Effect of Gamma-Oryzanol as Therapeutic Agent to Prevent Cardiorenal Metabolic Syndrome in Animals Submitted to High Sugar-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Protocol
2.2. Gamma-Oryzanol
2.3. Diets
2.4. Nutritional Analysis
2.5. Metabolic and Hormonal Analysis
2.6. Renal Function
2.7. Systolic Blood Pressure
2.8. Structural and Functional Cardiac Function by Echocardiogram
2.9. Statistical Analysis
3. Results
3.1. Nutritional, Metabolic, and Hormonal Analysis
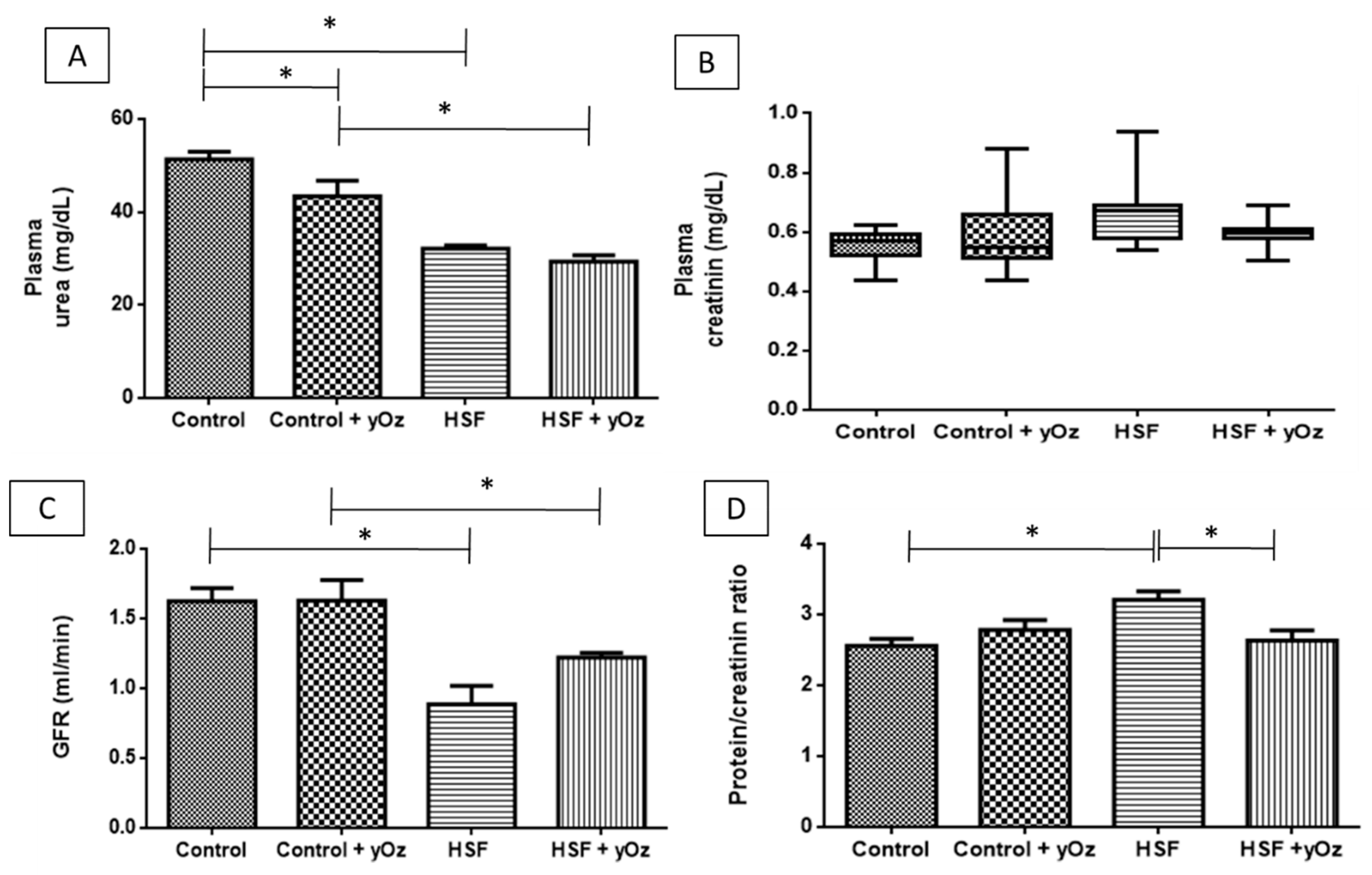
3.2. Renal Function
3.3. Cardiac Parameters and Systolic Blood Pressure
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Canella, D.S.; Novaes, H.M.D.; Levy, R.B. The influence of excess weight and obesity on health spending in Brazilian households. Cad. Saúde Pública 2015, 31, 2331–2341. (In Portugues) [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Chevalier, A.; D’Souza, M.; Baur, L.; Wen, L.M.; Simpson, J. Early childhood obesity: Association with healthcare expenditure in Australia. Obesity 2016, 24, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.L.; Hoelscher, D.M.; Kohl, H.W., III. Research contributions on childhood obesity from a public-private partnership. Int. J. Behav. Nutr. Phys. Act. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Swinburn, B.A.; Caterson, I.; Seidell, J.C.; James, W.P.T. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004, 7, 123–146. [Google Scholar] [PubMed]
- La Fleur, S.E.; Luijendijk, M.C.M.; van Rozen, A.J.; Kalsbeek, A.; Adan, R.A.H. A free-choice high-fat high-sugar diet induces glucose intolerance and insulin unresponsiveness to a glucose load not explained by obesity. Int. J. Obes. 2011, 35, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Adipose tissue inflammation: A cause or consequence of obesity-related insulin resistance? Clin. Sci. 2016, 130, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Lechi, A. The obesity paradox: Is it really a paradox? Hypertension. Eat. Weight Disord.–Stud. Anorex. Bulim. Obes. 2017, 22, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Monforte, M.; Sánchez, E.; Barrio, F.; Costa, B.; Flores-Mateo, G. Metabolic syndrome and dietary patterns: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2017, 56, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Sowers, J.R.; Whaley-Connell, A.; Hayden, M.R. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med. 2011, 1, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, M.K.; De Boer, R.A.; Navis, G.J.; Van Gilst, W.H.; Hillege, H.L. Animal models of cardiorenal syndrome: A review. Heart Fail. Rev. 2012, 17, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Cabandugama, P.K.; Gardner, M.J.; Sowers, J.R. The renin angiotensin aldosterone system in obesity and hypertension. Med. Clin. 2017, 101, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, L.; Bellasi, A.; Russo, D.; Cozzolino, M.; Ronco, C. Cardiorenal acute kidney injury: Epidemiology, presentation, causes, pathophysiology and treatment. Int. J. Cardiol. 2017, 227, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Van Dokkum, R.P.E.; Eijkelkamp, W.B.A.; Kluppel, A.C.A.; Henning, R.H.; van Goor, H.; Citgez, M.; Windt, W.A.K.M.; van Veldhuisen, D.J.; de Graeff, P.A.; de Zeeuw, D.; et al. Myocardial infarction enhances progressive renal damage in an experimental model for cardio-renal interaction. J. Am. Soc. Nephrol. 2004, 15, 3103–3110. [Google Scholar] [CrossRef] [PubMed]
