Growth, Gastrointestinal Tolerance and Stool Characteristics of Healthy Term Infants Fed an Infant Formula Containing Hydrolyzed Whey Protein (63%) and Intact Casein (37%): A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Study Formulas
2.4. Study Procedures
2.5. Sample Size
2.6. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Growth
3.3. Gastrointestinal Tolerance
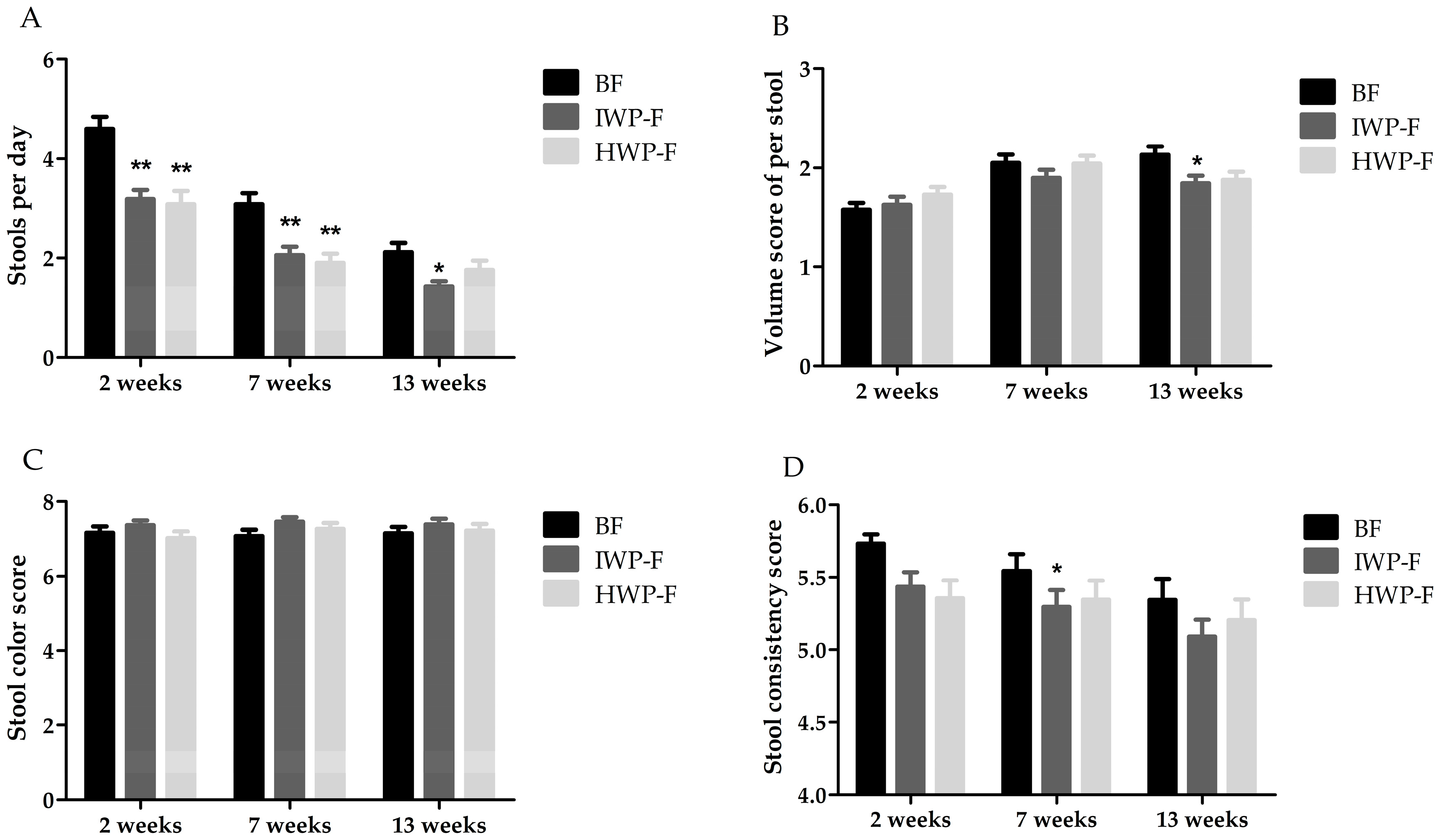
3.4. Stool Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Victora, C.G.; Bahl, R.; Barros, A.J.; Franca, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Le Huerou-Luron, I.; Blat, S.; Boudry, G. Breast- v. formula-feeding: Impacts on the digestive tract and immediate and long-term health effects. Nutr. Res. Rev. 2010, 23, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Spieldenner, J.; Farah, B.; Detzel, P.; Possner, M.; Iskedjian, M. Financial Budget Impact Analysis for Reimbursement of a 100% Whey-Based Partially-Hydrolyzed Infant Formula in Prevention of Atopic Dermatitis in Germany. Pediar. Res. 2011, 70, 787. [Google Scholar] [CrossRef]
- Mousan, G.; Kamat, D. Cow’s Milk Protein Allergy. Clin. Pediatr. 2016, 55, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Bongers, M.E.; de Lorijn, F.; Reitsma, J.B.; Groeneweg, M.; Taminiau, J.A.; Benninga, M.A. The clinical effect of a new infant formula in term infants with constipation: A double-blind, randomized cross-over trial. Nutr. J. 2007, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Iacono, G.; Merolla, R.; D’Amico, D.; Bonci, E.; Cavataio, F.; Di Prima, L.; Scalici, C.; Indinnimeo, L.; Averna, M.R.; Carroccio, A. Gastrointestinal symptoms in infancy: A population-based prospective study. Dig. Liver Dis. 2005, 37, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.A.; Hogel, J.; Pohlandt, F. Hydrolysed protein accelerates the gastrointestinal transport of formula in preterm infants. Acta Paediatr. 2001, 90, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Corvaglia, L.; Mariani, E.; Aceti, A.; Galletti, S.; Faldella, G. Extensively hydrolyzed protein formula reduces acid gastro-esophageal reflux in symptomatic preterm infants. Early Hum. Dev. 2013, 89, 453–455. [Google Scholar] [CrossRef] [PubMed]
