Prevalence and Predictors of Subclinical Micronutrient Deficiency in German Older Adults: Results from the Population-Based KORA-Age Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ethical Considerations
2.3. Blood Sample Processing
2.4. Biochemical Analyses of Nutritional Biomarkers
2.5. Cut-Off Points to Classify Subclinical Micronutrient Deficiency
2.6. Assesment of Predictors
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants
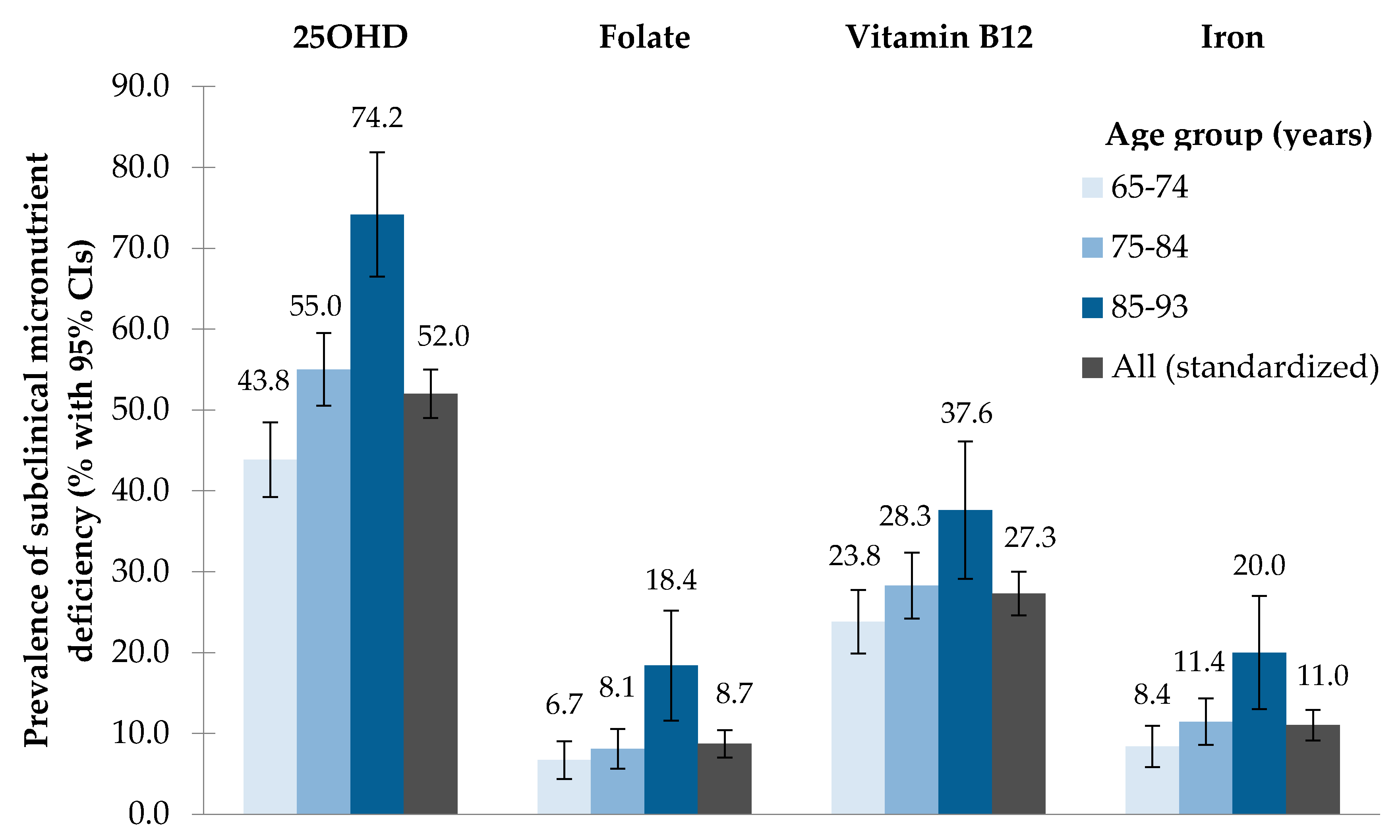
3.2. Prevalence of Subclinical Micronutrient Deficiency
3.3. Predictors of Subclinical Micronutrient Deficiency
4. Discussion
4.1. Prevalence of Subclinical Micronutrient Deficiency
4.2. Predictors of Subclinical Micronutrient Deficiency
4.2.1. Common Predictors of Subclinical Micronutrient Deficiency
Very Old Age
Physical Inactivity
Frailty
No/Irregular Use of Supplements
4.2.2. Further Predictors of Subclinical Micronutrient Deficiency
Vitamin D (Springtime, Female Sex, Obesity, Former Smoking)
Vitamin B12 (No Polypharmacy)
Folate (Alcohol Consumption)
Iron (Male Sex, GNRI, Polypharmacy)
4.3. Strengths and Weaknesses
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 25OHD | 25-hydroxyvitamin D |
| BMI | Body Mass Index |
| CI | Confidence interval |
| CVD | Cardiovascular disease |
| DEGS1 | German Health Interview and Examination Survey for Adults |
| DGE | German Nutrition Society |
| eGFR | Estimated Glomerular Filtration Rate |
| GNRI | Geriatric Nutritional Risk Index |
| IDOM | Instrument for Databased Assessment Of Medication |
| IQR | Interquartile range |
| KORA | Cooperative Health Research in the Region of Augsburg |
| MONICA | Monitoring of Trends and Determinants in Cardiovascular Disease |
| NNR | Nordic Nutrition Recommendations |
| NVS II | Second German National Nutrition Survey |
| OR | Odds ratio |
| Q1 | First quartile or 25th percentile |
| Q3 | Third quartile or 75th percentile |
| SCREEN II | Seniors in the Community Risk Evaluation for Eating and Nutrition, version II |
