The Association between Serum 25(OH)D Status and Blood Pressure in Participants of a Community-Based Program Taking Vitamin D Supplements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. The Pure North Community-Based Program (Intervention)
2.3. Program Measurements Included in the Dataset
2.4. Statistical Analysis
3. Results
3.1. Baseline Measurements
3.2. Longitudinal Analysis
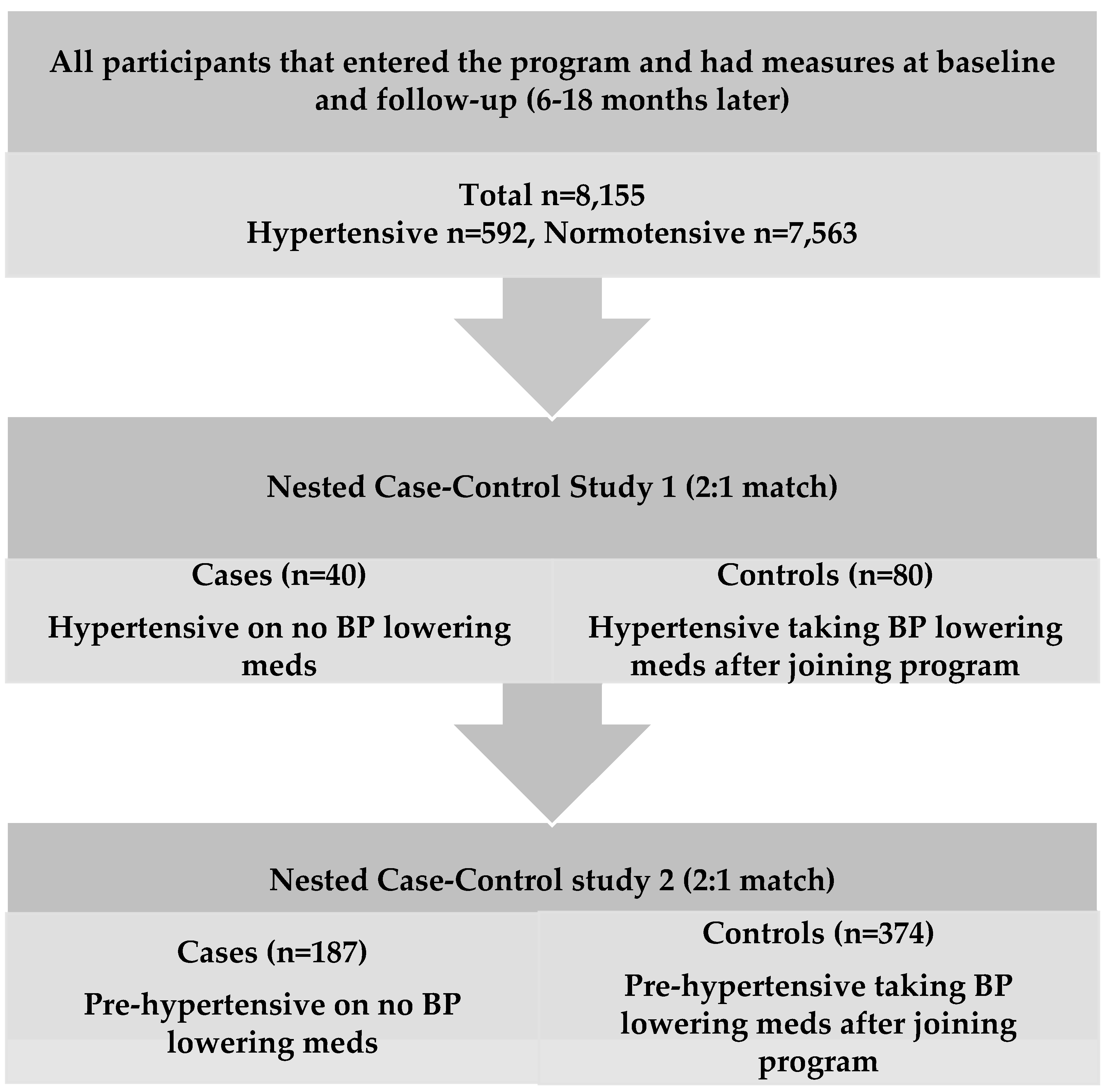
3.3. Nested Case-Control Study
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mittal, B.V.; Singh, A.K. Hypertension in the developing world: Challenges and opportunities. Am. J. Kidney Dis. 2010, 55, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: Important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med. J. 2005, 98, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Colley, R.C.; Garriguet, D.; Janssen, I.; Craig, C.L.; Clarke, J.; Trembla, M.S. Physical activity of Canadian adults: Accelerometer results from the 2007 to 2009 Canadian health measures survey. Health Rep. 2011, 22, 7–14. [Google Scholar] [PubMed]
- Warburton, D.E.; Charlesworth, S.; Ivey, A.; Nettlefold, L.; Bredin, S.S. A systematic review of the evidence for Canada’s physical activity guidelines for adults. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Elmer, P.J.; Obarzanek, E.; Vollmer, W.M.; Simons-Morton, D.; Stevens, V.J.; Young, D.R.; Lin, P.H.; Champagne, C.; Harsha, D.W.; Svetkey, L.P.; et al. Effects of Comprehensive Lifestyle Modification on Diet, Weight, Physical Fitness, and Blood Pressure Control: 18-Month Results of a Randomized Trial. Ann. Intern. Med. 2006, 144, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpanah, F.; Yarjanli, M.; Sheikholeslami, F.; Heibatollahi, M.; Eskandary, P.S.; Azizi, F. Associations between vitamin d and cardiovascular outcomes; Tehran lipid and glucose study. Atherosclerosis 2011, 218, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Sugden, J.A.; Davies, J.I.; Witham, M.D.; Morris, A.D.; Struthers, A.D. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet. Med. 2008, 25, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Tarcin, O.; Yavuz, D.G.; Ozben, B.; Telli, A.; Ogunc, A.V.; Yuksel, M.; Toprak, A.; Yazici, D.; Sancak, S.; Deyneli, O.; et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J. Clin. Endocrinol. Metab. 2009, 94, 4023–4030. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Forman, J.P. Vitamin D and hypertension: Current evidence and future directions. Hypertension 2010, 56, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Schleithoff, S.S.; Zittermann, A.; Tenderich, G.; Berthold, H.K.; Stehle, P.; Koerfer, R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 83, 754–759. [Google Scholar] [PubMed]