- McGraw, N.J.; Napawan, N.; Toland, M.R.; Schulze, J.; Tulk, B.M.; Krul, E.S. Partially Hydrolyzed Soy Protein Shows Enhanced Transport of Amino Acids Compared to Nonhydrolyzed Protein across an Intestinal Epithelial Cell Monolayer. J. Food Sci. 2014, 79, H1832–H1840. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Gottrand, F.; Veereman-Wauters, G.; De Greef, E.; Devreker, T.; Hauser, B.; Benninga, M.; Heymans, H.S. Gastrointestinal manifestations of cow’s milk protein allergy and gastrointestinal motility. Acta Paediatr. 2012, 101, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.A.; Franz, A.R.; Hogel, J.; Pohlandt, F. Hydrolyzed protein accelerates feeding advancement in very low birth weight infants. Pediatrics 2002, 110, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Crombach, N.; Gijsen, A.P.; Walrand, S.; Fauquant, J.; Kies, A.K.; Lemosquet, S.; Saris, W.H.; Boirie, Y.; van Loon, L.J. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am. J. Clin. Nutr. 2009, 90, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.Y.; Cao, R.M.; Chen, J.; Kaku, Y.; Wu, J.; Cheng, Y.; Shimizu, T.; Takase, M.; Wu, S.M.; Chen, T.X. Partially hydrolyzed cow’s milk formula has a therapeutic effect on the infants with mild to moderate atopic dermatitis: A randomized, double-blind study. Pediatr. Allergy Immunol. 2011, 22, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Benninga, M.; Broekaert, I.; Falconer, J.; Gottrand, F.; Guarino, A.; Lifschitz, C.; Lionetti, P.; Orel, R.; Papadopoulou, A.; et al. Functional gastro-intestinal disorder algorithms focus on early recognition, parental reassurance and nutritional strategies. Acta Paediatr. 2016, 105, 244–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Anthro (Version 3.2.2, January 2011) and Macros. Available online: http://www.who.int/childgrowth/software (accessed on 2 March 2017).
- Tseng, J.J.; Lai, M.S.; Lin, M.C.; Fu, Y.C. Stool color card screening for biliary atresia. Pediatrics 2011, 128, e1209–e1215. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.E.; Jung, H.K.; Lee, T.H.; Jo, Y.; Lee, H.; Song, K.H.; Hong, S.N.; Lim, H.C.; Lee, S.J.; Chung, S.S.; et al. Guidelines for the Diagnosis and Treatment of Chronic Functional Constipation in Korea, 2015 Revised Edition. J. Neurogastroenterol. Motil. 2016, 22, 383–411. [Google Scholar] [CrossRef] [PubMed]
- Clinical Testing of Infant Formulas with Respect to Nutritional Suitability for Term Infants. Available online: https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/InfantFormula/default.htm (accessed on 2 March 2017).