- Dikow, R.; Schmidt, U.; Kihm, L.P.; Schaier, M.; Schwenger, V.; Gross, M.-L.; Katus, H.A.; Zeier, M.; Hardt, S.E. Uremia aggravates left ventricular remodeling after myocardial infarction. Am. J. Nephrol. 2010, 32, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Windt, W.A.K.M.; Henning, R.H.; Kluppel, A.C.A.; Xu, Y.; de Zeeuw, D.; van Dokkum, R.P.E. Myocardial infarction does not further impair renal damage in 5/6 nephrectomized rats. Nephrol. Dial. Transp. 2008, 23, 3103–3110. [Google Scholar] [CrossRef] [PubMed]
- Noiri, E.; Nagano, N.; Negishi, K.; Doi, K.; Miyata, S.; Abe, M.; Tanaka, T.; Okamoto, K.; Hanafusa, N.; Kondo, Y.; et al. Efficacy of darbepoetin in doxorubicin-induced cardiorenal injury in rats. Nephron Exp. Nephrol. 2006, 104, e6–e14. [Google Scholar] [CrossRef] [PubMed]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Effect of oryzanol and ferulic acid on the glucose metabolism of mice fed with a high-fat diet. J. Food Sci. 2011, 76, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hua, N.; Godber, J.S. Antioxidant activity of tocopherols, tocotrienols, and γ-oryzanol components from rice bran against cholesterol oxidation accelerated by 2,2′-azobis(2-methylpropionamidine) dihydrochloride. J. Agric. Food Chem. 2001, 49, 2077–2081. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Godber, J.S. Purification and identification of components of γ-oryzanol in rice bran oil. J. Agric. Food Chem. 1999, 47, 2724–2728. [Google Scholar] [CrossRef] [PubMed]
- Minatel, I.O.; Han, S.; Aldini, G.; Colzani, M.; Matthan, N.R.; Correa, C.R.; Fecchio, D.; Yeum, K. Fat-soluble bioactive components in colored rice varieties. J. Med. Food 2014, 17, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Zolali, E.; Asgharian, P.; Hamishehkar, H.; Kouhsoltani, M.; Khodaii, H.; Hamishehkar, H. Effects of gamma oryzanol on factors of oxidative stress and sepsis-induced lung injury in experimental animal model. Iran. J. Basic Med. Sci. 2015, 18, 1257–1263. [Google Scholar] [PubMed]
- Candiracci, M.; Justo, M.L.; Castaño, A.; Rodriguez-Rodriguez, R.; Herrera, M.D. Rice bran enzymatic extract—Supplemented diets modulate adipose tissue inflammation markers in Zucker rats. Nutrition 2014, 30, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of ferulic acid and γ-oryzanol on high-fat and high-fructose diet-induced metabolic syndrome in rats. PLoS ONE 2015, 10, e0118135. [Google Scholar] [CrossRef] [PubMed]