| WHO | World Health Organization |
References
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 22–33. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 2013, 5, 111–148. [Google Scholar] [CrossRef] [PubMed]
- Trombetti, A.; Reid, K.F.; Hars, M.; Herrmann, F.R.; Pasha, E.; Phillips, E.M.; Fielding, R.A. Age-associated declines in muscle mass, strength, power, and physical performance: Impact on fear of falling and quality of life. Osteoporos. Int. 2016, 27, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Deeg, D.J.; Lips, P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.; Hoey, L.; Hughes, C.F.; Ward, M.; McNulty, H. Causes, Consequences and Public Health Implications of Low B-Vitamin Status in Ageing. Nutrients 2016, 8, 725. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Wawer, A.A.; Gillings, R.; Jennings, A.; Myint, P.K. Iron status in the elderly. Mech. Ageing Dev. 2014, 136–137, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.; Falvey, C.; Harris, T.B.; Simonsick, E.M.; Satterfield, S.; Ferrucci, L.; Metti, A.L.; Patel, K.V.; Yaffe, K. Anemia and risk of dementia in older adults: Findings from the Health ABC study. Neurology 2013, 81, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C. Vitamin D and Aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, R. Micronutrient requirements in older women. Am. J. Clin. Nutr. 2005, 81, 1240s–1245s. [Google Scholar] [PubMed]
- Ter Borg, S.; Verlaan, S.; Hemsworth, J.; Mijnarends, D.M.; Schols, J.M.; Luiking, Y.C.; de Groot, L.C. Micronutrient intakes and potential inadequacies of community-dwelling older adults: A systematic review. Br. J. Nutr. 2015, 113, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Rubner-Institut, M. Nationale Verzehrsstudie II, Ergebnisbericht Teil 2. Die Bundesweite Befragung zur Ernährung von Jugendlichen und Erwachsenen. 2008. Available online: https://www.mri.bund.de/fileadmin/MRI/Institute/EV/NVSII_Abschlussbericht_Teil_2.pdf (accessed on 14 November 2017).