- Hernell, O.; Lonnerdal, B. Nutritional evaluation of protein hydrolysate formulas in healthy term infants: Plasma amino acids, hematology, and trace elements. Am. J. Clin. Nutr. 2003, 78, 296–301. [Google Scholar] [PubMed]
- Vandenplas, Y.; Leluyer, B.; Cazaubiel, M.; Housez, B.; Bocquet, A. Double-Blind Comparative Trial with 2 Antiregurgitation Formulae. J. Pediatr. Gastr. Nutr. 2013, 57, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Florendo, K.N.; Bellflower, B.; van Zwol, A.; Cooke, R.J. Growth in preterm infants fed either a partially hydrolyzed whey or an intact casein/whey preterm infant formula. J. Perinatol. 2009, 29, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Ventura, A.K.; Beauchamp, G.K. Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics 2011, 127, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Rzehak, P.; Sausenthaler, S.; Koletzko, S.; Reinhardt, D.; von Berg, A.; Kramer, U.; Berdel, D.; Bollrath, C.; Grubl, A.; Bauer, C.P.; et al. Short- and long-term effects of feeding hydrolyzed protein infant formulas on growth at < or = 6 years of age: Results from the German Infant Nutritional Intervention Study. Am. J. Clin. Nutr. 2009, 89, 1846–1856. [Google Scholar] [PubMed]
- Rzehak, P.; Sausenthaler, S.; Koletzko, S.; Reinhardt, D.; von Berg, A.; Kramer, U.; Berdel, D.; Bollrath, C.; Grubl, A.; Bauer, C.P.; et al. Long-term effects of hydrolyzed protein infant formulas on growth—Extended follow-up to 10 y of age: Results from the German Infant Nutritional Intervention (GINI) study. Am. J. Clin. Nutr. 2011, 94, 1803S–1807S. [Google Scholar] [CrossRef] [PubMed]
- Von Berg, A.; Koletzko, S.; Grubl, A.; Filipiak-Pittroff, B.; Wichmann, H.E.; Bauer, C.P.; Reinhardt, D.; Berdel, D. The effect of hydrolyzed cow’s milk formula for allergy prevention in the first year of life: The German Infant Nutritional Intervention Study, a randomized double-blind trial. J. Allergy Clin. Immunol. 2003, 111, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A.; Hawthorne, K.M.; Pammi, M. A Systematic Review of Controlled Trials of Lower-Protein or Energy-Containing Infant Formulas for Use by Healthy Full-Term Infants. Adv. Nutr. 2015, 6, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Schmelzle, H.; Wirth, S.; Skopnik, H.; Radke, M.; Knol, J.; Bockler, H.M.; Bronstrup, A.; Wells, J.; Fusch, C. Randomized double-blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta-palmitic acid level, and nondigestible oligosaccharides. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, D.G.M.; D’Auria, E.; Peroni, D.; Palazzo, S.; Radaelli, G.; Comberiati, P.; Galdo, F.; Maiello, N.; Riva, E. Flavor, relative palatability and components of cow’s milk hydrolysed formulas and amino acid-based formula. Ital. J. Pediatr. 2015, 41, 42. [Google Scholar] [CrossRef] [PubMed]
