Tucum-do-Cerrado (Bactris setosa Mart.) May Promote Anti-Aging Effect by Upregulating SIRT1-Nrf2 Pathway and Attenuating Oxidative Stress and Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tucum-do-Cerrado Fruit Collection and Diet Preparation
2.2. Experimental Protocol
2.3. Hematological Parameters
2.4. Iron Status
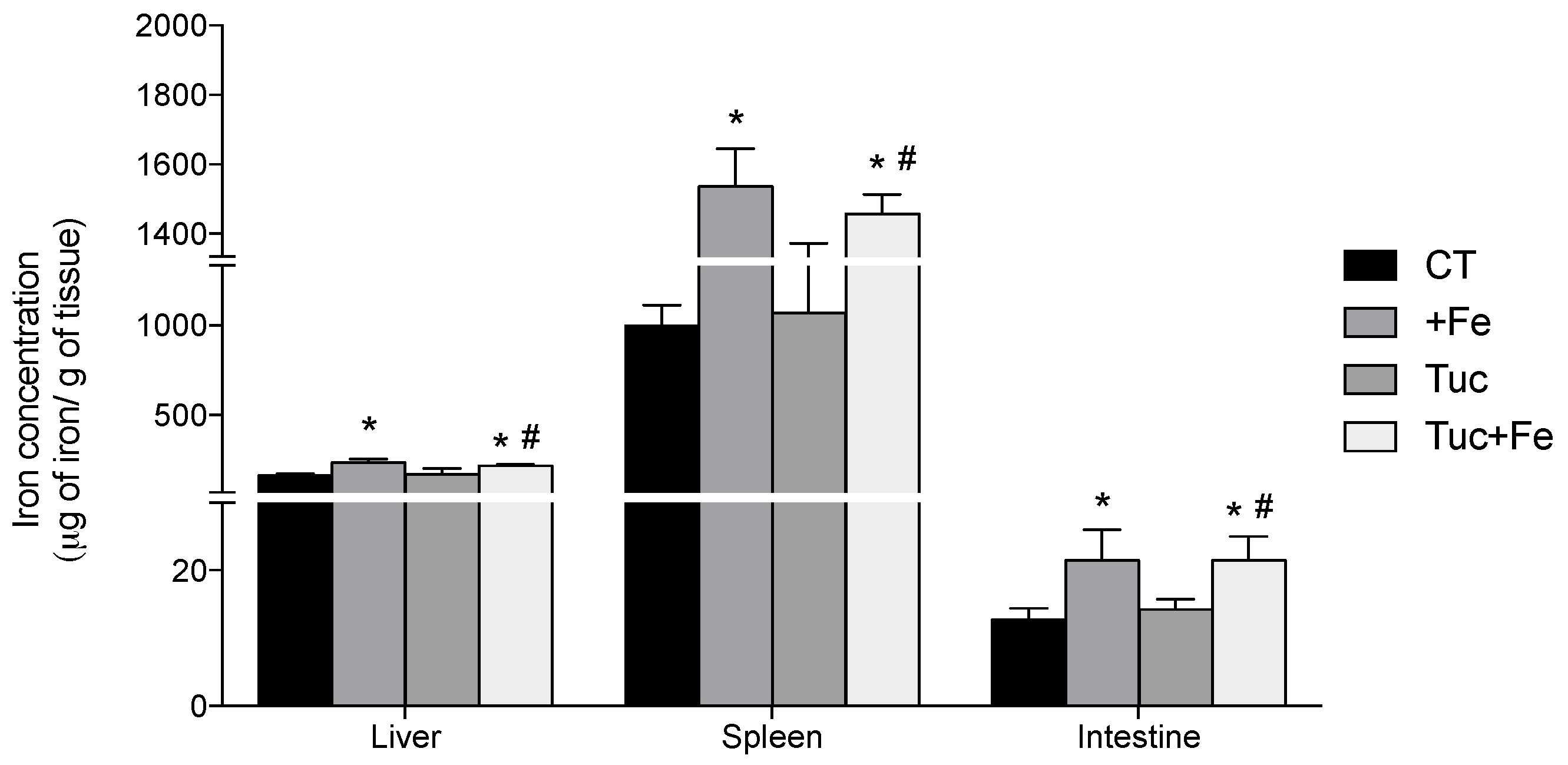
2.4.1. Total Iron Concentration in Tissues
2.4.2. Serum Iron Status
2.5. Oxidative Damage Markers and Antioxidant Capacity
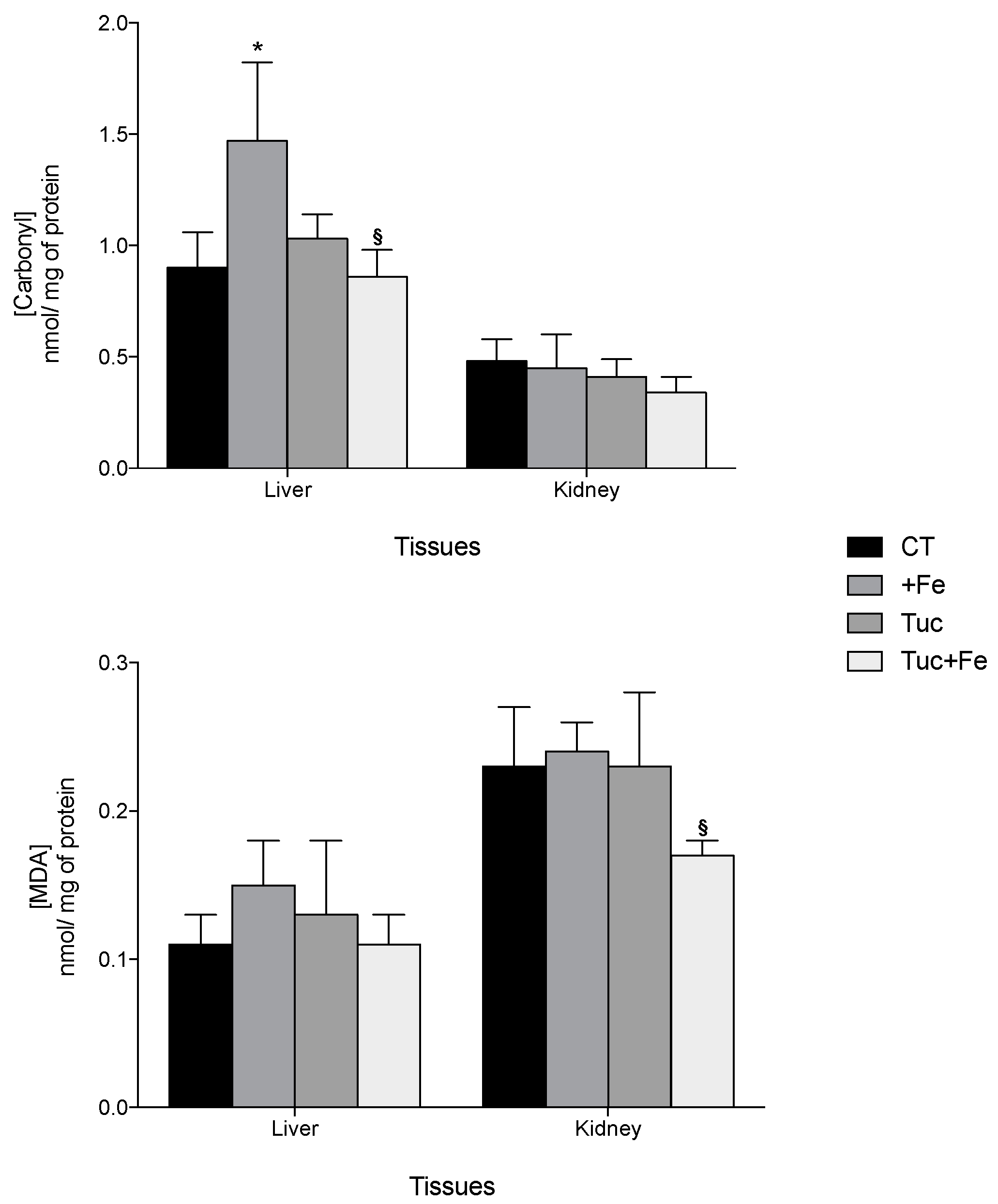
2.5.1. Carbonyl Protein and Lipid Peroxidation Levels
2.5.2. Activity of Antioxidant Enzymes in the Liver and Kidney
2.6. Proinflammatory Cytokine Serum Levels
2.7. Determination of Total RNA Extraction and Transcripts Levels
2.8. Immunoblot Analysis
2.9. Statistical Analysis
3. Results
3.1. Food Intake, Iron Intake, Weight Gain, and Hematological Parameters
3.2. Iron Status
3.3. Oxidative Damages and Antioxidant Capacity
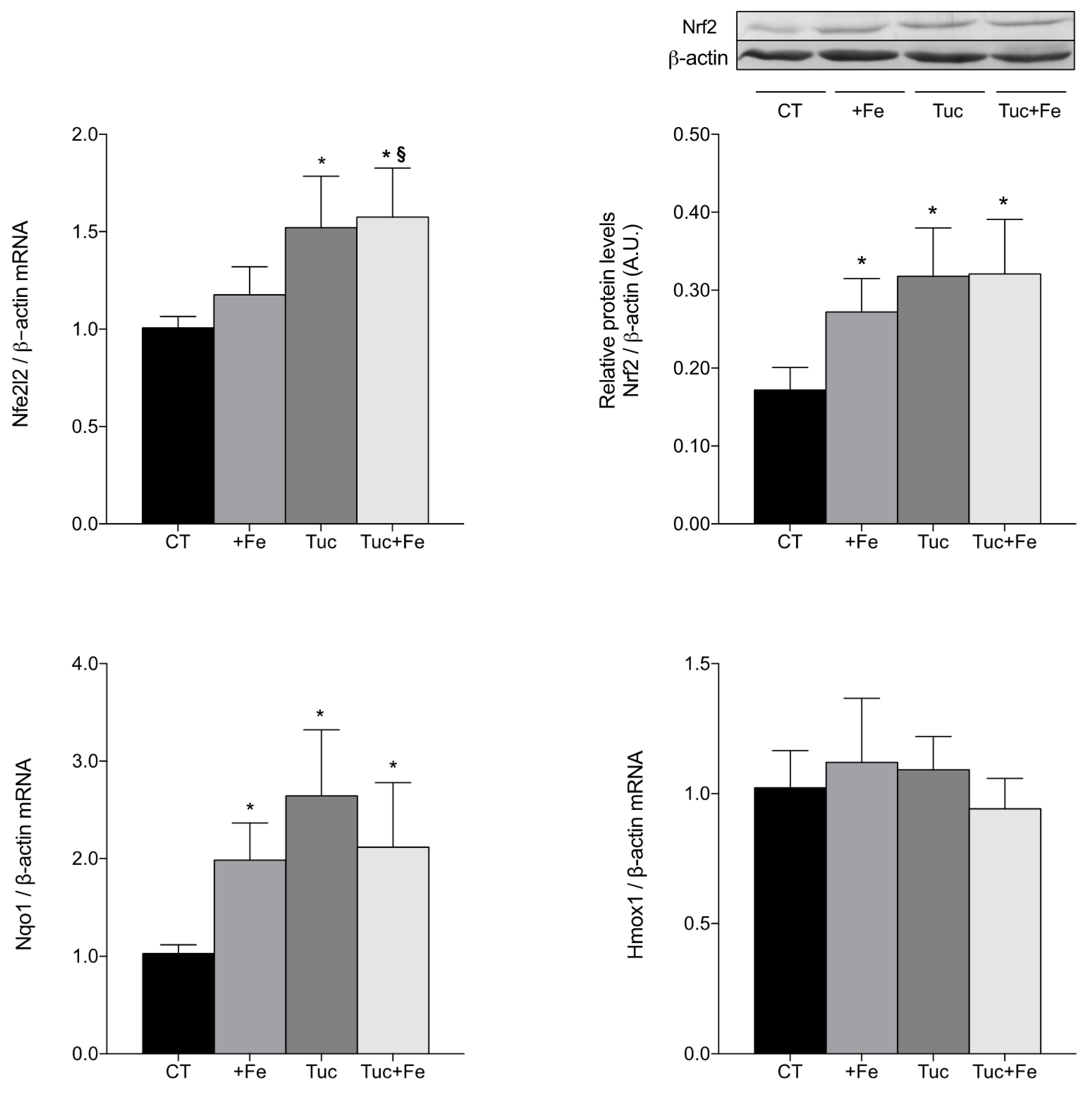
3.4. Nrf2-Related Pathway
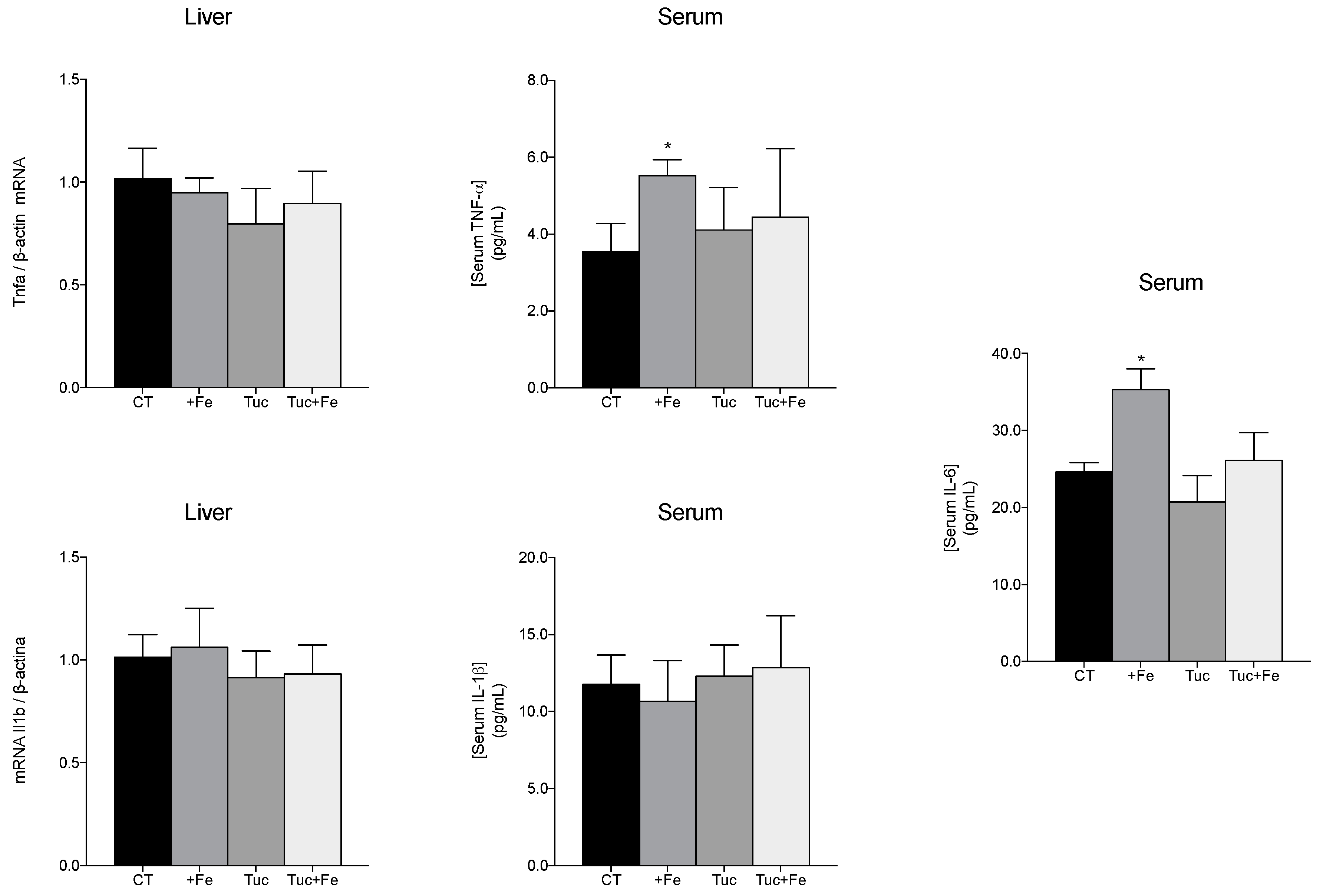
3.5. Inflammatory Markers
3.6. Aging-Related Markers
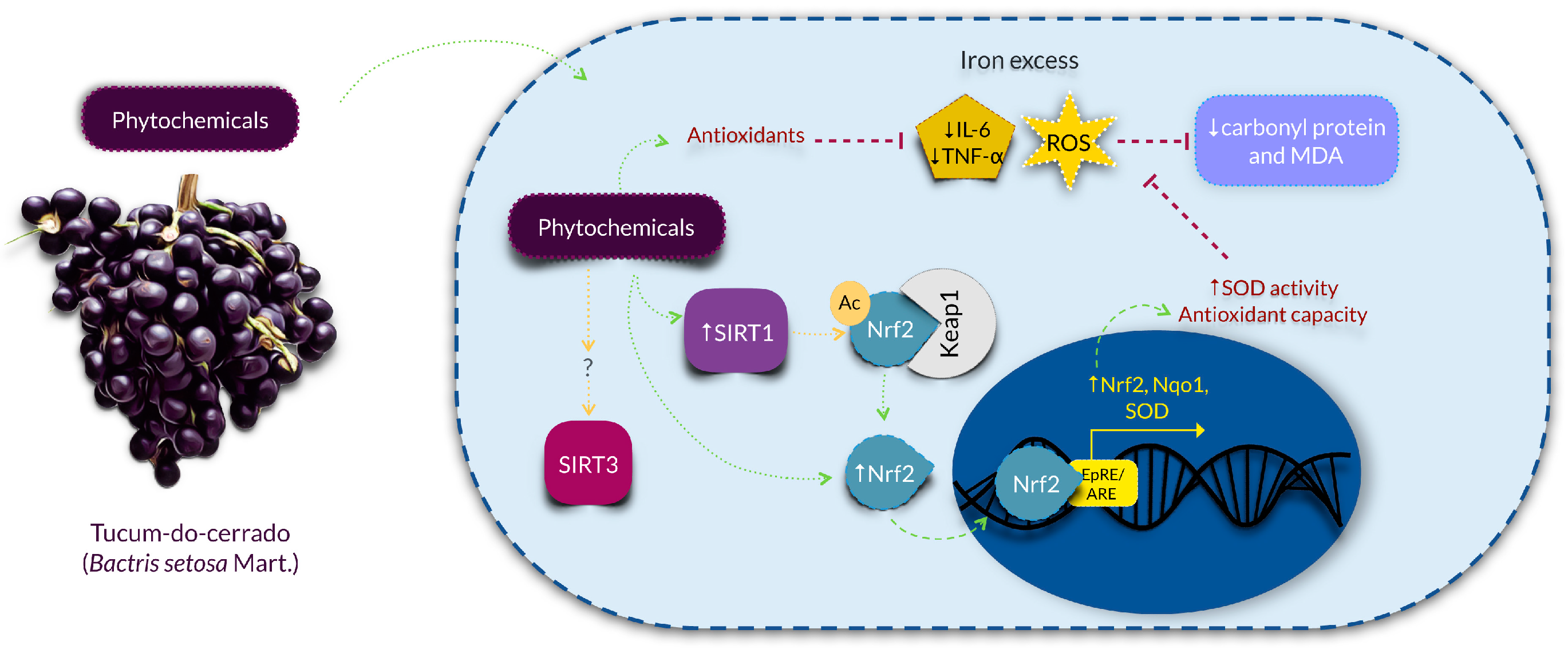
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO. Global Health and Aging—What Are the Public Health Implications of Global Ageing; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Monti, D.; Ostan, R.; Borelli, V.; Castellani, G.; Franceschi, C. Inflammaging and human longevity in the omics era. Mech. Ageing Dev. 2017, 165, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I. Theories of biological aging: Genes, proteins, and free radicals. Free Radic. Res. 2006, 40, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Edrey, Y.H.; Salmon, A.B. Revisiting an age-old question regarding oxidative stress. Free Radic. Biol. Med. 2014, 71, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Schottker, B.; Saum, K.U.; Jansen, E.H.; Boffetta, P.; Trichopoulou, A.; Holleczek, B.; Dieffenbach, A.K.; Brenner, H. Oxidative