- Rabenberg, M.; Scheidt-Nave, C.; Busch, M.A.; Rieckmann, N.; Hintzpeter, B.; Mensink, G.B. Vitamin D status among adults in Germany—Results from the German Health Interview and Examination Survey for Adults (DEGS1). BMC Public Health 2015, 15, 641. [Google Scholar] [CrossRef] [PubMed]
- McNeill, G.; Vyvyan, J.; Peace, H.; McKie, L.; Seymour, G.; Hendry, J.; MacPherson, I. Predictors of micronutrient status in men and women over 75 years old living in the community. Br. J. Nutr. 2002, 88, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.R.; Granic, A.; Davies, K.; Collerton, J.; Martin-Ruiz, C.; Siervo, M.; Mathers, J.C.; Adamson, A.J.; Francis, R.M.; Pearce, S.H.; et al. Serum 25-hydroxyvitamin D concentration and its determinants in the very old: The Newcastle 85+ Study. Osteoporos. Int. 2016, 27, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Hirani, V.; Tull, K.; Ali, A.; Mindell, J. Urgent action needed to improve vitamin D status among older people in England! Age Ageing 2010, 39, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Snijder, M.B.; Dekker, J.M.; Stehouwer, C.D.; Bouter, L.M.; Heine, R.J.; Lips, P. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: The hoorn study. Am. J. Clin. Nutr. 2007, 85, 755–761. [Google Scholar] [PubMed]
- McCarroll, K.; Beirne, A.; Casey, M.; McNulty, H.; Ward, M.; Hoey, L.; Molloy, A.; Laird, E.; Healy, M.; Strain, J.J.; et al. Determinants of 25-hydroxyvitamin D in older Irish adults. Age Ageing 2015, 44, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Jungert, A.; Neuhauser-Berthold, M. Sex-specific determinants of serum 25-hydroxyvitamin D3 concentrations in an elderly German cohort: A cross-sectional study. Nutr. Metab. Lond. 2015, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Doring, A.; Ladwig, K.H.; Meisinger, C.; Linkohr, B.; Autenrieth, C.; Baumeister, S.E.; Behr, J.; Bergner, A.; Bickel, H.; et al. Multimorbidity and successful aging: The population-based KORA-Age study. Z. Gerontol. Geriatr. 2011, 44 (Suppl. 2), 41–54. [Google Scholar] [CrossRef] [PubMed]
- German Nutrition Society. New reference values for vitamin D. Ann. Nutr. Metab. 2012, 60, 241–246. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C.; Brustad, M.; Meyer, H.E.; Steingrimsdottir, L. Vitamin D—A systematic literature review for the 5th edition of the Nordic Nutrition Recommendations. Food Nutr. Res. 2013, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauberlich, H. Laboratory Tests for the Assessment of Nutritional Status, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1999; p. 512. [Google Scholar]
- Green, R.; Allen, L.H.; Bjorke-Monsen, A.L.; Brito, A.; Gueant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Prim. 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, L.; Pesce, A. Clinical Chemistry: Theory, Analysis, and Correlation, 3rd ed.; Mosby, Inc.: St. Louis, MO, USA, 1996; p. 699. [Google Scholar]
- Ahrens, W.; Bellach, B.M.; Jöckel, K.H. Measurement and Quantification of Sociodemographic Characteristics in Epidemiological Studies. German Society for Medical Informatics, Biometry and Epidemiology (GMDS), 1998. Available online: https://dgepi.de/fileadmin/pdf/leitlinien/11_MessungUndQuantifizierungSoziodemographischerMerkmale_pdf2.pdf (accessed on 14 November 2017).
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric nutritional risk index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [PubMed]
- Keller, H.H.; Goy, R.; Kane, S.L. Validity and reliability of SCREEN II (Seniors in the community: Risk evaluation for eating and nutrition, Version II). Eur. J. Clin. Nutr. 2005, 59, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Lowel, H.; Thorand, B.; Doring, A. Leisure time physical activity and the risk of type 2 diabetes in men and women from the general population. The MONICA/KORA Augsburg Cohort Study. Diabetologia 2005, 48, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Doring, A.; Filipiak, B.; Stieber, J.; Keil, U. Trends in alcohol intake in a southern German population from 1984–1985 to 1989–1990: Results of the MONICA project augsburg. J. Stud. Alcohol 1993, 54, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Kirchberger, I.; Meisinger, C.; Heier, M.; Zimmermann, A.K.; Thorand, B.; Autenrieth, C.S.; Peters, A.; Ladwig, K.H.; Doring, A. Patterns of multimorbidity in the aged population. Results from the KORA-Age study. PLoS ONE 2012, 7, e30556. [Google Scholar] [CrossRef] [PubMed]
- Strobl, R.; Muller, M.; Emeny, R.; Peters, A.; Grill, E. Distribution and determinants of functioning and disability in aged adults–results from the German KORA-Age study. BMC Public Health 2013, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Pabst, G.; Zimmermann, A.K.; Huth, C.; Koenig, W.; Ludwig, T.; Zierer, A.; Peters, A.; Thorand, B. Association of low 25-hydroxyvitamin D levels with the frailty syndrome in an aged population: Results from the KORA-age Augsburg study. J. Nutr. Health Aging 2015, 19, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Mühlberger, N.; Behrend, C.; Stark, R.; Holle, R. Database-supported identification and entry of drug data in health studies–experience with the IDOM software. Inform. Biom. Epidemiol. Med. Biol. 2003, 34, 601–611. [Google Scholar]
- Schwab, S.; Heier, M.; Schneider, A.; Fischer, B.; Huth, C.; Peters, A.; Thorand, B. The use of dietary supplements among older persons in southern Germany–results from the KORA-age study. J. Nutr. Health Aging 2014, 18, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Mithal, A.; Wahl, D.A.; Bonjour, J.P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Austrian Nutrition Report. Österreichischer Ernährungsbericht 2012. Bundesministerium für Gesundheit, Wien. 2012. Available online: https://www.bmgf.gv.at/cms/home/attachments/4/5/3/CH1048/CMS1348749794860/oeb12.pdf (accessed on 14 November 2017).
