Dietary Pea Fiber Supplementation Improves Glycemia and Induces Changes in the Composition of Gut Microbiota, Serum Short Chain Fatty Acid Profile and Expression of Mucins in Glucose Intolerant Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Experimental Diets and Tissue Collection
2.2. Oral Glucose Tolerance Test
2.3. DNA Extraction and Microbial Profiling
Bioinformatics
2.4. RNA Extraction and Real-Time Polymerase Chain Reaction
2.5. SCFA Measurement
2.6. Statistical Analysis
3. Results
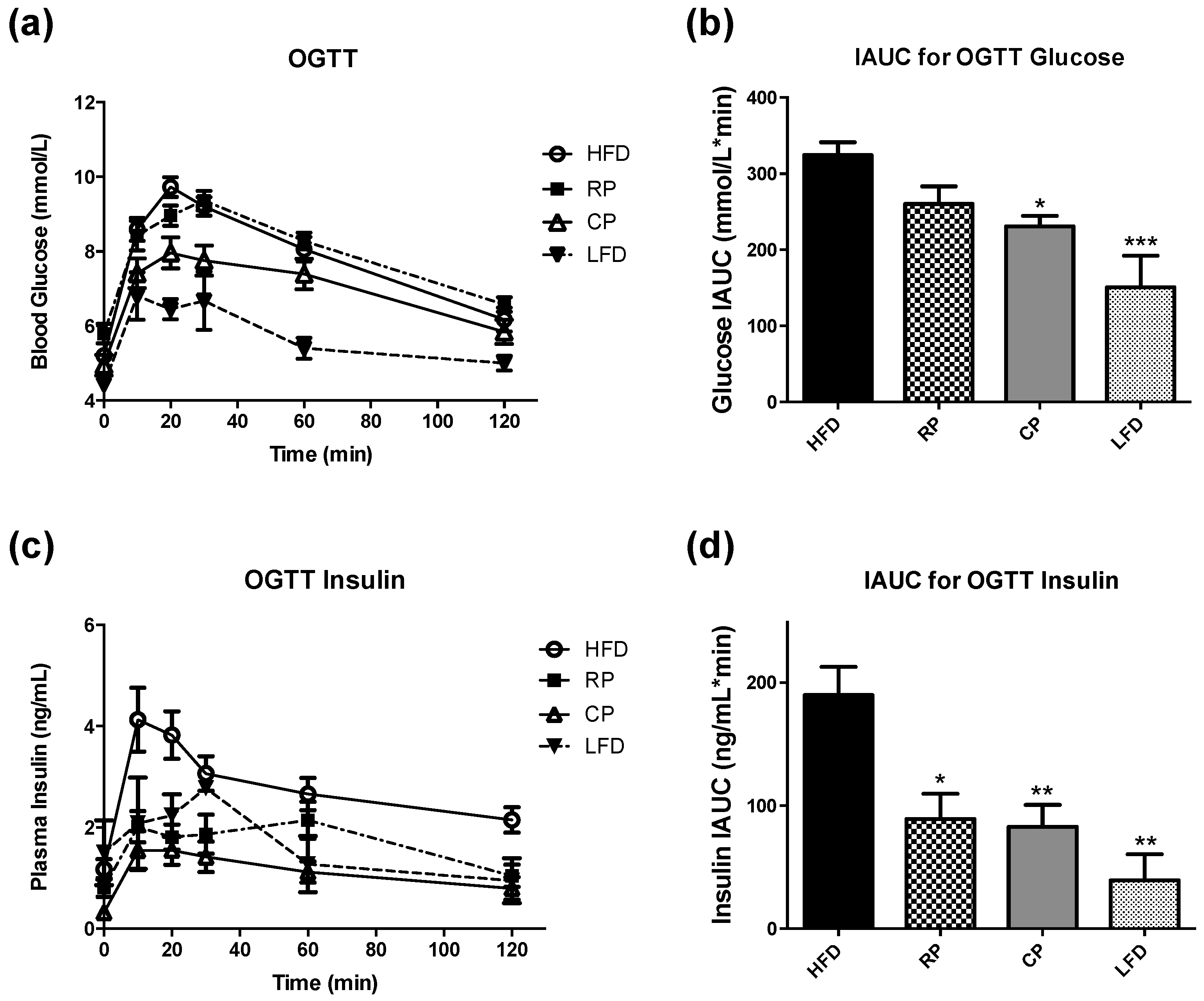
3.1. Oral Glucose Tolerance Test and Insulin Measurement
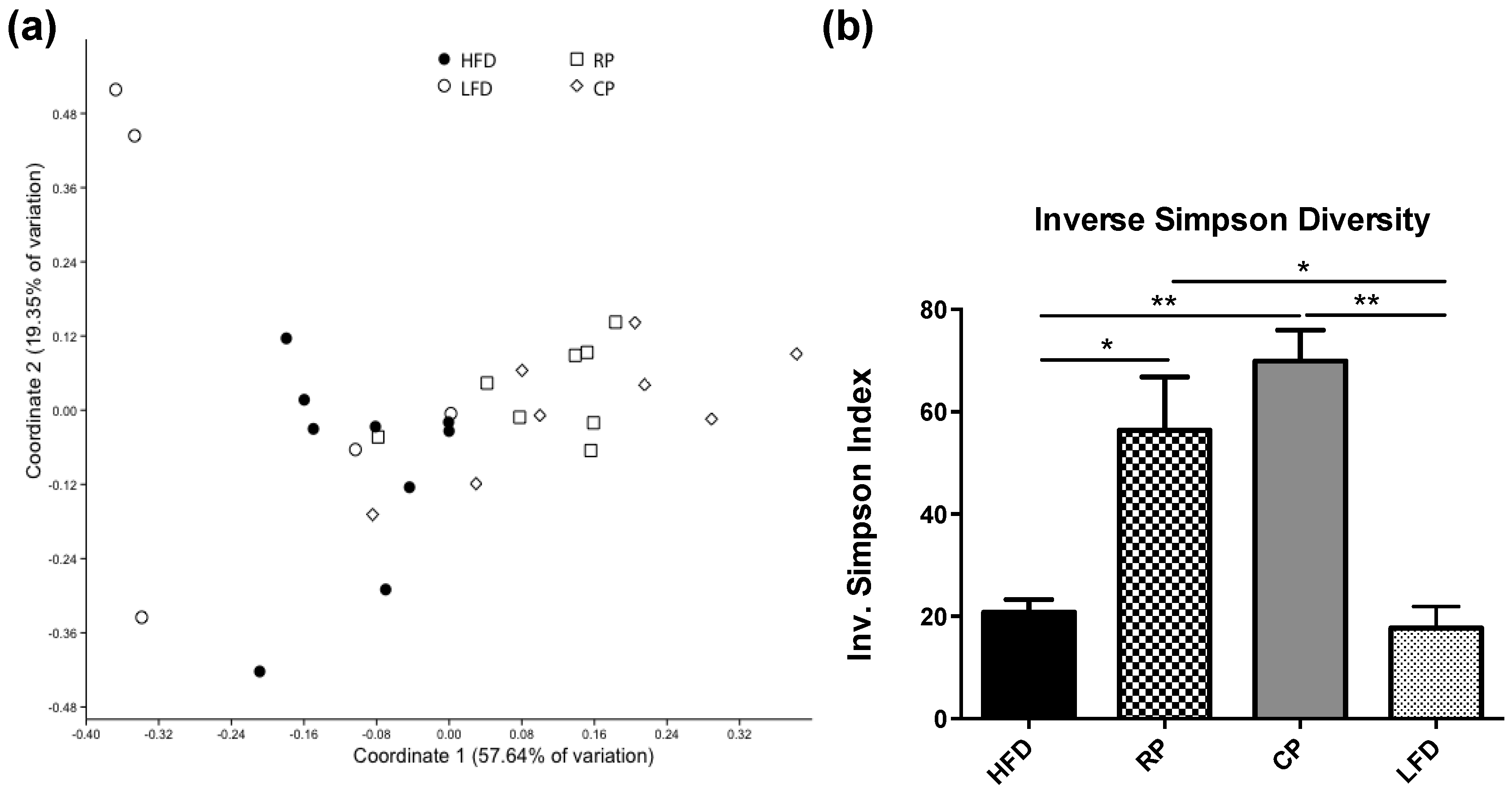
3.2. Microbial Community Structure
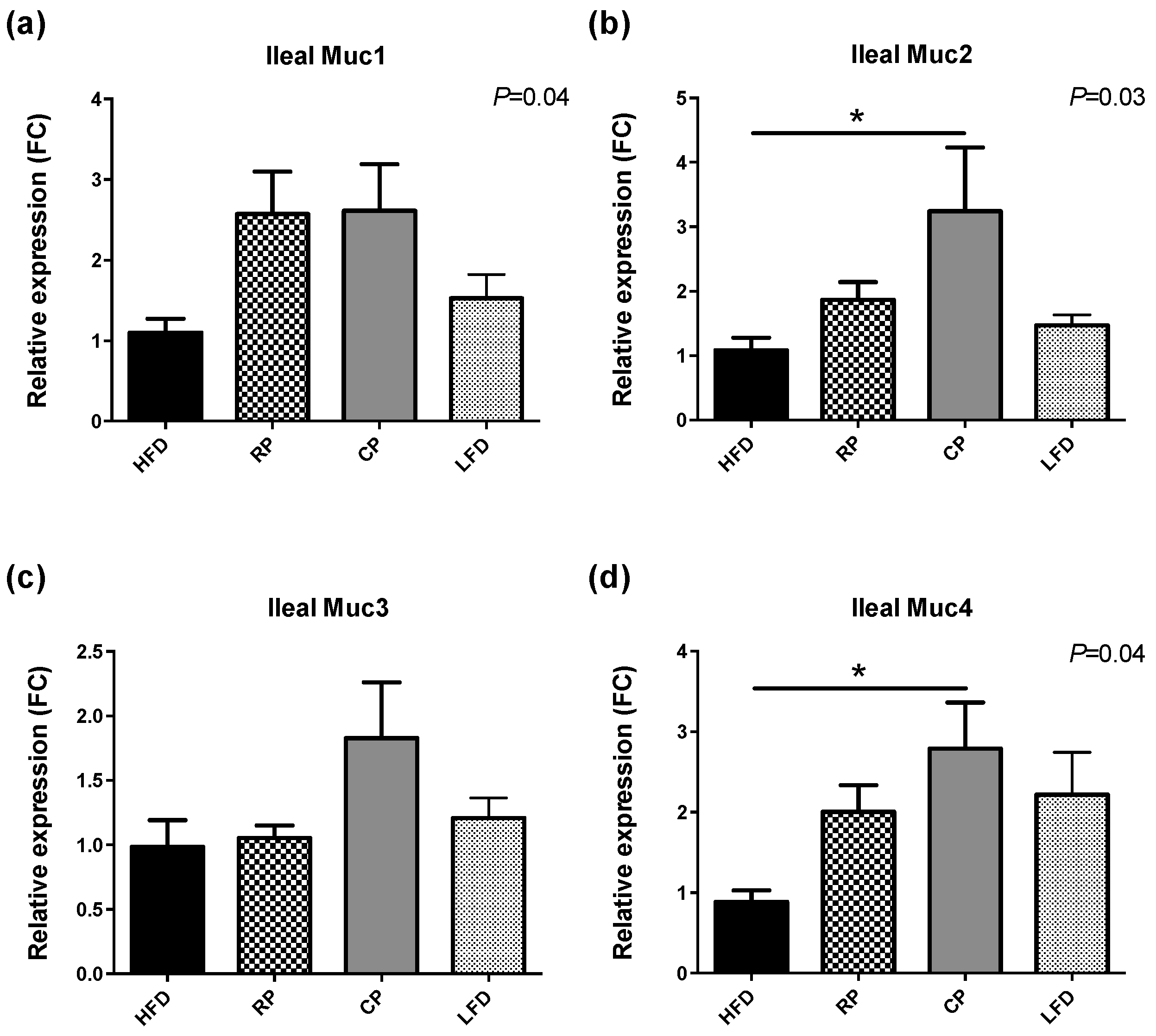
3.3. Gene Expression of TLR, Tight Junction Proteins, and Mucins
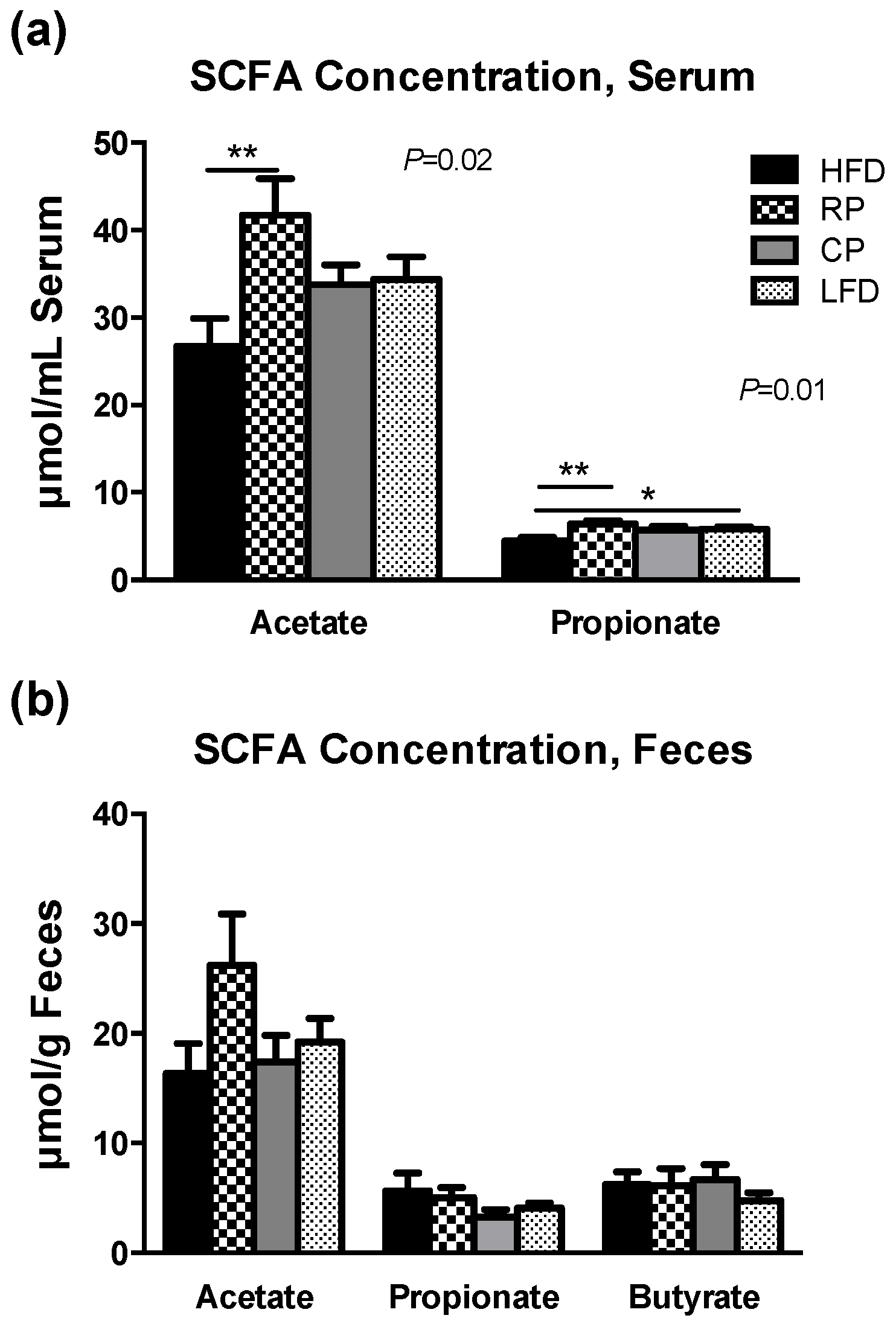
3.4. SCFA in Serum and Fecal Samples
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian diabetes association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Can. J. Diabetes 2013, 37 (Suppl. S1), S1–S212. [Google Scholar]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108 (Suppl. S1), S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Marinangeli, C.P.; Krause, D.; Harding, S.V.; Rideout, T.C.; Zhu, F.; Jones, P.J.H. Whole and fractionated yellow pea flours modulate insulin, glucose, oxygen consumption, and the caecal microbiome in Golden Syrian hamsters. Appl. Physiol. Nutr. Metab. 2011, 36, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, K.A.; Kozicky, L.; Jin, A.; Yee, H.; Ha, C.; Morris, J.; Field, C.J.; Bell, R.C.; Ozga, J.A.; Chan, C.B. Assessment of the mechanisms exerting glucose-lowering effects of dried peas in glucose-intolerant rats. Br. J. Nutr. 2012, 108 (Suppl. S1), S91–S102. [Google Scholar] [CrossRef] [PubMed]