- Brickman, A.; Nyby, M.; von Hungen, K.; Eggena, P.; Tuck, M. Parathyroid hormone platelet calcium and blood pressure in normotensive subjects. Hypertension 1991, 18, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.I.; Uwaifo, G.I.; Nicholas, W.C.; Koch, C.A. Does vitamin D deficiency cause hypertension? Current evidence from clinical studies and potential mechanisms. Int. J. Endocrinol. 2010, 2010, 579640. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.P.; Williams, J.S.; Fisher, N.D. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 2010, 55, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Sowers, M.; Bell, C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the third national health and nutrition examination survey. Am. J. Hypertens. 2007, 20, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Williams, D.; Lawlor, D.A. Associations of serum 25-hydroxyvitamin D, parathyroid hormone and calcium with cardiovascular risk factors: Analysis of 3 NHANES cycles (2001–2006). PLoS ONE 2010, 5, e13882. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Ho, S.C.; Zhong, L. Effects of vitamin D supplementation on blood pressure. South Med. J. 2010, 103, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, L.A.; Witham, M.D. Controversy in the link between vitamin D supplementation and hypertension. Expert Rev. Cardiovasc. Ther. 2015, 13, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Burgess, S.; Munroe, P.B.; Khan, H. Vitamin D and high blood pressure: Causal association or epiphenomenon? Eur. J. Epidemiol. 2014, 29, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Witham, M.D.; Nadir, M.A. Effect of vitamin d on blood pressure: A systematic review and meta-analysis. J. Hypertens. 2009, 27, 1948–1954. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Nachtigall, D.; Hansen, C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J. Clin. Endocrinol. Metab. 2001, 86, 1633–1637. [Google Scholar] [PubMed]
- Kunutsor, S.K.; Apekey, T.A.; Steur, M. Vitamin D and risk of future hypertension: Meta-analysis of 283,537 participants. Eur. J. Epidemiol. 2013, 28, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Island, P.E. Statistics Canada; High Blood Pressure 2013. Available online: http://www.statcan.gc.ca/pub/82-625-x/2014001/article/14020-eng.htm (accessed on 7 April 2016).