stress markers and all-cause mortality at older age: A population-based cohort study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Ortega, J.; Bordji, K.; Freret, T.; Rush, T.; Buisson, A. Iron overload accelerates neuronal amyloid-beta production and cognitive impairment in transgenic mice model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Real, J.M.; Manco, M. Effects of iron overload on chronic metabolic diseases. Lancet Diabetes Endocrinol. 2014, 2, 513–526. [Google Scholar] [CrossRef]
- Doulias, P.T.; Vlachou, C.; Boudouri, C.; Kanavaros, P.; Siamopoulos, K.C.; Galaris, D. Flow cytometric estimation of ’labile iron pool’ in human white blood cells reveals a positive association with ageing. Free Radic. Res. 2008, 42, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Arruda, L.F.; Arruda, S.F.; Campos, N.A.; de Valencia, F.F.; Siqueira, E.M. Dietary iron concentration may influence aging process by altering oxidative stress in tissues of adult rats. PLoS ONE 2013, 8, e61058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Hwang, J.C.; Lees, H.A.; Wohlgemuth, S.E.; Knutson, M.D.; Judge, A.R.; Dupont-Versteegden, E.E.; Marzetti, E.; Leeuwenburgh, C. Long-term perturbation of muscle iron homeostasis following hindlimb suspension in old rats is associated with high levels of oxidative stress and impaired recovery from atrophy. Exp. Gerontol. 2012, 47, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.; O’Neill, L.A. Oxidative stress and nuclear factor-kappab activation: A reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 2000, 59, 13–23. [Google Scholar] [CrossRef]
- Si, H.; Liu, D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J. Nutr. Biochem. 2014, 25, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Hou, D.X. Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol. Nutr. Food Res. 2016, 60, 1731–1755. [Google Scholar] [CrossRef] [PubMed]