- Unité de Surveillance et D’Epidémiologie Nutritionnelle (Usen). Étude Nationale Nutrition Santé (ENNS, 2006)–Situation Nutritionnelle en France en 2006 Selon les Indicateurs D’Objectif et les Repères du Programme National Nutrition Santé (PNNS). Institut de Veille Sanitaire, Université de Paris 13, Conservatoire National des Arts et Métiers. 2007. Available online: http://opac.invs.sante.fr/doc_num.php?explnum_id=3481 (accessed on 14 November 2017).
- Bailey, R.L.; Carmel, R.; Green, R.; Pfeiffer, C.M.; Cogswell, M.E.; Osterloh, J.D.; Sempos, C.T.; Yetley, E.A. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am. J. Clin. Nutr. 2011, 94, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Carmel, R.; Sarrai, M. Diagnosis and management of clinical and subclinical cobalamin deficiency: Advances and controversies. Curr. Hematol. Rep. 2006, 5, 23–33. [Google Scholar] [PubMed]
- Johnson, M.A.; Hausman, D.B.; Davey, A.; Poon, L.W.; Allen, R.H.; Stabler, S.P. Vitamin B12 deficiency in African American and white octogenarians and centenarians in Georgia. J. Nutr. Health Aging 2010, 14, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.M.; Sternberg, M.R.; Hamner, H.C.; Crider, K.S.; Lacher, D.A.; Rogers, L.M.; Bailey, R.L.; Yetley, E.A. Applying inappropriate cutoffs leads to misinterpretation of folate status in the US population. Am. J. Clin. Nutr. 2016, 104, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Steluti, J.; Selhub, J.; Paul, L.; Reginaldo, C.; Fisberg, R.M.; Marchioni, D.M.L. An overview of folate status in a population-based study from Sao Paulo, Brazil and the potential impact of 10 years of national folic acid fortification policy. Eur. J. Clin. Nutr. 2017, 71. [Google Scholar] [CrossRef] [PubMed]
- Corona, L.P.; Duarte, Y.A.; Lebrao, M.L. Prevalence of anemia and associated factors in older adults: Evidence from the SABE Study. Rev. Saude Publica 2014, 48, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Hinds, H.E.; Johnson, A.A.; Webb, M.C.; Graham, A.P. Iron, folate, and vitamin B12 status in the elderly by gender and ethnicity. J. Natl. Med. Assoc. 2011, 103, 870–877. [Google Scholar] [CrossRef]
- Milman, N.; Pedersen, A.N.; Ovesen, L.; Schroll, M. Iron status in 358 apparently healthy 80-year-old Danish men and women: Relation to food composition and dietary and supplemental iron intake. Ann. Hematol. 2004, 83, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 2009, 89, 693s–696s. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.; Strohle, A.; Hahn, A. Age-associated changes in the metabolism of vitamin B(12) and folic acid: Prevalence, aetiopathogenesis and pathophysiological consequences. Z. Gerontol. Geriatr. 