- Mollard, R.C.; Luhovyy, B.L.; Smith, C.; Anderson, G.H. Acute effects of pea protein and hull fibre alone and combined on blood glucose, appetite, and food intake in healthy young men—A randomized crossover trial. Appl. Physiol. Nutr. Metab. 2014, 39, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, Z.; Yang, K.; Yang, H.; Jin, A.; Ozga, J.; Chan, C.B. Cooking enhances beneficial effects of pea seed coat consumption on glucose tolerance, incretin, and pancreatic hormones in high-fat-diet-fed rats. Appl. Physiol. Nutr. Metab. 2015, 40, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Da S Queiroz-Monici, K.; Costa, G.E.; da Silva, N.; Reis, S.M.; de Oliveira, A.C. Bifidogenic effect of dietary fiber and resistant starch from leguminous on the intestinal microbiota of rats. Nutrition 2005, 21, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Eslinger, A.J.; Eller, L.K.; Reimer, R.A. Yellow pea fiber improves glycemia and reduces Clostridium leptum in diet-induced obese rats. Nutr. Res. 2014, 34, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Voreades, N.; Kozil, A.; Weir, T.L. Diet and the development of the human intestinal microbiome. Front. Microbiol. 2014, 5, 494. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Van Hee, V.F.; Piront, N.; De Backer, F.; Toussaint, O.; Cani, P.D.; Delzenne, N.M. Wheat-derived arabinoxylan oligosaccharides with prebiotic effect increase satietogenic gut peptides and reduce metabolic endotoxemia in diet-induced obese mice. Nutr. Diabetes 2012, 2, e28. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- De La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.Y.; Ha, C.W.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, O.; Atkinson, M.A.; Neu, J. The “perfect storm” for type 1 diabetes: The complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008, 57, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.P.; Texeira, T.F.; Ferreira, A.B.; Peluzio Mdo, C.; Alfenas Rde, C. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, I.S.; Choue, R. Obesity, inflammation and diet. Pediatr. Gastroenterol. Hepatol. Nutr. 2013, 16, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Geurts, L.; Neyrinck, A.M.; Delzenne, N.M.; Knauf, C.; Cani, P.D. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: Novel insights into molecular targets and interventions using prebiotics. Benef. Microbes 2014, 5, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Rinttila, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Gevers, D.; Westcott, S.L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE 2011, 6, e27310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontologial statistics software package for education. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Vasishtha, H.; Srivastava, R.P. Effect of soaking and cooking on dietary fibre components of different type of chickpea genotypes. J. Food Sci. Technol. 2013, 50, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.V.; Sapirstein, H.D.; Rideout, T.C.; Marinangeli, C.P.; Dona, A.K.; Jones, P.J. Consumption of wheat bran modified by autoclaving reduces fat mass in hamsters. Eur. J. Nutr. 2014, 53, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Marconi, E.; Ruggeri, S.; Cappelloni, M.; Leonardi, D.; Carnovale, E. Physicochemical, nutritional, and microstructural characteristics of chickpeas (Cicer arietinum L.) and common beans (Phaseolus vulgaris L.) following microwave cooking. J. Agric. Food Chem. 2000, 48, 5986–5994. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Umu, O.C.; Frank, J.A.; Fangel, J.U.; Oostindjer, M.; da Silva, C.S.; Bolhuis, E.J.; Bosch, G.; Willats, W.G.T.; Pope, P.B.; Diep, D.B. Resistant starch diet induces change in the swine microbiome and a predominance of beneficial bacterial populations. Microbiome 2015, 3, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.B.; Gupta, J.; Kozicky, L.; Hashemi, Z.; Yang, K. Improved glucose tolerance in insulin-resistant rats after pea hull feeding is associated with changes in lipid metabolism-targeted transcriptome. Appl. Physiol. Nutr. Metab. 2014, 39, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [PubMed]
- Boets, E.; Deroover, L.; Houben, E.; Vermeulen, K.; Gomand, S.V.; Delcour, J.A.; Verbeke, K. Quantification of in Vivo Colonic Short Chain Fatty Acid Production from Inulin. Nutrients 2015, 7, 8916–8929. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, E.; Roos, S.; Liu, H.Y.; Lindberg, J.E. Fermentable non-starch polysaccharides increases the abundance of Bacteroides-Prevotella-Porphyromonas in ileal microbial community of growing pigs. Animal 2014, 8, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.L.; Hergert, N.; Rompato, G.; Lefevre, M. Whole grain oats improve insulin sensitivity and plasma cholesterol profile and modify gut microbiota composition in C57BL/6J mice. J. Nutr. 2015, 145, 222–230. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Tsai, Y.H.; Chow, C.J. Water-insoluble fiber-rich fraction from pineapple peel improves intestinal function in hamsters: Evidence from cecal and fecal indicators. Nutr. Res. 2014, 34, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mao, X.; He, J.; Yu, B.; Huang, Z.; Yu, J.; Zheng, P.; Chen, D. Dietary fibre affects intestinal mucosal barrier function and regulates intestinal bacteria in weaning piglets. Br. J. Nutr. 2013, 110, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Kinght, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 2009, 137, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.F.; Cotter, P.D.; Healy, S.; Marques, T.M.; O’Sullivan, O.; Fouhy, F.; Clarke, S.F.; O’Toole, P.W.; Quigley, E.M.; Stanton, C.; et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Abu AI-Soud, W.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Taras, D.; Schafer, K.; Beijer, S.; Bos, N.A.; Donus, C.; Hardt, P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity 2010, 18, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ma, C.; Han, L.; Nawaz, M.; Gao, F.; Zhang, X.Y.; Yu, P.B.; Zhao, C.A.; Li, L.C.; Zhou, A.P.; et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr. Microbiol. 2010, 61, 69–78. [Google Scholar] [CrossRef] [PubMed]
- McCusker, R.H.; Kelley, K.W. Immune-neural connections: How the immune system’s response to infectious agents influences behavior. J. Exp. Biol. 2013, 216, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Toll-like receptors; their physiological role and signal transduction system. Int. Immunopharmacol. 2001, 1, 625–635. [Google Scholar] [CrossRef]
- Murakami, K.; Bujo, H.; Unoki, H.; Saito, Y. High fat intake induces a population of adipocytes to co-express TLR2 and TNFalpha in mice with insulin resistance. Biochem. Biophys. Res. Commun. 2007, 354, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Adam, C.L.; Gratz, S.W.; Peinado, D.I.; Thomson, L.M.; Garden, K.E.; Williams, P.A.; Richardson, A.J.; Ross, A.W. Effects of Dietary Fibre (Pectin) and/or Increased Protein (Casein or Pea) on Satiety, Body Weight, Adiposity and Caecal Fermentation in High Fat Diet-Induced Obese Rats. PLoS ONE 2016, 11, e0155871. [Google Scholar] [CrossRef] [PubMed]