- Porquet, D.; Casadesus, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegri, C.; Sanfeliu, C.; Camins, A.; Pallas, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. Age 2013, 35, 1851–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Bo, C.; Martini, D.; Porrini, M.; Klimis-Zacas, D.; Riso, P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015, 6, 2890–2917. [Google Scholar] [CrossRef] [PubMed]
- Stefanson, A.L.; Bakovic, M. Dietary regulation of Keap1/Nrf2/are pathway: Focus on plant-derived compounds and trace minerals. Nutrients 2014, 6, 3777–3801. [Google Scholar] [CrossRef] [PubMed]
- Asseburg, H.; Schafer, C.; Muller, M.; Hagl, S.; Pohland, M.; Berressem, D.; Borchiellini, M.; Plank, C.; Eckert, G.P. Effects of grape skin extract on age-related mitochondrial dysfunction, memory and life span in C57BL/6J mice. Neuromol. Med. 2016, 18, 378–395. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Na, L.; Feng, R.; Gong, L.; Zhao, Y.; Li, Q.; Li, Y.; Sun, C. The phytochemical, EGCG, extends lifespan by reducing liver and kidney function damage and improving age-associated inflammation and oxidative stress in healthy rats. Aging Cell 2013, 12, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Cruzat, V.F.; Newsholme, P.; Cheng, J.; Chen, Y.; Lu, Y. Regulation of SIRT1 in aging: Roles in mitochondrial function and biogenesis. Mech. Ageing Dev. 2016, 155, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Rahman, M.S.; Saha, S.K.; Saikot, F.K.; Deep, A.; Kim, K.H. Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell 2017, 16, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Chen, C.; Hao, J.; Huang, J.; Wang, S.; Liu, P.; Huang, H. Polydatin promotes Nrf2-are anti-oxidative pathway through activating SIRT1 to resist ages-induced upregulation of fibronetin and transforming growth factor-beta1 in rat glomerular messangial cells. Mol. Cell. Endocrinol. 