- Kuczmarski, R.J.; Ogden, C.L.; Grummer-Strawn, L.M.; Flegal, K.M.; Guo, S.S.; Wei, R.; Mei, Z.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. CDC growth charts: United States. Adv. Data 2000, 314, 1–27. [Google Scholar]
- Picaud, J.C.; Rigo, J.; Normand, S.; Lapillonne, A.; Reygrobellet, B.; Claris, O.; Salle, B.L. Nutritional efficacy of preterm formula with a partially hydrolyzed protein source: A randomized pilot study. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, J.B.; Koo, W.W.; Walters, J.; Neylan, M.; Luebbers, S. Growth, tolerance and biochemical measures in healthy infants fed a partially hydrolyzed rice protein-based formula: A randomized, blinded, prospective trial. J. Am. Coll. Nutr. 2006, 25, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lucassen, P.L.; Assendelft, W.J.; Gubbels, J.W.; van Eijk, J.T.; Douwes, A.C. Infantile colic: Crying time reduction with a whey hydrolysate: A double-blind, randomized, placebo-controlled trial. Pediatrics 2000, 106, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Abkari, A.; Bellaiche, M.; Benninga, M.; Chouraqui, J.P.; Çokura, F.; Harb, T.; Hegar, B.; Lifschitz, C.; Ludwig, T.; et al. Prevalence and Health Outcomes of Functional Gastrointestinal Symptoms in Infants from Birth to 12 Months of Age. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Martinelli, M.; Sciorio, E.; Botta, C.; Miele, E.; Vallone, G.; Staiano, A. Stool Consistency, but Not Frequency, Correlates with Total Gastrointestinal Transit Time in Children. J. Pediatr. 2013, 162, 1188–1192. [Google Scholar] [CrossRef] [PubMed]

| IWP-F (per 100 mL) | HWP-F (per 100 mL) | |
|---|---|---|
| Energy, kJ | 285 | 287 |
| Protein, g | 1.47 | 1.55 |
| Intact whey protein, g | 0.90 | / |
| Hydrolyzed whey protein, g | / | 0.97 |
| intact casein, g | 0.57 | 0.58 |
| Whey/casein | 61:39 | 63:37 |
| Fat, g | 3.74 | 3.73 |
| Linoleic acid, g | 0.57 | 0.57 |
| ɑ-linolenic acid, mg | 57 | 57 |
| DHA, mg | 7.5 | 7.5 |
| ARA, mg | 10.6 | 10.8 |
| Carbohydrate, g | 7.0 | 7.0 |
| Lactose, g | 6.6 | 6.6 |
| GOS, FOS, g | 0.4 | 0.4 |
| Vitamins (vits) | ||
| Vitamin A, μgRE | 51 | 52 |
| Vitamin D, μg | 1.12 | 1.14 |
| Vitamin E, mg α-TE | 0.92 | 0.81 |
| Vitamin K1, μg | 9.2 | 8.1 |
| Vitamin B1, μg | 73 | 70 |
| Vitamin B2, μg | 92 | 88 |
| Vitamin B6, μg | 55.4 | 51.3 |
| Vitamin B12, μg | 0.198 | 0.203 |
| Vitamin C, mg | 7.9 | 7.4 |
| Minerals | ||
| Calcium, mg | 46 | 41 |
| Phosphorus, mg | 29 | 27 |
| Magnesium, mg | 4.8 | 5.4 |
| Sodium, mg | 17 | 18 |
| Kalium, mg | 49 | 43 |
| Chlorine, mg | 42 | 51 |
| Iron, mg | 0.63 | 0.63 |
| Zinc, mg | 0.50 | 0.50 |
| Copper, μg | 46.2 | 47.3 |
| Iodine, μg | 7.7 | 8.3 |
| Manganese, μg | 4.0 | 4.4 |
| Selenium, μg | 1.91 | 1.73 |
| Group | p | |||
|---|---|---|---|---|
| BF (n = 63) | IWP-F (n = 65) | HWP-F (n = 61) | ||
| Infant characteristics | ||||
| Gender, male, n (%) | 29 (46.0) | 29 (44.6) | 41 (67.2) 1 | 0.019 |
| Age at study entry, days, median(IQR) | 5.0 (3.0,7.0) | 6.0 (3.0,9.0) | 6.0 (2.0,9.0) | 0.236 |