- Spreckley, E.; Murphy, K.G. The L-Cell in Nutritional Sensing and the Regulation of Appetite. Front. Nutr. 2015, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Mastrodonato, M.; Mentino, D.; Portincasa, P.; Calamita, G.; Liquori, G.E.; Ferri, D. High-fat diet alters the oligosaccharide chains of colon mucins in mice. Histochem. Cell Biol. 2014, 142, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.D.; Atay, C.; Heringer, J.; Romrig, F.K.; Schwitalla, S.; Aydin, B.; Ziegler, P.K.; Varga, J.; Reindl, W.; Pommerenke, C.; et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature 2014, 514, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.K.; Florin, T.H.; McGuckin, M.A. Mucin dynamics in intestinal bacterial infection. PLoS ONE 2008, 3, e3952. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. Accurate taxonomy assignments from 16S rRNA sequences produced by highly parallel pyrosequencers. Nucleic Acids Res. 2008, 36, e120. [Google Scholar] [CrossRef] [PubMed]
- Claesson, M.J.; Wang, Q.; O’Sullivan, O.; Greene-Diniz, R.; Cole, J.R.; Ross, R.P.; O’Toole, P.W. Comparison of two next-generation sequencing technologies for resolving highly complex microbiota composition using tandem variable 16S rRNA gene regions. Nucleic Acids Res. 2010, 38, e200. [Google Scholar] [CrossRef] [PubMed]

| HFD | HFD + PSC | LFD | |
|---|---|---|---|
| Canola sterine | 99.5 | 99.5 | 29.85 |
| Flaxseed oil | 6 | 6 | 1.8 |
| Sunflower oil | 94.5 | 94.5 | 28.35 |
| Casein | 270 | 263 | 270 |
| Dextrose | 189 | 189 | 255 |
| Corn starch | 169 | 169 | 245 |
| Cellulose | 100 | 0 | 100 |
| Pea seed coat (dwt) 1 | 0 | 107 | 0 |
| L-methionine | 2.5 | 2.5 | 2.5 |
| Mineral mix | 51 | 51 | 51 |
| Vitamin mix | 10 | 10 | 7.6 |
| Inositol | 6.3 | 6.3 | 6.3 |
| Choline chloride | 2.8 | 2.8 | 2.8 |
| Carbohydrate (% dwt) | 36 | 36 | 51 |