2004, 37, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Brock, K.; Cant, R.; Clemson, L.; Mason, R.S.; Fraser, D.R. Effects of diet and exercise on plasma vitamin D (25(OH)D) levels in Vietnamese immigrant elderly in Sydney, Australia. J. Steroid Biochem. Mol. Biol. 2007, 103, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.; Decke, S.; de Las Heras Gala, T.; Linkohr, B.; Koenig, W.; Ladwig, K.H.; Peters, A.; Thorand, B. Prospective association of vitamin D with frailty status and all-cause mortality in older adults: Results from the KORA-Age Study. Prev. Med. 2015, 73, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Pires Corona, L.; Drumond Andrade, F.C.; de Oliveira Duarte, Y.A.; Lebrao, M.L. The Relationship between Anemia, Hemoglobin Concentration and Frailty in Brazilian Older Adults. J. Nutr. Health Aging 2015, 19, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Yannakoulia, M.; Ntanasi, E.; Anastasiou, C.A.; Scarmeas, N. Frailty and nutrition: From epidemiological and clinical evidence to potential mechanisms. Metabolism 2017, 68, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Fabian, E.; Bogner, M.; Kickinger, A.; Wagner, K.H.; Elmadfa, I. Vitamin status in elderly people in relation to the use of nutritional supplements. J. Nutr. Health Aging 2012, 16, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Bor, M.V.; Lydeking-Olsen, E.; Moller, J.; Nexo, E. A daily intake of approximately 6 microg vitamin B-12 appears to saturate all the vitamin B-12-related variables in Danish postmenopausal women. Am. J. Clin. Nutr. 2006, 83, 52–58. [Google Scholar] [PubMed]
- Whiting, S.J.; Bonjour, J.P.; Payen, F.D.; Rousseau, B. Moderate amounts of vitamin D3 in supplements are effective in raising serum 25-hydroxyvitamin D from low baseline levels in adults: A systematic review. Nutrients 2015, 7, 2311–2323. [Google Scholar] [CrossRef] [PubMed]
- Eussen, S.J.; de Groot, L.C.; Clarke, R.; Schneede, J.; Ueland, P.M.; Hoefnagels, W.H.; van Staveren, W.A. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency: A dose-finding trial. Arch. Intern. Med. 2005, 165, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.M.; Kazantzidis, A.; Ryan, M.J.; Barber, N.; Sempos, C.T.; Durazo-Arvizu, R.A.; Jorde, R.; Grimnes, G.; Eiriksdottir, G.; Gudnason, V.; et al. Seasonal Changes in Vitamin D-Effective UVB Availability in Europe and Associations with Population Serum 25-Hydroxyvitamin D. Nutrients 2016, 8, 533. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [PubMed]
- Shirazi, L.; Almquist, M.; Malm, J.; Wirfalt, E.; Manjer, J. Determinants of serum levels of vitamin D: A study of life-style, menopausal status, dietary intake, serum calcium, and PTH. BMC Womens Health 2013, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Keil, U.; Chambless, L.E.; Doring, A.; Filipiak, B.; Stieber, J. The relation of alcohol intake to coronary heart disease and all-cause mortality in a beer-drinking population. Epidemiology 1997, 8, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Halsted, C.H.; Villanueva, J.A.; Devlin, A.M.; Chandler, C.J. Metabolic interactions of alcohol and folate. J. Nutr. 2002, 132, 2367s–2372s. [Google Scholar] [PubMed]