2015, 399, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, G.; Chen, L.; Ding, Y.; Lian, J.; Hong, G.; Lu, Z. Resveratrol protects mice from paraquat-induced lung injury: The important role of SIRT1 and Nrf2 antioxidant pathways. Mol. Med. Rep. 2016, 13, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Masutomi, H.; Noda, Y.; Ozawa, Y.; Takahashi, K.; Handa, S.; Maruyama, N.; Shimizu, T.; Ishigami, A. Senescence marker protein-30/superoxide dismutase 1 double knockout mice exhibit increased oxidative stress and hepatic steatosis. FEBS Open Bio 2014, 4, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.J.; Lee, E.K.; Kim, S.J.; Song, C.W.; Maruyama, N.; Ishigami, A.; Kim, N.D.; Im, D.S.; Yu, B.P.; Chung, H.Y. Anti-inflammatory activity of SMP30 modulates NF-kappab through protein tyrosine kinase/phosphatase balance. J. Mol. Med. 2015, 93, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit polyphenols: A review of anti-inflammatory effects in humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 419–444. [Google Scholar] [CrossRef] [PubMed]
- Oyebode, O.; Gordon-Dseagu, V.; Walker, A.; Mindell, J.S. Fruit and vegetable consumption and all-cause, cancer and CVD mortality: Analysis of health survey for england data. J. Epidemiol. Community Health 2014, 68, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, D.R.; Terzibasi, E.; Genade, T.; Cattaneo, A.; Domenici, L.; Cellerino, A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006, 16, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Boeing, J.S.; Ribeiro, D.; Chiste, R.C.; Visentainer, J.V.; Costa, V.M.; Freitas, M.; Fernandes, E. Chemical characterization and protective effect of the bactris setosa Mart. fruit against oxidative/nitrosative stress. Food Chem. 2017, 220, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Rosa, F.R.; Arruda, A.F.; Siqueira, E.M.; Arruda, S.F. Phytochemical compounds and antioxidant capacity of tucum-do-cerrado (bactris setosa mart), Brazil’s native fruit. Nutrients 2016, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Fustinoni-Reis, A.M.; Arruda, S.F.; Dourado, L.P.; da