Validity of Dietary Assessment in Athletes: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection of Studies
2.3. Data Extraction and Conversions
2.4. Assessment of Methodological Quality
2.5. Meta-Analysis
3. Results
3.1. Identification and Selection of Studies
3.2. Demographic and Anthropometric Characteristics
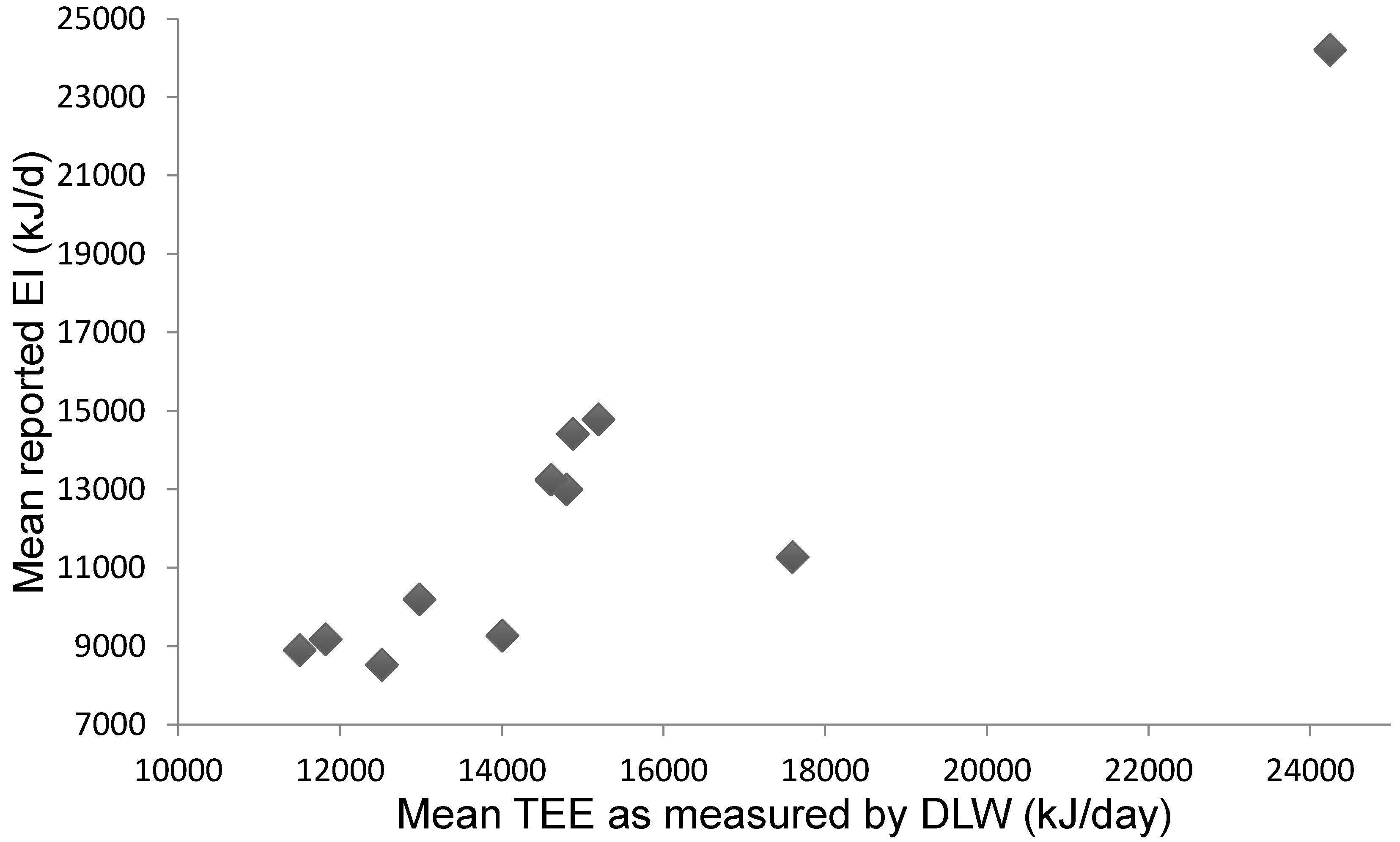
3.3. Studies Comparing Reported Energy Intake to Energy Expenditure as Measured by DLW
Mean Difference between EI and TEE: Reporting Bias
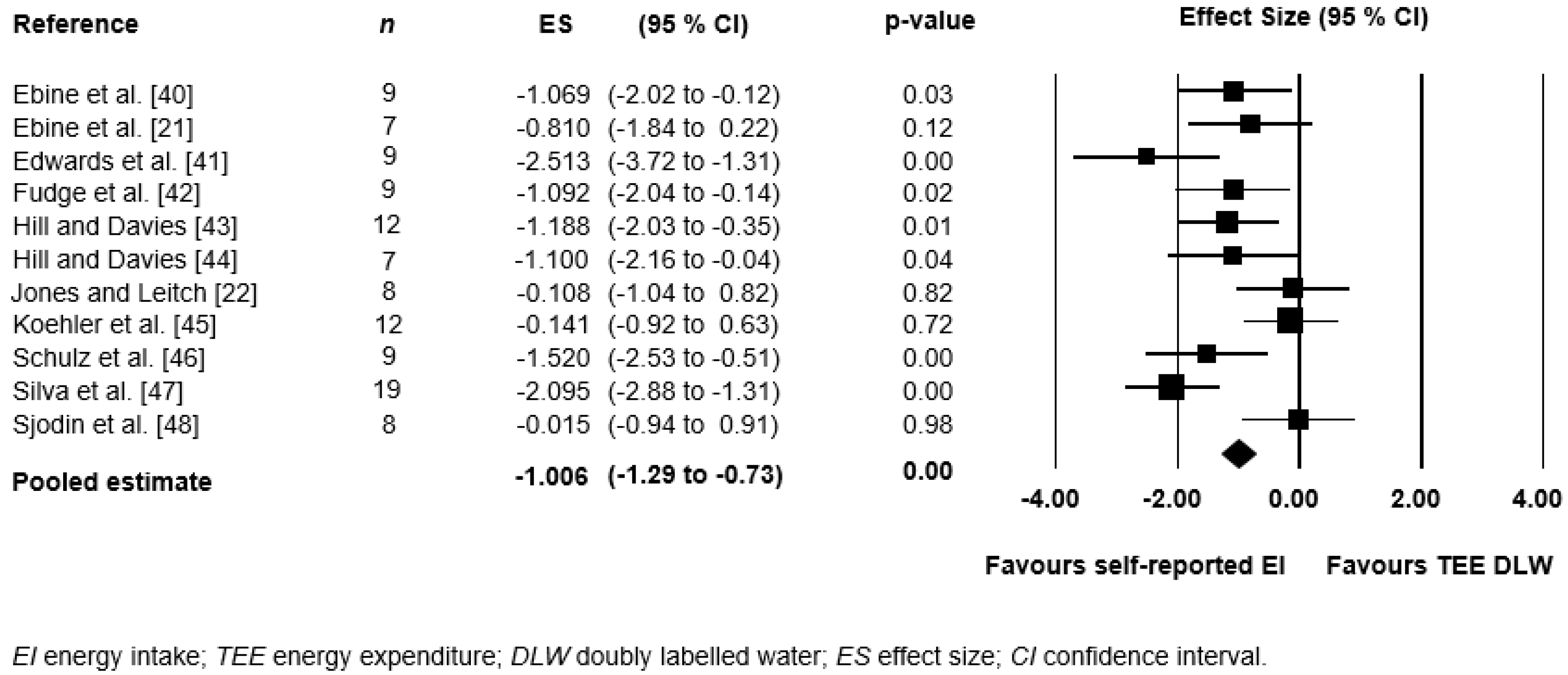
3.4. Meta-Analysis
3.5. Studies Comparing Reported Dietary Intake by Two or More Methods of Dietary Assessment
3.5.1. Reported Mean Energy Intake
3.5.2. Reported Mean Macronutrient Intake
3.5.3. Other Nutrients, Food Groups and Dietary Patterns Reported
3.6. Evaluation of Methodological Quality
4. Discussion
4.1. Studies Comparing EI to TEE as Measured by DLW
4.1.1. Methodological Issues
4.1.2. Assessment of Dietary Intake
4.1.3. Variability of Intake and Expenditure in Athletes
4.1.4. Influence of Body Mass, Body Image, and Energy Demands
4.2. Studies Comparing Dietary Intake by Two or More Methods of Dietary Assessment
4.2.1. Dietary Reference Methods
4.2.2. Evaluation Using Biomarkers
4.2.3. Nutrients, Food Groups and Dietary Patterns
4.3. Qualitative Assessment of Methodological Quality
5. Limitations, Strengths and Future Directions
6. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Manore, M.M.; Thompson, J.L. Energy requirements of the athlete: Assessment and evidence of energy efficiency. In Clinical Sports Nutrition, 5th ed.; Burke, L., Deakin, V., Eds.; McGraw-Hill: North Ryde, Australia, 2015; pp. 114–139. [Google Scholar]
- Burke, L.M. Energy needs of athletes. Can. J. Appl. Physiol. 2001, 26, S202–S219. [Google Scholar] [CrossRef] [PubMed]
- Deakin, V.; Kerr, D.; Boushey, C. Measuring nutritional status of athletes: Clinical and research perspectives. In Clinical Sports Nutrition, 5th ed.; Burke, L.M., Deakin, V., Eds.; McGraw-Hill: North Ryde, Australia, 2015; pp. 27–53. [Google Scholar]