| Baseline Characteristics | All (n = 1079) | Men (n = 537) | Women (n = 542) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Season of blood collection | ||||||
| Months of blood collection a | ||||||
| February–May | 440 | 42.3 | 242 | 46.2 | 198 | 38.4 |
| June–August | 360 | 34.6 | 173 | 33 | 187 | 36.2 |
| September–November | 240 | 23.1 | 109 | 20.8 | 131 | 25.4 |
| Socio-demographic factors | ||||||
| Age groups (years) | ||||||
| 65–74 | 457 | 42.4 | 233 | 43.4 | 224 | 41.3 |
| 75–84 | 486 | 45 | 243 | 45.3 | 243 | 44.8 |
| 85–93 | 136 | 12.6 | 61 | 11.4 | 75 | 13.8 |
| Family status b | ||||||
| Living with a partner | 663 | 62.1 | 424 | 79.3 | 239 | 44.9 |
| Living alone, divorced or widowed | 404 | 37.9 | 111 | 20.8 | 293 | 55.1 |
| Educational level (years) | ||||||
| Medium to high (10 to 17) | 854 | 79.2 | 490 | 91.3 | 364 | 67.2 |
| Low (8 to 9) | 225 | 20.9 | 47 | 8.8 | 178 | 32.8 |
| Lifestyle factors | ||||||
| Nutritional status | ||||||
| Geriatric Nutritional Risk Index (GNRI) c | ||||||
| No risk (>98) | 960 | 92.3 | 482 | 92.3 | 478 | 92.3 |
| Low risk (92 to 98) | 59 | 5.7 | 29 | 5.6 | 30 | 5.8 |
| Moderate or major risk (<92) | 21 | 2 | 11 | 2.1 | 10 | 1.9 |
| Nutrition Score (SCREEN II) d | ||||||
| Low risk (41 to 48) | 408 | 38.3 | 239 | 44.8 | 169 | 31.7 |
| Medium risk (36 to <41) | 377 | 35.4 | 181 | 34 | 196 | 36.8 |
| High risk (<36) | 281 | 26.4 | 113 | 21.2 | 168 | 31.5 |
| Physical activity e | ||||||
| Very active or moderately active | 564 | 52.3 | 304 | 56.6 | 260 | 48.1 |
| Less active or inactive | 514 | 47.7 | 233 | 43.4 | 281 | 51.9 |
| Alcohol consumption (g/day) f | ||||||
| 0 | 393 | 36.6 | 126 | 23.5 | 267 | 49.7 |
| >0 to <20 | 373 | 34.8 | 164 | 30.6 | 209 | 38.9 |
| ≥20 | 307 | 28.6 | 246 | 45.9 | 61 | 11.4 |
| Smoking status | ||||||
| Never smoker | 617 | 57.2 | 204 | 38 | 413 | 76.2 |
| Former smoker | 413 | 38.3 | 303 | 56.4 | 110 | 20.3 |
| Current smoker | 49 | 4.5 | 30 | 5.6 | 19 | 3.5 |
| Health factors | ||||||
| Body mass index (BMI) (kg/m2) | ||||||
| Normal (18.5 to <25) | 228 | 21.1 | 99 | 18.4 | 129 | 23.8 |
| Overweight (25 to <30) | 525 | 48.7 | 281 | 52.3 | 244 | 45 |
| Obese (≥30) | 326 | 30.2 | 157 | 29.2 | 169 | 31.2 |
| Frailty g | ||||||
| Non-frail | 572 | 57.5 | 290 | 57.4 | 282 | 57.6 |
| Pre-frail | 377 | 37.9 | 194 | 38.4 | 183 | 37.4 |
| Frail | 46 | 4.6 | 21 | 4.2 | 25 | 5.1 |
| Polypharmacy (≥5 medications) | ||||||
| No | 712 | 66 | 356 | 66.3 | 356 | 65.7 |
| Yes | 367 | 34 | 181 | 33.7 | 186 | 34.3 |
| eGFR (mL/min/1.73 m2) | ||||||
| Normal (≥60) | 709 | 65.7 | 366 | 68.2 | 343 | 63.3 |
| Low (<60) | 370 | 34.3 | 171 | 31.8 | 199 | 36.7 |
| Multi-morbidity h | ||||||
| No disease | 91 | 8.5 | 56 | 10.5 | 35 | 6.6 |
| One disease | 263 | 24.7 | 138 | 25.9 | 125 | 23.4 |
| Two or more diseases | 712 | 66.8 | 338 | 63.5 | 374 | 70 |
| Use of supplements | ||||||
| Vitamin D | ||||||
| Regular intake | 140 | 13 | 36 | 6.7 | 104 | 19.2 |
| No/irregular intake | 939 | 87 | 501 | 93.3 | 438 | 80.8 |