- Whiting, S.J.; Langlois, K.; Vatanparast, H.; Greene-Finestone, L.S. The vitamin D status of Canadians relative to the 2011 dietary reference intakes: An examination in children and adults with and without supplement use. Am. J. Clin. Nutr. 2011, 94, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Statistics Canada. Canadian Health Measures Survey-Data User Guide: Cycle 1, Definitions, Data Sources and Methods. Ottawa 2011. Available online: http://www23.statcan.gc.ca/imdb-bmdi/document/5071_D2_T1_V1-eng.htm (accessed on 2 May 2016).
- Statistics Canada. Canadian Health Measures Survey (chms) Data User Guide: Cycle 2. Ottawa 2012. Available online: http://data.library.utoronto.ca/datapub/codebooks/cstdli/chms/CHMS_User_Guide_Cycle2_E.pdf (accessed on 2 May 2016).
- Heaney, R.P. Toward a physiological referent for the vitamin d requirement. J. Endocrinol. Investig. 2014, 37, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. Joint National Committee on Prevention, Detection, Evaluation, Treatment of High Blood Pressure. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed]
- Smeltzer, S.C.; Bare, B.G.; Hinkle, J.L.; Cheever, K.H. Brunner & Suddarth’s Textbook of Medical-Surgical Nursing, 12th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am. J. Clin. Nutr. 2004, 80, 1706S–1709S. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Yetley, E.A.; Pfeiffer, C.M.; Phinney, K.W.; Bailey, R.L.; Blackmore, S.H.; Bock, J.L.; Brody, L.C.; Carmel, R.; Curtin, L.R.; Durazo-Arvizu, R.A.; et al. Biomarkers of vitamin B-12 status in NHANES: A roundtable summary. Am. J. Clin. Nutr. 2011, 94, 313S–321S. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B. Vitamins, Trace Minerals, and other Micronutrients; Elsevier Saunders: Philadelphia, PA, USA, 2011. [Google Scholar]
- Kimball, S.M.; Mirhosseini, N.; Holick, M.F. Evaluation of vitamin D3 intakes up to 15,000 international units/day and serum 25-hydroxyvitamin D concentrations up to 300 nmol/L on calcium metabolism in a community setting. Dermato-Endocrinology 2017, 9, e1300213. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Loloei, S.; Mirjalili, M.R.; Barzegar, K. The effect of vitamin D supplementation on blood pressure in patients with elevated blood pressure and vitamin d deficiency: A randomized, double-blind, placebo-controlled trial. Blood Press Monit. 2015, 20, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Judd, S.E.; Raiser, S.N.; Kumari, M.; Tangpricha, V. 1,25 dihydroxyvitamin D3 reduces systolic blood pressure in hypertensive adults: A pilot feasibility study. J. Steroid Biochem. Mol. Biol. 2010, 121, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Kienreich, K.; Grubler, M.; Tomaschitz, A.; Schmid, J.; Verheyen, N.; Rutters, F.; Dekker, J.M.; Pilz, S. Vitamin D, arterial hypertension & cerebrovascular disease. Indian J. Med. Res. 2013, 137, 669–679. [Google Scholar] [PubMed]