- Economos, C.D.; Bortz, S.S.; Nelson, M.E. Nutritional practices of elite athletes. Practical recommendations. Sports Med. 1993, 16, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Dennis, S.C.; Lindsay, F.H.; Noakes, T.D. Nutritional practices of athletes: Are they sub-optimal? J. Sports Sci. 1995, 13, S75–S81. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Cox, G.R.; Cummings, N.K.; Desbrow, B. Guidelines for daily carbohydrate intake: Do athletes achieve them? Sports Med. 2001, 31, 267–299. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Slater, G.; Broad, E.M.; Haukka, J.; Modulon, S.; Hopkins, W.G. Eating patterns and meal frequency of elite Australian athletes. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Wardenaar, F.; Brinkmans, N.; Ceelen, I.; Van Rooji, B.; Mensink, M.; Witkamp, R.; De Vries, J. Macronutrient intakes in 553 Dutch elite and sub-elite endurance, team, and strength athletes: Does intake differ between sport disciplines? Nutrients 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Jonnalagadda, S.S.; Benardot, D.; Dill, M.N. Assessment of under-reporting of energy intake by elite female gymnasts. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Farajian, P.; Kavouras, S.A.; Yannakoulia, M.; Sidossis, L.S. Dietary Intake and Nutritional Practices of Elite Greek Aquatic Athletes. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Julian-Almarcegui, C.; Gómez-Cabello, A.; González-Agüero, A.; Olmedillas, H.; Gómez-Bruton, A.; Matute-Llorente, A.; Casajús, J.A.; Vicente-Rodríguez, G. The nutritional status in adolescent Spanish cyclists. Nutr. Hosp. 2013, 28, 1184–1189. [Google Scholar] [PubMed]
- Shriver, L.H.; Betts, N.M.; Wollenberg, G. Dietary intakes and eating habits of college athletes: Are female college athletes following the current sports nutrition standards? J. Am. Coll. Health Assoc. 2013, 61, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Calleja-González, J.; Urdampilleta, A.; Ostojic, S.M. Dietary intake habits and controlled training on body composition and strength in elite female volleyball players during the season. Appl. Physiol. Nutr. Metab. 