| Folic acid | ||||||
| Regular intake | 116 | 10.8 | 51 | 9.5 | 65 | 12 |
| No/irregular intake | 963 | 89.3 | 486 | 90.5 | 477 | 88 |
| Vitamin B12 | ||||||
| Regular intake | 114 | 10.6 | 48 | 8.9 | 66 | 12.2 |
| No/irregular intake | 965 | 89.4 | 489 | 91.1 | 476 | 87.8 |
| Iron | ||||||
| Regular intake | 36 | 3.3 | 15 | 2.8 | 21 | 3.9 |
| No/irregular intake | 1043 | 96.7 | 522 | 97.2 | 521 | 96.1 |
| Nutritional Biomarkers | Biomarker Concentrations (Percentile Scale) | Prevalence of Subclinical Deficiency | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Min | 1st | 10th | Q1 | Median | Q3 | 90th | 99th | Max | % | % (Standardized *) | 95% CI | |
| All | |||||||||||||
| 25OHD [nmol/L] | 1040 | 3.7 | 7.7 | 21.3 | 31.4 | 48.3 | 69.6 | 93.1 | 136.3 | 174.7 | 52.5 | 52.0 | 49.0–55.0 |
| Folate [nmol/L] | 1043 | 5.8 | 9.0 | 14.0 | 18.2 | 24.5 | 33.3 | 45.3 | 45.3 | 90.6 | 8.7 | 8.7 | 7.0–10.4 |
| Vitamin B12 [pmol/L] | 1044 | 66 | 104 | 173 | 214 | 277 | 376 | 523 | 1476 | 1476 | 27.5 | 27.3 | 24.6–30.0 |
| Iron [μmol/L] | 1050 | 3.4 | 5.2 | 10.0 | 13.1 | 16.1 | 20.0 | 22.8 | 32.9 | 40.5 | 11.1 | 11.0 | 9.1–12.9 |
| Men | |||||||||||||
| 25OHD [nmol/L] | 524 | 3.7 | 3.7 | 22.5 | 36.2 | 51.8 | 75.0 | 96.8 | 145.0 | 174.7 | 46.4 | 44.4 | 40.2–48.6 |
| Folate [nmol/L] | 524 | 5.8 | 10.2 | 14.4 | 17.9 | 23.8 | 31.3 | 45.3 | 45.3 | 63.9 | 8.2 | 8.0 | 5.7–10.3 |
| Vitamin B12 [pmol/L] | 525 | 68 | 104 | 170 | 210 | 263 | 355 | 494 | 1441 | 1476 | 29.3 | 28.5 | 24.6–32.4 |
| Iron [μmol/L] | 527 | 3.4 | 5.7 | 10.7 | 13.6 | 16.8 | 19.9 | 24.2 | 33.7 | 40.5 | 14.2 | 13.5 | 10.6–16.4 |
| Women | |||||||||||||
| 25OHD [nmol/L] | 516 | 3.7 | 8.7 | 20.6 | 27.7 | 45.3 | 65.9 | 86.4 | 119.8 | 156.3 | 58.7 | 59.0 | 54.8–63.2 |
| Folate [nmol/L] | 519 | 7.3 | 8.9 | 13.8 | 18.4 | 25.2 | 35.4 | 45.3 | 45.3 | 90.6 | 9.2 | 9.4 | 6.9–12.0 |
| Vitamin B12 [pmol/L] | 519 | 66 | 108 | 175 | 218 | 292 | 392 | 542 | 1476 | 1476 | 25.6 | 26.0 | 22.3–29.3 |
| Iron [μmol/L] | 523 | 3.6 | 5.0 | 9.3 | 12.4 | 15.6 | 18.8 | 22.0 | 28.6 | 38.0 | 8.0 | 8.4 | 6.0–10.8 |
| Predictor | Predictor Categories | Low 25OHD (n = 525) | Low Folate (n = 86) | Low Vitamin B12 (n = 283) | Low Iron (n = 106) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | ||
| Season of blood collection | |||||||||||||
| Months of blood collection | February–May vs. June–August | 2.1 | 1.5–2.8 | <0.001 | . | . | . | . | . | . | . | . | . |
| Months of blood collection | September–November vs. June–August | 0.8 | 0.5–1.1 | 0.135 | . | . | . | . | . | . | . | . | . |
| Socio-demographic factors | |||||||||||||
| Sex | Women vs. men | 1.9 | 1.4–2.5 | <0.001 | 0.8 | 0.5–1.4 | 0.459 | 0.8 | 0.6–1.1 | 0.231 | 0.4 | 0.3–0.7 | <0.001 |
| Age groups (years) | 75–84 vs. 65–74 | 1.3 | 1.0–1.8 | 0.058 | 0.9 | 0.6–1.6 | 0.818 | 1.3 | 0.9–1.8 | 0.110 | 1.0 | 0.6–1.7 | 0.911 |
| Age groups (years) | 85–93 vs. 65–74 | 2.2 | 1.3–3.8 | 0.003 | 2.3 | 1.2–4.3 | 0.011 | 2.0 | 1.2–3.2 | 0.004 | 1.2 | 0.6–2.3 | 0.564 |