| Birth weight, kg, mean ± SD | 3.4 ± 0.4 | 3.4 ± 0.4 | 3.4 ± 0.3 | 0.532 |
| Gestational age at birth, week, mean ± SD | 39.2 ± 1.1 | 39.6 ± 1.1 | 39.4 ± 1.0 | 0.252 |
| Caesarean delivery, n (%) | 34 (54.8) | 39 (60.0) | 32 (52.5) | 0.682 |
| Maternal characteristics | ||||
| Age at study entry, years, mean ± SD | 28.5 ± 4.1 | 29.1 ± 4.7 | 28.4 ± 4.7 | 0.687 |
| BMI at study entry, kg/m2, median(IQR) | 22.8 (19.6,27.9) | 23.3 (21.5,25.8) | 23.4 (21.3,26.1) | 0.396 |
| Parity, primiparous, n (%) | 21 (33.3) | 27 (42.2) | 25 (41.0) | 0.556 |
| Education level, n (%) | ||||
| Primary school or below | 10 (15.9) | 18 (27.7) | 16 (26.2) | 0.434 |
| Secondary school | 20 (31.7) | 22 (33.8) | 19 (31.1) | |
| High school or above | 33 (52.4) | 25 (38.5) | 26 (42.6) | |
| Active or passive smoking during pregnancy, n (%) | 13 (20.6) | 20 (30.8) | 16 (26.2) | 0.424 |
| Baseline | p 3 | 7 Weeks | p 3 | 13 Weeks | p 3 | p 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BF (n = 63) | IWP-F (n = 65) | HWP-F (n = 61) | BF (n = 63) | IWP-F (n = 65) | HWP-F (n = 61) | BF (n = 63) | IWP-F (n = 65) | HWP-F (n = 61) | |||||
| Absolute values 1 | |||||||||||||
| Weight, kg | 3.6 ± 0.5 | 3.5 ± 0.4 | 3.6 ± 0.5 | 0.708 | 5.3 ± 0.5 | 5.3 ± 0.5 | 5.2 ± 0.7 | 0.759 | 6.5 ± 0.6 | 6.5 ± 0.6 | 6.5 ± 0.8 | 0.927 | 0.379 |
| Crown–heel length, cm | 51.3 ± 2.1 | 51.3 ± 2.0 | 51.4 ± 2.2 | 0.506 | 57.4 ± 2.0 | 57.5 ± 2.1 | 57.6 ± 2.3 | 0.893 | 61.5 ± 2.0 | 61.7 ± 2.1 | 61.6 ± 2.7 | 0.855 | 0.857 |
| Head circumference, cm | 34.9 ± 1.4 | 34.8 ± 1.2 | 35.1 ± 1.3 | 0.605 | 38.1 ± 1.2 | 37.7 ± 1.1 | 38.1 ± 1.3 | 0.099 | 39.9 ± 1.1 | 39.8 ± 1.1 | 39.9 ± 1.2 | 0.915 | 0.405 |
| BMI, kg/m2 | 13.5 ± 1.3 | 13.3 ± 1.2 | 13.4 ± 1.4 | 0.830 | 16.0 ± 1.3 | 15.9 ± 1.2 | 15.7 ± 1.6 | 0.345 | 17.1 ± 1.5 | 17.1 ± 1.4 | 17.1 ± 1.6 | 0.995 | 0.543 |
| z-scores 1,2 | |||||||||||||
| Weight-for age z-score | 0.22 ± 0.87 | 0.21 ± 0.76 | 0.29 ± 0.96 | 0.852 | 0.33 ± 0.70 | 0.40 ± 0.73 | 0.11 ± 1.03 | 0.129 | 0.43 ± 0.77 | 0.50 ± 0.65 | 0.31 ± 0.91 | 0.393 | 0.157 |
| Length-for-age z-score | 0.30 ± 1.08 | 0.39 ± 1.09 | 0.52 ± 1.24 | 0.551 | 0.36 ± 0.94 | 0.53 ± 0.97 | 0.37 ± 1.04 | 0.543 | 0.41 ± 0.92 | 0.52 ± 1.02 | 0.29 ± 1.21 | 0.480 | 0.401 |
| Weight-for-length z-score | −0.32 ± 1.18 | −0.43 ± 1.17 | −0.42 ± 1.20 | 0.832 | 0.12 ± 1.05 | 0.02 ± 1.01 | −0.11 ± 1.11 | 0.482 | 0.25 ± 1.02 | 0.25 ± 0.95 | 0.22 ± 1.23 | 0.983 | 0.886 |
| Head circumference-for-age z-score | 0.06 ± 1.11 | 0.03 ± 0.98 | 0.15 ± 1.05 | 0.798 | −0.04 ± 0.95 | −0.30 ± 0.93 | −0.20 ± 1.05 | 0.345 | −0.11 ± 0.84 | −0.16 ± 0.88 | −0.31 ± 0.96 | 0.460 | 0.302 |
| BMI-for-age z-score | 0.06 ± 1.01 | −0.03 ± 0.89 | −0.01 ± 1.07 | 0.871 | 0.18 ± 0.89 | 0.15 ± 0.83 | −0.05 ± 1.00 | 0.309 | 0.28 ± 0.96 | 0.29 ± 0.85 | 0.20 ± 1.11 | 0.861 | 0.740 |
| Group | p 2 | p 3 | |||
|---|---|---|---|---|---|
| BF (n = 63) | IWP-F (n = 65) | HWP-F (n = 61) | |||
| Weight, g/day 1 | |||||
