Giltay, Gerjan Navis, Stephan J. L. Bakker, Johanna M. Geleijnse, Daan Kromhout, and Sabita S. Soedamah-Muthu. 2017. "Effect of Omega-3 Fatty Acid Supplementation on Plasma Fibroblast Growth Factor 23 Levels in Post-Myocardial Infarction Patients with Chronic Kidney Disease: The Alpha Omega Trial" Nutrients 9, no. 11: 1233. https://doi.org/10.3390/nu9111233