Cunha, M.S.; Siqueira, E.M. Tucum-do-cerrado (Bactris setosa Mart.) consumption modulates iron homeostasis and prevents iron-induced oxidative stress in the rat liver. Nutrients 2016, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Yang, L.; Zu, Y.; Lu, Q. Effects of rosmarinic acid on liver and kidney antioxidant enzymes, lipid peroxidation and tissue ultrastructure in aging mice. Food Funct. 2015, 6, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, Y.H.; Wang, S.T.; Ren, J.; Camer, D.; Hua, Y.Z.; Zhang, Q.; Huang, J.; Xue, D.L.; Zhang, X.F.; et al. Chlorogenic acid protects d-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm. Biol. 2016, 54, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
- Baranowska, I.; Czernicki, K.; Aleksandrowicz, R. The analysis of lead, cadmium, zinc, copper and nickel content in human bones from the upper silesian industrial district. Sci. Total Environ. 1995, 159, 155–162. [Google Scholar] [CrossRef]
- Richert, S.; Wehr, N.B.; Stadtman, E.R.; Levine, R.L. Assessment of skin carbonyl content as a noninvasive measure of biological age. Arch. Biochem. Biophys. 2002, 397, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Candan, N.; Tuzmen, N. Very rapid quantification of malondialdehyde (MDA) in rat brain exposed to lead, aluminium and phenolic antioxidants by high-performance liquid chromatography-fluorescence detection. Neurotoxicology 2008, 29, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Hartree, E.F. Determination of protein: A modification of the lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- McCord, J.M. Analysis of superoxide dismutase activity. In Current Protocols in Toxicology; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta cT) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.O.; Felipe, M.S.S.; Brígido, M.M.; Maranhão, A.Q.; De-Souza, M.T. Técnicas Básicas Em Biologia Molecular; Editora Universidade de Brasília: Brasília, Brazil, 2010. [Google Scholar]