| Fat (% dwt) | 20 | 20 | 6 |
| Protein (% dwt) | 27 | 27 | 27 |
| Fiber (% dwt) | 10 | 10 | 10 |
| Title 1 | HFD | RP | CP | LFD | SEM | p Value |
|---|---|---|---|---|---|---|
| Phyla | ||||||
| Firmicutes | 63.9 | 71.3 | 77.4 | 65.6 | 2.01 | 0.06 |
| Bacteroidetes | 34.2 | 25.4 | 19.5 * | 29.6 | 1.62 | 0.023 |
| Proteobacteria | 0.49 | 0.36 | 0.49 | 2.43 | 0.14 | 0.09 |
| TM7 | 0.17 | 0.77 * | 0.65 | 0.34 | 0.19 | 0.024 |
| Tenericutes | 0.11 | 0.57 | 0.35 | 0.31 | 0.31 | 0.36 |
| Deferribacteres | 0.17 | 0.11 | 0.13 | 0.02 | 0.04 | 0.39 |
| Actinobacteria | 0.05 | 0.00 | 0.05 | 0.04 | 0.03 | 0.34 |
| Verrucomicrobia | 0.00 | 0.00 | 0.02 | 0.04 | 0.03 | 0.15 |
| Family | ||||||
| Lachnospiraceae | 34.6 | 51.0 *,† | 55.0 *,† | 24.8 | 2.72 | <0.001 |
| Bacteroidaceae | 12.88 | 10.41 | 6.61 | 17.53 | 2.21 | 0.06 |
| Ruminococcaceae | 19.63 | 11.92 | 12.72 | 16.50 | 1.42 | 0.46 |
| Porphyromonadaceae | 13.4 | 5.45 * | 4.10 * | 3.8 * | 1.49 | 0.01 |
| Clostridiales 1 | 3.53 | 5.48 | 7.01 * | 4.70 | 0.60 | 0.02 |
| Bacteroidales 1 | 4.44 | 5.51 | 5.09 | 4.13 | 0.58 | 0.58 |
| Erysipelotrichaceae | 0.39 † | 0.3 † | 0.17 † | 12.8 | 0.09 | 0.001 |
| Acidaminococcaceae | 0.60 | 0.00 | 0.00 | 3.17 * | 1.64 | 0.001 |
| Lactobacillaceae | 2.33 | 1.31 | 1.16 | 1.13 | 0.50 | 0.98 |
| Peptostreptococcaceae | 2.16 | 1.24 | 0.38 | 0.31 | 0.50 | 0.15 |
| Firmicutes 2 | 0.58 † | 0.36 † | 0.50 † | 1.55 | 0.08 | 0.011 |
| Sutterellaceae | 0.20 | 0.08 | 0.17 | 0.48 | 0.04 | 0.28 |
| Prevotellaceae | 0.11 | 1.23 *,† | 0.69 *,† | 0.02 | 0.39 | <0.001 |
| TM7_family_incertae_sedis | 0.24 | 1.23 | 0.54 | 0.34 | 0.12 | 0.16 |
| Verrucomicrobiaceae | 0.00 | 0.00 | 0.03 | 0.02 | 0.07 | 0.27 |
| Pasteurellaceae | 0.06 | 0.14 | 0.02 | 1.39 | 0.06 | 0.08 |
| Rikenellaceae | 0.16 | 0.17 | 0.02 | 0.06 | 0.32 | 0.14 |