- Arora, P.; Song, Y.; Dusek, J.; Plotnikoff, G.; Sabatine, M.; Cheng, S.; Valcour, A.; Swales, H.; Taylor, B.; Carney, E.; et al. Vitamin D therapy in individuals with prehypertension or hypertension: The daylight trial. Circulation 2015, 131, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Gaksch, M.; O’Hartaigh, B.; Tomaschitz, A.; Marz, W. The role of vitamin D deficiency in cardiovascular disease: Where do we stand in 2013? Arch. Toxicol. 2013, 87, 2083–2103. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.; Buhring, M.; Sharma, A.M.; Hopfenmuller, W.; Chen, T.C.; Holick, M.F. UV irradiation and blood pressure—The role of vitamin D in essential hypertension. In Biologic Effects of Light 1998; Springer: New York, NY, USA, 1999; pp. 249–255. [Google Scholar]
- Forman, J.P.; Scott, J.B.; Ng, K.; Drake, B.F.; Suarez, E.G.; Hayden, D.L.; Bennett, G.G.; Chandler, P.D.; Hollis, B.W.; Emmons, K.M.; et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension 2013, 61, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, J.D.; Camargo, C.A.; Alistair, W.; Stewart, A.W.; Waayer, D.; Lawes, C.M.M.; Toop, L.; Khaw, K.T.; Simon, A.; Thom, S.A.; et al. Effect of Monthly, High-Dose, Long-Term Vitamin D Supplementation on Central Blood Pressure Parameters: A Randomized Controlled Trial Substudy. J. Am. Heart Assoc. 2017, 6, e006802. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.L.; Ray, R.M.; van Horn, L.; Manson, J.E.; Allison, M.A.; Black, H.R.; Beresford, S.A.; Connelly, S.A.; Curb, J.D.; Grimm, R.H.; et al. Women’s Health Initiative Investigators. Effect of calcium and vitamin D supplementation on blood pressure: The women’s health initiative randomized trial. Hypertension 2008, 52, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Sneve, M.; Torjesen, P.; Figenschau, Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J. Intern. Med. 2010, 267, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Witham, M.D.; Price, R.J.; Struthers, A.D.; Donnan, P.T.; Messow, C.M.; Ford, I.; McMurdo, M.E. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: The VitDISH randomized controlled trial. JAMA Intern. Med. 2013, 173, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.S.; Oviatt, S. Relationship of mineral metabolism and long-term calcium and cholecalciferol supplementation on blood pressure in normotensive men. Am. J. Clin. Nutr. 1990, 52, 717–721. [Google Scholar] [PubMed]
- Scragg, R.; Slow, S.; Stewart, A.W.; Jennings, L.C.; Chamber, S.T.; Priest, P.C.; Florkowski, C.M.; Camargo, C.A.; Murdoch, D.R. Long-term high-dose vitamin D3 supplementation and blood pressure in healthy adults a randomized controlled trial. Hypertension 2014, 64, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.H.; Wang, C.Y.; Li, L.A.; Kao, L.S.; Yeh, S.H. No significant effect of calcium and vitamin D supplementation on blood pressure and calcium metabolism in elderly Chinese. Chin. J. Physiol. 1993, 36, 85–94. [Google Scholar] [PubMed]
- Scragg, R.; Khaw, K.T.; Murphy, S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur. J. Clin. Nutr. 1995, 49, 640–646. [Google Scholar] [PubMed]