| Baseline–7 weeks | 40.5 ± 10.3 | 42.1 ± 11.4 | 38.2 ± 13.7 | 0.195 | 0.079 |
| Baseline–13 weeks | 34.6 ± 6.4 | 35.3 ± 7.0 | 34.1 ± 8.4 | 0.618 | 0.325 |
| Crown–heel length, cm/day 1 | |||||
| Baseline–7 weeks | 0.14 ± 0.05 | 0.15 ± 0.05 | 0.14 ± 0.05 | 0.268 | 0.119 |
| Baseline–13 weeks | 0.12 ± 0.02 | 0.12 ± 0.03 | 0.12 ± 0.03 | 0.343 | 0.313 |
| Head circumference, cm/day 1 | |||||
| Baseline–7 weeks | 0.07 ± 0.03 | 0.07 ± 0.03 | 0.07 ± 0.03 | 0.535 | 0.396 |
| Baseline–13 weeks | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.568 | 0.566 |
| Group | p 2 | |||
|---|---|---|---|---|
| BF (n = 63) | IWP-F (n = 65) | HWP-F (n = 61) | ||
| Number of daily feedings 1 | ||||
| 2 weeks | 9.7 ± 1.8 | 8.7 ± 1.7 3 | 8.6 ± 1.8 3 | 0.002 |
| 7 weeks | 9.4 ± 1.7 | 8.4 ± 1.7 3 | 8.4 ± 1.6 3 | 0.002 |
| 13 weeks | 8.7 ± 1.7 | 7.7 ± 1.6 3 | 7.8 ± 1.6 3 | 0.002 |
| Volume of formula intake, mL/kg/day 1 | ||||
| 2 weeks | N/A | 128.8 ± 47.8 | 135.3 ± 52.2 | 0.445 |
| 7 weeks | N/A | 127.9 ± 37.4 | 153.4 ± 51.7 | 0.002 |
| 13 weeks | N/A | 117.7 ± 34.1 | 140.9 ± 39.3 | 0.001 |
| Number of daily crying 1 | ||||
| 2 weeks | 1.3 ± 2.2 | 1.2 ± 2.1 | 1.1 ± 1.7 | 0.905 |
| 7 weeks | 0.9 ± 1.9 | 1.3 ± 2.2 | 1.0 ± 1.8 | 0.441 |
| 13 weeks | 0.4 ± 1.2 | 0.8 ± 1.9 | 0.7 ± 1.5 | 0.385 |
| Episodes of adverse events, n (%) | ||||
| Baseline–7 weeks | 2 (3.2) | 5 (7.7) | 2 (3.3) | 0.515 |
| 7 weeks–13 weeks | 7 (11.1) | 8 (12.3) | 5 (8.2) | 0.745 |
| Episodes of difficult defecation, n (%) | ||||
| Baseline–7 weeks | 5 (7.9) | 13 (20.0) | 11 (18.0) | 0.130 |
| 7 weeks–13 weeks | 7 (11.1) | 12 (18.5) | 6 (9.8) | 0.300 |
| Episodes of spit-up, n (%) | ||||
| Baseline–7 weeks | 21 (33.3) | 28 (43.1) | 18 (29.5) | 0.257 |
| 7 weeks–13 weeks | 19 (30.2) | 24 (36.9) | 17 (27.9) | 0.522 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.-L.; Ding, D.; Fang, A.-P.; Chen, P.-Y.; Chen, S.; Jing, L.-P.; Chen, Y.-M.; Zhu, H.-L. Growth, Gastrointestinal Tolerance and Stool Characteristics of Healthy Term Infants Fed an Infant Formula Containing Hydrolyzed Whey Protein (63%) and Intact Casein (37%): A Randomized Clinical Trial. Nutrients 2017, 9, 1254. https://doi.org/10.3390/nu9111254
Wu S-L, Ding D, Fang A-P, Chen P-Y, Chen S, Jing L-P, Chen Y-M, Zhu H-L. Growth, Gastrointestinal Tolerance and Stool Characteristics of Healthy Term Infants Fed an Infant Formula Containing Hydrolyzed Whey Protein (63%) and Intact Casein (37%): A Randomized Clinical Trial. Nutrients. 2017; 9(11):1254. https://doi.org/10.3390/nu9111254
Chicago/Turabian StyleWu, Shang-Ling, Ding Ding, Ai-Ping Fang, Pei-Yan Chen, Si Chen, Li-Peng Jing, Yu-Ming Chen, and Hui-Lian Zhu. 2017. "Growth, Gastrointestinal Tolerance and Stool Characteristics of Healthy Term Infants Fed an Infant Formula Containing Hydrolyzed Whey Protein (63%) and Intact Casein (37%): A Randomized Clinical Trial" Nutrients 9, no. 11: 1254. https://doi.org/10.3390/nu9111254