| Lifestyle factors | |||||||||||||
| Nutritional status | GNRI: Low (92 to 98) vs. no risk (>98) | - | - | - | - | - | - | - | - | - | 2.7 | 1.3–5.4 | 0.005 |
| Nutritional status | GNRI: Moderate/major (<92) vs. no risk (>98) | - | - | - | - | - | - | - | - | - | 4.0 | 1.2–12.0 | 0.015 |
| Physical activity | Less active or inactive vs. very active or moderately active | 1.6 | 1.2–2.2 | 0.001 | 2.0 | 1.2–3.4 | 0.006 | 1.4 | 1.0–1.8 | 0.042 | - | - | - |
| Alcohol consumption (g/day) | >0 to <20 vs. 0 | - | - | - | 1.0 | 0.6–1.6 | 0.876 | - | - | - | - | - | - |
| Alcohol consumption (g/day) | ≥20 vs. 0 | - | - | - | 0.4 | 0.2–0.8 | 0.017 | - | - | - | - | - | - |
| Smoking status | Current smoker vs. never smoker | 0.8 | 0.4–1.5 | 0.516 | - | - | - | - | - | - | - | - | - |
| Smoking status | Former smoker vs. never smoker | 0.6 | 0.4–0.8 | 0.002 | - | - | - | - | - | - | - | - | - |
| Health factors | |||||||||||||
| BMI (kg/m2) | Overweight (25 to <30) vs. normal (18.5 to <25) | 0.9 | 0.7–1.4 | 0.763 | - | - | - | - | - | - | - | - | - |
| BMI (kg/m2) | Obese (≥30) vs. normal (18.5 to <25) | 1.8 | 1.2–2.6 | 0.005 | - | - | - | - | - | - | - | - | - |
| Frailty | Missing value vs. non-frail | 1.1 | 0.6–2.2 | 0.736 | - | - | - | - | - | - | 3.4 | 1.3–8.0 | 0.008 |
| Frailty | Pre-frail vs. non-frail | 1.8 | 1.3–2.5 | <0.001 | - | - | - | - | - | - | 2.7 | 1.7–4.5 | <0.001 |
| Frailty | Frail vs. non-frail | 2.5 | 1.2–5.4 | 0.022 | - | - | - | - | - | - | 4.2 | 1.6–10.2 | 0.002 |
| Polypharmacy | Yes vs. no | - | - | - | - | - | - | 0.5 | 0.4–0.7 | <0.001 | 1.6 | 1.0–2.4 | 0.045 |
| Use of supplements | Vitamin D: No/irregular intake vs. regular intake | 4.8 | 3.1–7.6 | <0.001 | . | . | . | . | . | . | . | . | . |
| Use of supplements | Folic acid: No/irregular intake vs. regular intake | . | . | . | 3.9 | 1.4–16.1 | 0.024 | . | . | . | . | . | . |
| Use of supplements | Vitamin B12: No/irregular intake vs. regular intake | . | . | . | . | . | . | 4.7 | 2.5–10.2 | <0.001 | . | . | . |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conzade, R.; Koenig, W.; Heier, M.; Schneider, A.; Grill, E.; Peters, A.; Thorand, B. Prevalence and Predictors of Subclinical Micronutrient Deficiency in German Older Adults: Results from the Population-Based KORA-Age Study. Nutrients 2017, 9, 1276. https://doi.org/10.3390/nu9121276
Conzade R, Koenig W, Heier M, Schneider A, Grill E, Peters A, Thorand B. Prevalence and Predictors of Subclinical Micronutrient Deficiency in German Older Adults: Results from the Population-Based KORA-Age Study. Nutrients. 2017; 9(12):1276. https://doi.org/10.3390/nu9121276
Chicago/Turabian StyleConzade, Romy, Wolfgang Koenig, Margit Heier, Andrea Schneider, Eva Grill, Annette Peters, and Barbara Thorand. 2017. "Prevalence and Predictors of Subclinical Micronutrient Deficiency in German Older Adults: Results from the Population-Based KORA-Age Study" Nutrients 9, no. 12: 1276. https://doi.org/10.3390/nu9121276