- Ekwaru, J.P.; Ohinmaa, A.; Veugelers, P.J. The effectiveness of a preventive health program 400 and vitamin D status in improving health-related quality of life of older Canadians. Qual. Life Res. 2016, 25, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, D. A review of the biochemistry, metabolism and clinical benefits of thiamin (e) and its derivatives. Evid. Based Complement. Alternat. Med. 2006, 3, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, sufficiency and toxicity. Nutrients 2013, 5, 3605–3616. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Qiao, G.; Uskokovic, M.; Xiang, W.; Zheng, W.; Kong, J. Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Bonakdaran, S.; Hami, M.; Hatefi, A. The effects of calcitriol on albuminuria in patients with type-2 diabetes mellitus. Saudi J. Kidney Dis. Transpl. 2012, 23, 1215–1220. [Google Scholar] [PubMed]
- Dalbeni, A.; Scaturro, G.; Degan, M.; Minuz, P.; Delva, P. Effects of six months of vitamin D supplementation in patients with heart failure: A randomized double-blind controlled trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Mose, F.H.; Bech, J.N.; Hansen, A.B.; Pedersen, E.B. Effect of cholecalciferol supplementation during winter months in patients with hypertension: A randomized, placebo-controlled trial. Am. J. Hypertens. 2012, 25, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Kumar, T.D.; Singh, N.P.; Saha, R. Seasonal variation of blood pressure in normotensive females aged 18 to 40 years in an urban slum of Delhi, India. Asia Pac. J. Public Health 2010, 22, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Shahar, D.R.; Froom, P.; Harari, G.; Yerushalmi, N.; Lubrin, F.; Kristal-Boneh, E. Change in dietary intake account for seasonal changes in cardiovascular disease risk factors. Eur. J. Clin. Nutr. 1999, 53, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Ajabshir, S.; Asif, A.; Nayer, A. The effect of vitamin D on the renin-angiotensin system. J. Nephropathol. 2014, 3, 41–43. [Google Scholar] [PubMed]
| Parameter at Baseline | All | Serum 25(OH)D | |||||
|---|---|---|---|---|---|---|---|
| 0–50 | 50–100 | 100–150 | 150–200 | 200–250 | ≥250 | ||
| Number | 8155 | 867 | 5059 | 1809 | 306 | 69 | 45 |
| 25(OH)D, nmol/L | 87 ± 37 | 39 ± 8 f | 75 ± 13 e | 118 ± 13 d | 169 ± 14 c | 220 ± 16 b | 292 ± 38 a |
| Vitamin D supplement dose, IU/day | 1600 ± 2500 | 380 ± 1300 f | 1200 ± 2000 d | 2600 ± 2800 c | 4100 ± 3800 b | 5400 ± 4200 a | 6300 ± 500 a |
| Vitamin B12, pmol/L | 470 ± 310 | 320 ± 170 e | 440 ± 280 d | 580 ± 370 c | 680 ± 380 bc | 820 ± 500 a | 720 ± 350 b |
| AA/EPA ratio | 16 ± 8 | 21 ± 7 a | 17 ± 8 b | 14 ± 8 c | 12 ± 7 cd | 11 ± 7 d | 12 ± 10 cd |
| Systolic BP, mmHg | 125 ± 17 | 126 ± 16 a | 126 ± 17 a | 124 ± 17 ab | 123 ± 17 ab | 119 ± 18 b | 118 ± 14 b |
| Diastolic BP, mmHg | 77 ± 10 | 79 ± 10 a | 77 ± 10 ab | 76 ± 10 ab | 76 ± 10 ab | 75 ± 10 b | 75 ± 9 b |