- IBGE—Instituto Brasileiro de Geografia e Estatística Pesquisa de Orçamentos Familiares (POF) 2008–2009: Análise do Consumo Alimentar Pessoal No Brasil. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv50063.pdf/ (accessed on 26 September 2017).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Anna, V.O.; Mátyus, J.; Sárkány, E.; Horváth, A.; Fodor, B. New trends in the laboratory diagnostics of proteinuria and albuminuria. Orv. Hetil. 2010, 151, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.P.; Rafacho, B.P.M.; de Freitas Gonçalves, A.; Jaldin, R.G.; do Nascimento, T.B.; Silva, M.A.B.; Cau, S.B.A.; Roscani, M.G.; Azevedo, P.S.; Minicucci, M.F.; et al. Vitamin D induces increased systolic arterial pressure via vascular reactivity and mechanical properties. PLoS ONE 2014, 9, e98895. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Bolton-Smith, C.; Woodward, M. Dietary composition and fat to sugar ratios in relation to obesity. Int. J. Obes. Relat. Metab. Disord. 1994, 18, 820–828. [Google Scholar] [PubMed]
- Oliveira Junior, S.A.; Padovani, C.R.; Rodrigues, S.A.; Silva, N.R.; Martinez, P.F.; Campos, D.H.; Okoshi, M.P.; Okoshi, K.; Dal-Pai, M.; Cicogna, A.C. Extensive impact of saturated fatty acids on metabolic and cardiovascular profile in rats with diet-induced obesity: A canonical analysis. Cardiovasc. Diabetol. 2013, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.J. Digestion and absorption of carbohydrates—From molecules and membranes to humans. Am. J. Clin. Nutr. 1994, 59, 690S–698S. [Google Scholar] [PubMed]
- Stanhope, K.L.; Havel, P.J. Fructose consumption: Potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr. Opin. Lipidol. 2008, 19, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.A.; Powell, P.C.; Agarwal, A.; Dell’Italia, L.J.; Chatham, J.C. Cardiovascular dysfunction in Zucker obese and Zucker diabetic fatty rats: Role of hydronephrosis. Am. J. Physiol.–Heart Circ. Physiol. 2007, 293, H292–H298. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Hohl, M.; Schutze, J.; Mahfoud, F.; Speer, T.; Linz, B.; Hubschle, T.; Juretschke, H.-P.; Dechend, R.; Geisel, J.; et al. Progression of kidney injury and cardiac remodeling in obese spontaneously hypertensive rats: The role of renal sympathetic innervation. Am. J. Hypertens. 2015, 28, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.H.; Neckář, J.; Cummens, B.; Wahl, G.M.; Imig, J.D. Azilsartan decreases renal and cardiovascular injury in the spontaneously hypertensive obese rat. Cardiovasc. Drugs Ther. 2014, 28, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Minatel, I.O.; Lee, Y.-M.; Yoon, H.; Yoon, Y.; Han, S.-I.; Correa, C.R.; Fecchio, D.; Yeum, K.-J. Antiadipogenic activity of γ-oryzanol and its stability in pigmented rice. J. Med. Food 2016, 19, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Justo, M.L.L.; Rodriguez-Rodriguez, R.; Claro, C.M.M.; De Sotomayor, M.A.; Parrado, J.; Herrera, M.D.D. Water-soluble rice bran enzymatic extract attenuates dyslipidemia, hypertension and insulin resistance in obese Zucker rats. Eur. J. Nutr. 2013, 52, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Minatel, I.; Francisqueti, F.; Corrêa, C.; Lima, G. Antioxidant activity of γ-oryzanol: A complex network of interactions. Int. J. Mol. Sci. 2016, 17, 1107. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, S.; Singh, R.; Chatterjee, B.; Zhang, B.; Ali, A. A blend of sesame oil and rice bran oil lowers blood pressure and improves the lipid profile in mild-to-moderate hypertensive patients. J. Clin. Lipidol. 2016, 10, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.Z.; Varghese, Z.; Moorhead, J.F. An update on the lipid nephrotoxicity hypothesis. Nat. Rev. Nephrol. 2009, 5, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.; Furth, S.; Zoccali, C. World kidney day obesity and kidney disease: Hidden consequences of the epidemic. Indian J. Nephrol. 2017, 27, 85. [Google Scholar] [PubMed]
- Yang, P.; Xiao, Y.; Luo, X.; Zhao, Y.; Zhao, L.; Wang, Y.; Wu, T.; Wei, L.; Chen, Y. Inflammatory stress promotes the development of obesity-related chronic kidney disease via CD36 in mice. J. Lipid Res. 2017, 58, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Lopaschuk, G.D. Cardiac fatty acid oxidation in heart failure associated with obesity and diabetes. Biochim. Biophys. Acta(BBA)–Mol. Cell Biol. Lipids 2016, 1861, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.P.C.; Sugasini, D.; Lokesh, B.R. Dietary gamma oryzanol plays a significant role in the anti-inflammatory activity of rice bran oil by decreasing pro-inflammatory mediators secreted by peritoneal macrophages of rats. Biochem. Biophys. Res. Commun. 2016, 479, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Murata, T.; Fujisawa, M.; Nagasaka, R.; Ushio, H.; Bari, A.M.; Hori, M.; Ozaki, H. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br. J. Pharmacol. 2008, 154, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
| Components | Control | HSF |
|---|---|---|
| Soybean meal (g/kg) | 335 | 340 |
| Sorghum (g/kg) | 278 | 80 |
| Soy hulls (g/kg) | 188 | 116 |
| Dextrin (g/kg) | 146 | 20 |
| Sucrose (g/kg) | - | 80 |
| Fructose (g/kg) | - | 180 |
| Soybean oil (g/kg) | 14 | - |
| Lard (g/kg) | - | 154 |
| Minerals (g/kg) | 25 | 25 |
| Salt (g/kg) | 4 | 8 |
| Nutritional values | ||
| Protein (% of ingredients) | 20.0 | 18.0 |
| Carbohydrate (% of ingredients) | 60.0 | 53.5 |
| Fat (% of ingredients) | 4.00 | 16.5 |
| % of unsaturated | 69.0 | 47.0 |
| % of saturated | 31.0 | 53.0 |
| % Energy from protein | 22.9 | 16.6 |
| % Energy from carbohydrate | 66.8 | 49.2 |
| % Energy from fat | 10.4 | 34.2 |
| Energy (kcal/g) | 3.59 | 4.35 |
| Groups | Effects | ||||||
|---|---|---|---|---|---|---|---|
| Control | Control + yOz | HSF | HSF + yOz | Diet | yOz | Interaction | |
| Final body weight | 461 ± 54 | 475 ± 57 | 540 ± 48 * | 447 ± 66 † | 0.212 | 0.061 | 0.013 |
| Chow fed (g/day) | 24.2 ± 2.4 | 25.4 ± 2.4 | 12.2 ± 2.4 * | 10.1 ± 1.5 # | <0.001 | 0.030 | 0.018 |
| Water intake (mL/day) | 35.0 ± 5.1 | 35.2 ± 6.3 | 43.8 ± 3.8 * | 43.3 ± 6.5 # | <0.001 | 0.280 | 0.236 |