- Zhao, L.; Wang, Y.; Wang, Z.; Xu, Z.; Zhang, Q.; Yin, M. Effects of dietary resveratrol on excess-iron-induced bone loss via antioxidative character. J. Nutr. Biochem. 2015, 26, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, D.; Ghate, N.B.; Panja, S.; Das, A.; Mandal, N. Wild edible fruit of prunus nepalensis ser. (steud), a potential source of antioxidants, ameliorates iron overload-induced hepatotoxicity and liver fibrosis in mice. PLoS ONE 2015, 10, e0144280. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Kauppinen, A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: Impact on the aging process and age-related diseases. Cell. Mol. Life Sci. 2012, 69, 2999–3013. [Google Scholar] [CrossRef] [PubMed]
- Piloni, N.E.; Perazzo, J.C.; Fernandez, V.; Videla, L.A.; Puntarulo, S. Sub-chronic iron overload triggers oxidative stress development in rat brain: Implications for cell protection. Biometals 2016, 29, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Silva-Gomes, S.; Santos, A.G.; Caldas, C.; Silva, C.M.; Neves, J.V.; Lopes, J.; Carneiro, F.; Rodrigues, P.N.; Duarte, T.L. Transcription factor NRF2 protects mice against dietary iron-induced liver injury by preventing hepatocytic cell death. J. Hepatol. 2014, 60, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, S.; Fujii, M.; Hou, D.X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic. Biol. Med. 2007, 42, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; Macia, A.; Romero, M.P.; Angles, N.; Morello, J.R.; Motilva, M.J. Distribution of procyanidins and their metabolites in rat plasma and tissues after an acute intake of hazelnut extract. Food Funct. 2011, 2, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, D.H.; Ahn, J.; Chung, W.J.; Jang, Y.J.; Seong, K.S.; Moon, J.H.; Ha, T.Y.; Jung, C.H. Pharmacokinetics, tissue distribution, and anti-lipogenic/adipogenic effects of allyl-isothiocyanate metabolites. PLoS ONE 2015, 10, e0132151. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; DesAulniers, J.; Dyck, J.R.; Kassiri, Z.; Oudit, G.Y. Resveratrol mediates therapeutic hepatic effects in acquired and genetic murine models of iron-overload. Liver Int. 2016, 36, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Seong, R.K.; Kim, J.A.; Son, S.J.; Kim, Y.; Yokozawa, T.; Shin, O.S. Oligonol promotes anti-aging pathways via modulation of SIRT1-AMPK-autophagy pathway. Nutr. Res. Pract. 2016, 10, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Giblin, W.; Skinner, M.E.; Lombard, D.B. Sirtuins: Guardians of mammalian healthspan. Trends Genet. 2014, 30, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Rose, G.; Dato, S.; Altomare, K.; Bellizzi, D.; Garasto, S.; Greco, V.; Passarino, G.; Feraco, E.; Mari, V.; Barbi, C.; et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp. Gerontol. 2003, 38, 1065–1070. [Google Scholar] [CrossRef]
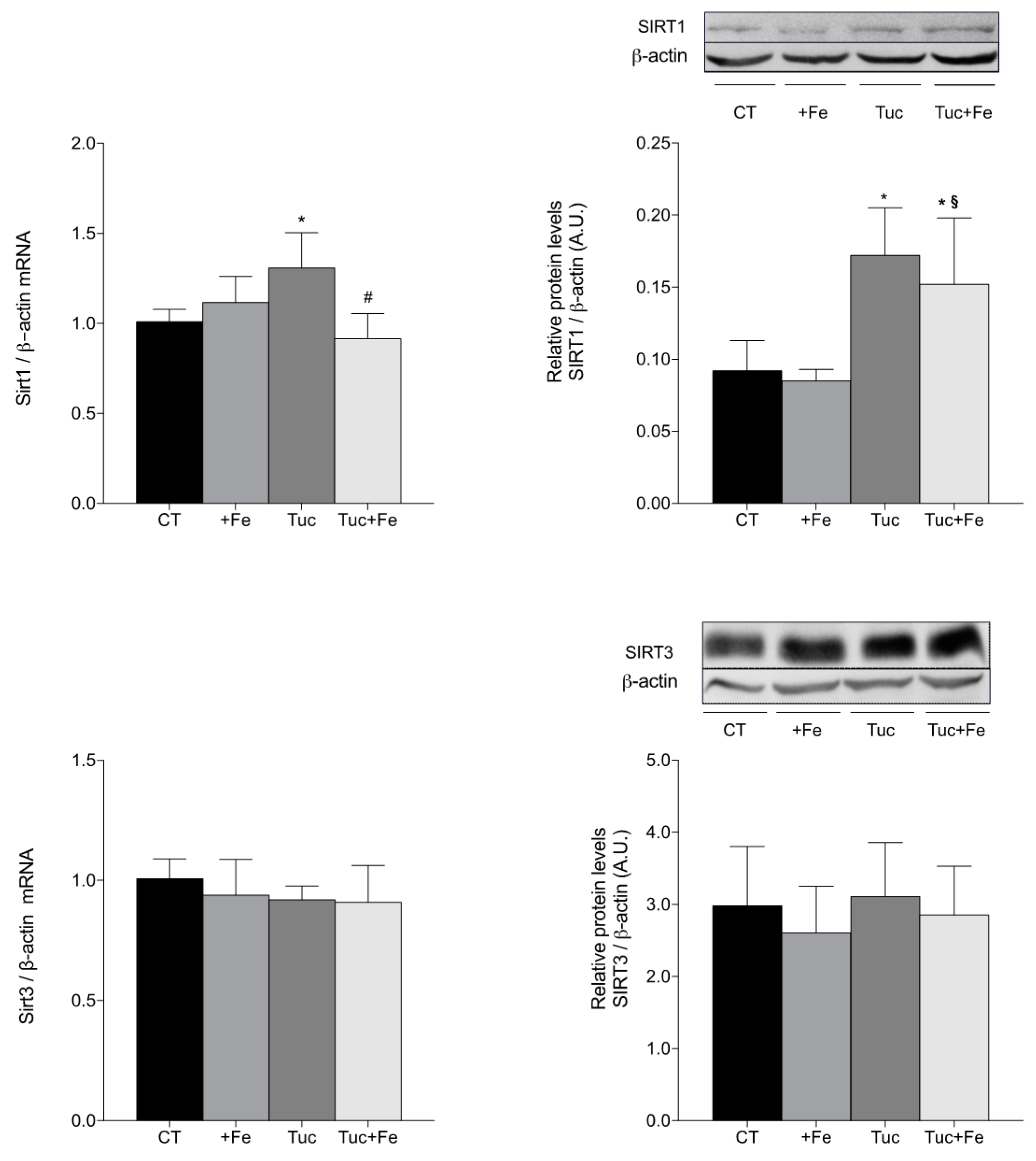

| CT | +Fe | Tuc | Tuc + Fe | |
|---|---|---|---|---|
| Body weight gain (g) | 218.7 ± 29.2 | 223.8 ± 26.8 | 195.3 ± 31.4 | 197.2 ± 39.8 |
| Dietary intake (g/12 weeks) | 1558.6 ± 106.8 | 1642.0 ± 89.6 | 1534.7 ± 73.7 | 1604.7 ± 188.9 |
| Dietary iron intake (mg/12 weeks) | 38.5 ± 2.6 | 352.4 ± 19.2 * | 44.0 ± 2.1 | 347.7 ± 40.9 *,# |
| CT | +Fe | Tuc | Tuc + Fe | |
|---|---|---|---|---|
| RBC (×106/μL) | 7.95 ± 0.63 | 7.84 ± 0.60 | 7.77 ± 0.60 | 7.29 ± 0.48 |
| Hb (g/dL) | 15.07 ± 0.94 | 14.77 ± 0.31 | 15.29 ± 0.95 | 15.19 ± 1.10 |
| HCT (%) | 42.88 ± 1.03 | 41.28 ± 1.35 | 42.50 ± 1.50 | 40.50 ± 1.66 |
| PTL (×103/μL) | 590.08 ± 56.53 | 544.08 ± 85.26 | 524.17 ± 16.68 | 589.10 ± 48.06 |
| WBC (×103/μL) | 4.86 ± 1.35 | 4.36 ± 0.69 | 4.18 ± 0.89 | 4.85 ± 0.88 |
| CT | +Fe | Tuc | Tuc + Fe | |
|---|---|---|---|---|
| Serum iron (μg/dL) | 107.68 ± 5.85 | 202.46 ± 78.42 * | 142.17 ± 32.98 | 177.60 ± 42.02 |
| UIBC (μg/dL) | 286.18 ± 59.85 | 199.64 ± 41.21 * | 256.15 ± 20.93 | 260.98 ± 37.59 |