| Pulse Pressure, mmHg | 48 ± 13 | 47 ± 12 ab | 48 ± 13 a | 48 ± 13 a | 47 ± 14 ab | 44 ± 14 b | 44 ± 9 b |
| MAP, mmHg | 93.2 ± 10.9 | 94.6 ± 10.9 a | 93.5 ± 10.9 a | 92.0 ± 10.8 ab | 91.6 ± 11.0 ab | 89.3 ± 11.4 b | 89.3 ± 9.6 b |
| Age, year | 56 ± 15 | 51 ± 15 c | 57 ± 15 a | 57 ± 15 a | 56 ± 15 ab | 53 ± 17 b | 52 ± 14 bc |
| BMI, kg/m2 | 27 ± 6 | 30 ± 6 a | 28 ± 6 b | 26 ± 5 bc | 25 ± 5 cd | 24 ± 4 d | 24 ± 4 d |
| WC, cm | 93 ± 15 | 100 ± 16 a | 94 ± 15 b | 88 ± 13 c | 86 ± 14 cd | 84 ± 12 cd | 81 ± 11 d |
| Female, n (%) | 4890 (60) | 403 (46) | 2968 (59) | 1229 (68) | 209 (68) | 48 (70) | 33 (73) |
| Ethnicity, n (%) | 7640 | 788 | 4756 | 1704 | 287 | 64 | 41 |
| North American | 5782 (76) | 478 (61) | 3562 (75) | 1409 (83) | 243 (85) | 57 (89) | 33 (80) |
| Asian | 776 (10) | 109 (14) | 540 (11) | 107 (6) | 15 (5) | 3 (5) | 3 (7) |
| Aboriginal | 304 (4) | 112 (14) | 159 (4) | 30 (2) | 3 (1) | 0 | 0 |
| European | 492 (6.4) | 29 (4) | 300 (6) | 129 (7) | 26 (9) | 4 (6) | 4 (10) |
| Others | 286 (3.7) | 60 (7) | 195 (4) | 29 (2) | 1 (0) | 0 | 1 (3) |
| BMI status, n (%) | 8155 | 867 | 5059 | 1809 | 306 | 69 | 45 |
| Underweight | 101 (1.2) | 7 (1) | 48 (1) | 32 (2) | 6 (2) | 6 (9) | 2 (4) |
| Normal weigh | 2927 (36) | 203 (23.2) | 1693 (33.5) | 815 (45) | 159 (52) | 31 (44) | 26 (58) |
| Overweight | 2970 (36.4) | 293 (33.8) | 1884 (37) | 647 (36) | 105 (34) | 26 (38) | 15 (33) |
| Obese | 2157 (26.4) | 364 (42) | 1434 (28.5) | 315 (17) | 36 (12) | 6 (9) | 2 (5) |
| Self-reported HTN, n (%) | 1378 (27.5) | 119 (26.5) | 939 (29) | 281 (25) | 30 (17) | 7 (17) | 2 (8) |
| Male: Female (if hypertensive) | 1.25:1 | 2.69:1 | 1.32:1 | 0.60:1 | 1.83:1 | 1.50:1 | 0 |
| History of cardiac disease, n (%) | 381 (7.6) | 32 (7) | 242 (7.5) | 93 (8.4) | 12 (7) | 1 (2.4) | 1 (2.4) |
| BP lowering medication, n (%) | 1291 (32) | 94 (31) | 906 (34) | 255 (29) | 27 (20) | 7 (23) | 2 (13) |
| Season, n (%) | |||||||
| Winter (November–April) | 3836 (47) | 523 (60) | 2162 (43) | 900 (50) | 181 (59) | 42 (61) | 28 (62) |
| Summer (May–October) | 4319 (53) | 344 (40) | 2897 (57) | 909 (50) | 125 (41) | 27 (39) | 17 (38) |
| Parameter | n | Baseline Mean ± SD | One-Year Mean ± SD | p-Value * |
|---|---|---|---|---|
| BMI (kg/m2) | 8155 | 27 ± 6 | 28 ± 6 | <0.001 |
| Serum 25(OH)D (nmol/L) | 8155 | 87 ± 37 | 113 ± 39 | <0.001 |
| Vitamin D supplementation dose (IU/day) | 8127 | 1600 ± 2500 | 5200 ± 4300 | <0.001 |
| Vitamin B12 (pmol/L) | 7966 | 470 ± 310 | 1200 ± 1100 | <0.001 |
| Systolic BP (mmHg) | 8155 | 125 ± 17 | 125 ± 17 | 0.10 |
| Diastolic BP (mmHg) | 8155 | 77 ± 10 | 77 ± 9 | 0.10 |
| Pulse Pressure (mmHg) | 8155 | 48 ± 13 | 48 ± 14 | 0.09 |
| MAP (mmHg) | 8155 | 93 ± 11 | 93 ± 11 | 0.10 |
| AA:EPA | 1821 | 16 ± 8 | 11 ± 7 | <0.001 |
| Cases (n = 40) Hypertensive on No BP Meds | Controls (n = 80) Hypertensive on BP Meds after Joining Program | Cases (n = 187) Pre-Hypertensive on No BP Meds | Controls (n = 374) Pre-Hypertensive on BP Meds after Joining Program | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Mean ± SD | 95% CI | Mean ± SD | 95% CI | p Value a | Mean ± SD | 95% CI | Mean ± SD | 95% CI | p Value a |