| Caloric intake (kcal/day) | 86.9 ± 8.7 | 91.0 ± 8.6 | 96.8 ± 8.7 | 87.4 ± 9.6 | 0.342 | 0.846 | 0.147 |
| Glucose (mg/dL) 1 | 85.2 (11.7) | 89.7 (5.2) | 115.0 (15.5) * | 132.1 ± 30.9 # | <0.001 | 0.294 | 0.522 |
| Triglycerides (mg/dL) | 79.9 ± 14.3 | 63.2 ± 14.3 | 113.0 ± 24.1 * | 89.7 ± 24.1 †,# | <0.001 | 0.008 | 0.638 |
| Insulin (ng/mL) | 2.66 ± 1.27 | 3.42 ± 1.62 | 5.85 ± 1.25 * | 5.32 ± 1.93 # | <0.001 | 0.84 | 0.249 |
| HOMA-IR | 23.0 ± 11.6 | 27.3 ± 15.2 | 68.7 ± 11.6 * | 69.1 ± 26.7 # | <0.001 | 0.744 | 0.786 |
| Uric acid | 1.08 ± 0.11 | 1.14 ± 0.41 | 1.23 ± 0.18 | 1.32 ± 0.19 | 0.198 | 0.311 | 0.619 |
| Groups | Effect | ||||||
|---|---|---|---|---|---|---|---|
| Control | Control + yOz | HSF | HSF + yOz | Diet | yOz | Interaction | |
| HR | 239 ± 44 | 230 ± 40 | 280 ± 61 | 271 ± 50 | 0.008 | 0.737 | 0.884 |
| SBP | 129 ± 4 | 129 ± 5 | 137 ± 7 * | 139 ± 5 # | <0.001 | 0.915 | 0.815 |
| LVDD (mm) | 7.05 ± 0.30 | 6.74 ± 0.30 | 6.42 ± 0.35 | 7.05 ± 0.35 | 0.777 | 0.789 | 0.0037 |
| LA (mm) | 4.69 ± 0.23 | 4.63 ± 0.52 | 5.67 ± 0.21 * | 4.76 ± 0.19 † | <0.001 | <0.001 | <0.001 |
| LA/AO | 1.24 ± 0.09 | 1.18 ± 0.20 | 1.49 ± 0.11 * | 1.24 ± 0.08 † | 0.696 | 0.950 | 0.024 |
| RWT | 0.44 ± 0.02 | 0.44 ± 0.02 | 0.59 ± 0.09 * | 0.43 ± 0.01 † | <0.001 | <0.001 | <0.001 |
| FS (%) | 59.8 ± 4.0 | 58.9 ± 4.2 | 53.8 ± 5.6 * | 56.6 ± 4.0 # | <0.001 | 0.197 | 0.439 |
| S′m (cm/s) | 5.61 ± 0.28 | 5.67 ± 0.27 | 4.92 ± 0.55 * | 5.66 ± 0.33 † | <0.001 | <0.001 | 0.003 |
| PWSV (cm/s) | 60.8 ± 4.3 | 60.6 ± 2.4 | 52.8 ± 5.9 * | 61.9 ± 3.6 † | 0.005 | <0.001 | 0.003 |
| E wave (cm/s) | 74.3 ± 3.8 | 73.8 ± 4.9 | 76.7 ± 7.6 | 76.8 ± 6.1 | 0.102 | 0.748 | 0.484 |
| E/A | 1.72 ± 0.24 | 1.95 ± 0.30 | 1.84 ± 0.57 | 1.77 ± 0.19 | 0.339 | 0.262 | 0.239 |
| E/E′ | 13.8 ± 0.8 | 13.8 ± 1.5 | 17.6 ± 3.5 * | 14.2 ± 1.2 † | 0.002 | 0.008 | 0.041 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francisqueti, F.V.; Minatel, I.O.; Ferron, A.J.T.; Bazan, S.G.Z.; Silva, V.D.S.; Garcia, J.L.; De Campos, D.H.S.; Ferreira, A.L.; Moreto, F.; Cicogna, A.C.; et al. Effect of Gamma-Oryzanol as Therapeutic Agent to Prevent Cardiorenal Metabolic Syndrome in Animals Submitted to High Sugar-Fat Diet. Nutrients 2017, 9, 1299. https://doi.org/10.3390/nu9121299
Francisqueti FV, Minatel IO, Ferron AJT, Bazan SGZ, Silva VDS, Garcia JL, De Campos DHS, Ferreira AL, Moreto F, Cicogna AC, et al. Effect of Gamma-Oryzanol as Therapeutic Agent to Prevent Cardiorenal Metabolic Syndrome in Animals Submitted to High Sugar-Fat Diet. Nutrients. 2017; 9(12):1299. https://doi.org/10.3390/nu9121299
Chicago/Turabian StyleFrancisqueti, Fabiane Valentini, Igor Otávio Minatel, Artur Junio Togneri Ferron, Silméia Garcia Zanati Bazan, Vanessa Dos Santos Silva, Jéssica Leite Garcia, Dijon Henrique Salomé De Campos, Ana Lúcia Ferreira, Fernando Moreto, Antonio Carlos Cicogna, and et al. 2017. "Effect of Gamma-Oryzanol as Therapeutic Agent to Prevent Cardiorenal Metabolic Syndrome in Animals Submitted to High Sugar-Fat Diet" Nutrients 9, no. 12: 1299. https://doi.org/10.3390/nu9121299