| Anaeroplasmataceae | 0.14 | 0.63 | 0.41 | 0.21 | 0.02 | 0.50 |
| Proteobacteria 2 | 0.08 | 0.03 | 0.08 | 0.00 | 0.06 | 0.61 |
| Peptostreptococcaceae | 0.09 | 0.17 | 0.22 | 0.15 | 0.05 | 0.73 |
| Deferribacteraceae | 0.20 | 0.09 | 0.08 | 0.06 | 0.02 | 0.60 |
| Streptococcaceae | 0.05 | 0.08 | 0.09 | 0.27 | 0.02 | 0.11 |
| Clostrideaceae | 0.05 | 0.02 | 0.02 | 0.38 | 0.03 | 0.66 |
| Enteriobacteriaceae | 0.08 | 0.06 | 0.03 | 0.15 | 0.04 | 0.66 |
| Clostridiales_Incertae_Sedis_XIII | 0.08 | 0.00 | 0.05 | 0.10 | 0.04 | 0.06 |
| Desulfovibrionaceae | 0.02 | 0.09 | 0.03 | 0.10 | 0.06 | 0.37 |
| Coriobacteriaceae | 0.02 | 0.00 | 0.02 | 0.08 | 0.04 | 0.08 |
| Bifidobacteria 3 | 0.123 | 0.004 | 0.003 | 0.003 | 0.09 | 0.72 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemi, Z.; Fouhse, J.; Im, H.S.; Chan, C.B.; Willing, B.P. Dietary Pea Fiber Supplementation Improves Glycemia and Induces Changes in the Composition of Gut Microbiota, Serum Short Chain Fatty Acid Profile and Expression of Mucins in Glucose Intolerant Rats. Nutrients 2017, 9, 1236. https://doi.org/10.3390/nu9111236
Hashemi Z, Fouhse J, Im HS, Chan CB, Willing BP. Dietary Pea Fiber Supplementation Improves Glycemia and Induces Changes in the Composition of Gut Microbiota, Serum Short Chain Fatty Acid Profile and Expression of Mucins in Glucose Intolerant Rats. Nutrients. 2017; 9(11):1236. https://doi.org/10.3390/nu9111236
Chicago/Turabian StyleHashemi, Zohre, Janelle Fouhse, Hyun Seun Im, Catherine B. Chan, and Benjamin P. Willing. 2017. "Dietary Pea Fiber Supplementation Improves Glycemia and Induces Changes in the Composition of Gut Microbiota, Serum Short Chain Fatty Acid Profile and Expression of Mucins in Glucose Intolerant Rats" Nutrients 9, no. 11: 1236. https://doi.org/10.3390/nu9111236