| Systolic blood pressure (mmHg) | ||||||||||
| Baseline | 156 ± 15 | 151 to 161 | 155 ± 16 | 151 to 158 | 0.61 | 133 ± 10 | 131 to 134 | 136 ± 12 | 134 to 137 | 0.01 |
| Follow-up | 138 ± 21 * | 131 to 145 | 141 ± 17 * | 137 to 145 | 0.40 | 130 ± 15 * | 128 to 132 | 135 ± 17 | 133 to 137 | <0.01 |
| Changes | −18 ± 19 | −24 to −12 | −14 ± 21 | −18 to −9 | 0.25 | −3 ± 16 | −5 to −0.28 | −1 ± 19 | −1 to 1.3 | 0.23 |
| Diastolic blood pressure (mmHg) | ||||||||||
| Baseline | 96 ± 8 | 94 to 99 | 96 ± 7 | 94 to 97 | 0.92 | 81 ± 7 | 80 to 82 | 81 ± 8 | 80 to 82 | 0.59 |
| Follow-up | 84 ± 12 * | 80 to 87 | 84 ± 11 * | 81 to 86 | 0.97 | 79 ± 10 * | 78 to 80 | 79 ± 10 * | 78 to 80 | 0.74 |
| Changes | −12 ± 12 | −16 to −9 | −12 ± 12 | −15 to −10 | 0.97 | −2 ± 8 | −4 to −1.3 | −2 ± 10 | −3 to −0.85 | 0.46 |
| Pulse Pressure (mmHg) | ||||||||||
| Baseline | 60 ± 12 | 60 to 64 | 59 ± 13 | 56 to 62 | 0.56 | 52 ± 12 | 50 to 53 | 55 ± 14 | 53 to 56 | 0.01 |
| Follow-up | 55 ± 16 * | 49 to 60 | 58 ± 13 | 55 to 61 | 0.27 | 52 ± 13 | 50 to 54 | 56 ± 16 | 54 to 58 | <0.01 |
| Changes | −5 ± 17 | −11 to −0.44 | −1 ± 17 | −5 to 2 | 0.17 | 0 ± 15 | −2 to 2 | 1 ± 17 | −0.5 to 3 | 0.39 |
| Mean Arterial Pressure (mmHg) | ||||||||||
| Baseline | 116 ± 10 | 113 to 119 | 116 ± 9 | 114 to 118 | 0.73 | 98 ± 6 | 98 to 99 | 99 ± 6 | 98 to 100 | 0.24 |
| Follow-up | 102 ± 14 * | 97 to 106 | 103 ± 12 * | 100 to 105 | 0.69 | 96 ± 8 * | 95 to 97 | 98 ± 10 * | 97 to 99 | 0.05 |
| Changes | −14 ± 12 | −18 to −11 | −13 ± 13 | −16 to −10 | 0.53 | −2 ± 9 | −4 to −1 | −1 ± 11 | −3 to −0.34 | 0.26 |
| Serum 25(OH)D (nmol/L) | ||||||||||
| Baseline | 82 ± 38 | 70 to 95 | 83 ± 42 | 73 to 92 | 0.93 | 83 ± 32 | 79 to 88 | 87 ± 36 | 83 to 91 | 0.24 |
| Follow-up | 113 ± 35 * | 102 to 124 | 110 ± 37 * | 102 to 119 | 0.67 | 113 ± 36 * | 108 to 118 | 112 ± 34 * | 108 to 115 | 0.67 |
| Changes | 31 ± 35 | 20 to 43 | 27 ± 43 | 18 to 37 | 0.64 | 30 ± 36 | 24 to 35 | 25 ± 38 | 21 to 29 | 0.15 |
| Vitamin D supplementation dose | ||||||||||
| Baseline | 1900 ± 2800 | 970 to 2800 | 2000 ± 2800 | 1300 to 2600 | 0.87 | 1400 ± 2000 | 1200 to 1700 | 1700 ± 2500 | 1500 to 2000 | 0.17 |
| Follow-up | 5900 ± 4600 * | 4400 to 7300 | 6600 ± 4900 * | 5500 to 7700 | 0.42 | 5500 ± 4100 * | 5000 to 6100 | 5600 ± 4200 * | 5100 to 6000 | 0.88 |
| Changes | 4000 ± 5000 | 2400 to 5600 | 4700 ± 5500 | 3400 to 6000 | 0.52 | 4100 ± 4400 | 3500 to 4700 | 3800 ± 4700 | 3400 to 4300 | 0.43 |
| Vitamin B12 (pmol/L) | ||||||||||
| Baseline | 460 ± 310 | 360 to 560 | 440 ± 260 | 380 to 500 | 0.63 | 440 ± 270 | 400 to 480 | 520 ± 370 | 480 to 560 | 0.01 |
| Follow-up | 1500 ± 1300 * | 1000 to 1900 | 1300 ± 960 * | 1100 to 1500 | 0.45 | 1300 ± 1000 * | 1100 to 1400 | 1400 ± 1300 * | 1200 to 1500 | 0.38 |