| TIBC (μg/dL) | 405.67 ± 48.94 | 380.64 ± 74.93 | 398.32 ± 45.08 | 438.58 ± 30.92 |
| TS (%) | 26.77 ± 3.76 | 40.80 ± 9.27 * | 37.13 ± 3.16 | 40.40 ± 8.50 * |
| Tf (mg/dL) | 283.97 ± 34.26 | 266.45 ± 52.45 | 278.82 ± 31.55 | 307.01 ± 21.64 |
| Enzyme | Tissue | CT | +Fe | Tuc | Tuc + Fe |
|---|---|---|---|---|---|
| CAT | Liver | 208.89 ± 24.96 | 167.53 ± 40.80 | 266.28 ± 51.32 | 223.87 ± 28.11 |
| Kidney | 103.85 ± 13.03 | 116.95 ± 15.46 | 91.05 ± 11.57 | 91.16 ± 8.94 § | |
| GPx | Liver | 1.07 ± 0.11 | 0.74 ± 0.03 | 1.33 ± 0.29 | 1.13 ± 0.18 § |
| Kidney | 0.64 ± 0.14 | 0.63 ± 0.10 | 0.54 ± 0.10 | 0.57 ± 0.11 | |
| SOD | Liver | 17.23 ± 1.16 | 37.83 ± 4.49 * | 29.99 ± 7.89 * | 28.17 ± 3.74 |
| Kidney | 19.44 ± 1.76 | 21.99 ± 3.14 | 13.13 ± 1.30 * | 10.67 ± 1.12 *,§ | |
| GR | Liver | 38.37 ± 10.84 | 36.35 ± 6.67 | 42.48 ± 5.45 | 22.81 ± 3.80 * |
| Kidney | 133. 68 ± 30.21 | 139.90 ± 17.72 | 108.33 ± 21.24 | 97.00 ± 6.36 | |
| GST | Liver | 340.09 ± 76.14 | 428.47 ± 74.26 | 454.54 ± 93.64 | 427.16 ± 73.22 |
| Kidney | 124.16 ± 17.62 | 139.10 ± 24.83 | 125.56 ± 23.67 | 118.42 ± 12.22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Cunha, M.D.S.B.; Arruda, S.F. Tucum-do-Cerrado (Bactris setosa Mart.) May Promote Anti-Aging Effect by Upregulating SIRT1-Nrf2 Pathway and Attenuating Oxidative Stress and Inflammation. Nutrients 2017, 9, 1243. https://doi.org/10.3390/nu9111243
Da Cunha MDSB, Arruda SF. Tucum-do-Cerrado (Bactris setosa Mart.) May Promote Anti-Aging Effect by Upregulating SIRT1-Nrf2 Pathway and Attenuating Oxidative Stress and Inflammation. Nutrients. 2017; 9(11):1243. https://doi.org/10.3390/nu9111243
Chicago/Turabian StyleDa Cunha, Marcela De Sá Barreto, and Sandra Fernandes Arruda. 2017. "Tucum-do-Cerrado (Bactris setosa Mart.) May Promote Anti-Aging Effect by Upregulating SIRT1-Nrf2 Pathway and Attenuating Oxidative Stress and Inflammation" Nutrients 9, no. 11: 1243. https://doi.org/10.3390/nu9111243