| Changes | 1040 ± 1300 | 580 to 1500 | 860 ± 960 | 670 to 1100 | 0.55 | 860 ± 1900 | 680 to 970 | 880 ± 1300 | 720 to 980 | 0.30 |
| AA:EPA ratio # | ||||||||||
| Baseline | 15 ± 6.4 | 10 to 19 | 15 ± 7.5 | 5.6 to 18 | 0.93 | 16 ± 7.1 | 12 to 16 | 15 ± 7.1 | 12 to 19 | 0.15 |
| Follow-up | 6.1 ± 2.8 * | 2.8 to 9.2 | 7.9 ± 4.3 * | 4.1 to 10 | 0.37 | 8.6 ± 4.4 * | 7.0 to 10 | 9.7 ± 6.9 | 7.9 to 11 * | 0.39 |
| Changes | −8.7 ± 4.7 | −14 to −3.7 | −6.8 ± 5.7 | −11 to 0.89 | 0.26 | −7.2 ± 5.1 | −7.4 to −3.5 | −4.9 ± 7.7 | −10 to −5.5 | 0.17 |
| Dependent Variable | Model | Co-Variables | Exp (B) | p-Value | 95% CI |
|---|---|---|---|---|---|
| Multivariate analysis | |||||
| HTN (R2 = 0.03, p < 0.001) | 1 | Age | 1.02 * | <0.001 | 1.01–1.03 |
| Sex (male) | 1.91 * | <0.01 | 1.50–2.43 | ||
| 25(OH)D (BL < 50 and FL ≥ 100) | Ref | 0.07 | |||
| 25(OH)D (BL 50–100 and FL ≥ 100) | 0.64 * | 0.04 | 0.41–0.99 | ||
| 25(OH)D (BL ≥ 100 and FL ≥ 100) | 0.58 * | 0.02 | 0.36–0.92 | ||
| HTN (R2 = 0.27, p < 0.001) | 2 | Age | 1.03 | 0.34 | 0.97–1.1 |
| Sex (male) | 3.5 | 0.11 | 0.73–17 | ||
| 25(OH)D (BL < 50 and FL ≥ 100) | Ref | 0.08 | |||
| 25(OH)D (BL 50–100 and FL ≥ 100) | 0.10 * | 0.03 | 0.01–0.87 | ||
| 25(OH)D (BL ≥ 100 and FL ≥ 100) | 0.46 | 0.43 | 0.07–3.2 | ||
| Season (Winter) | 1.0 | 0.98 | 0.27–3.7 | ||
| Vitamin B12 (BL < 450 and FL ≥ 450) | 1.2 | 0.76 | 0.30–5.1 | ||
| Ethnicity (other ethnics) | Ref | 0.12 | |||
| North American | 0.45 * | 0.03 | 0.21–0.96 | ||
| Asian | 0.25 * | 0.01 | 0.08–0.78 | ||
| European | 0.44 | 0.15 | 0.17–1.5 | ||
| Aboriginal | 0.53 | 0.23 | 0.19–1.5 | ||
| BMI (Normal weight) | Ref | 0.11 | |||
| Overweight | 0.67 | 0.65 | 0.12–3.8 | ||
| Obese | 3.6 | 0.11 | 0.75–17 | ||
| AA:EPA ratio | 0.88 | 0.24 | 0.71–1.1 | ||
| Inflammation (hs-CRP) | 0.85 | 0.62 | 0.41–1.7 | ||
| Fruit and vegetable consumption (≥5 servings/day) | 0.28 | 0.07 | 0.07–1.1 | ||
| Physical activity (none) | Ref | 0.95 | |||
| Mild | 1.8 | 0.64 | 0.15–23 | ||
| Moderate | 1.3 | 0.82 | 0.11–16 | ||
| Strenuous | 1.6 | 0.77 | 0.06–45 | ||
| Tobacco use (yes) | 2.1 | 0.40 | 0.36–13 | ||
| BP lowering medication (yes) | 0.20 | 0.21 | 0.02–2.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirhosseini, N.; Vatanparast, H.; Kimball, S.M. The Association between Serum 25(OH)D Status and Blood Pressure in Participants of a Community-Based Program Taking Vitamin D Supplements. Nutrients 2017, 9, 1244. https://doi.org/10.3390/nu9111244
Mirhosseini N, Vatanparast H, Kimball SM. The Association between Serum 25(OH)D Status and Blood Pressure in Participants of a Community-Based Program Taking Vitamin D Supplements. Nutrients. 2017; 9(11):1244. https://doi.org/10.3390/nu9111244
Chicago/Turabian StyleMirhosseini, Naghmeh, Hassanali Vatanparast, and Samantha M. Kimball. 2017. "The Association between Serum 25(OH)D Status and Blood Pressure in Participants of a Community-Based Program Taking Vitamin D Supplements" Nutrients 9, no. 11: 1244. https://doi.org/10.3390/nu9111244