2015, 40, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, G.M.; Kukkonen-Harjuyla, T.K.; Taipale, S.A.; Sievänen, H.T.; Oja, P.; Vuori, I.M. Resting metabolic rate and energy intake in female gymnasts, figure-skaters and soccer players. Int. J. Sports Med. 1995, 16, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Hassapidou, M.N.; Manstrantoni, A. Dietary intakes of elite female athletes in Greece. J. Hum. Nutr. Diet. 2001, 14, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.; Harries, S.K.; Williams, R.L.; Lum, C.; Callister, R. The diet quality of competitive adolescent male rugby union players with energy balance estimated using different physical activity coefficients. Nutrients 2016, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J.; Davies, P. The validity of self-reported energy intake as determined using the doubly labelled water technique. Br. J. Nutr. 2001, 85, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Yannakoulia, M. Methodology of dietary assessment in athletes: Concepts and pitfalls. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Trabulsi, J.; Schoeller, D.A. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am. J. Physiol. 2001, 281, E891–E899. [Google Scholar]
- Ebine, N.; Rafamantanantsoa, H.; Nayuki, Y.; Yamanaka, K.; Tashima, K.; Ono, T.; Saitoh, S.; Jones, P.J.H. Measurement of total energy expenditure by the doubly labelled water method in professional soccer players. J. Sports Sci. 2002, 20, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.; Leitch, C.A. Validation of doubly labeled water for measurement of caloric expenditure of collegiate swimmers. J. Appl. Physiol. 1993, 74, 2909–2914. [Google Scholar] [PubMed]
- Livingstone, M.B.; Prentice, A.M.; Coward, W.A.; Strain, J.J.; Black, A.E.; Davies, P.S.; Stewart, C.M.; McKenna, P.G.; Whitehead, R.G. Validation of estimates of energy intake by weighed dietary records and diet history in children and adolescents. Am. J. Clin. Nutr. 1992, 56, 29–35. [Google Scholar] [PubMed]
- Prentice, A.M.; Black, A.E.; Coward, W.A.; Davies, H.L.; Goldberg, G.R.; Murgatroyd, P.R.; Ashford, J.; Sawyer, M.; Whitehead, R.G. High levels of energy expenditure in obese women. Br. Med. J. 1986, 292, 983–987. [Google Scholar] [CrossRef]
- Westerterp, K.R.; Saris, W.H.M.; van Es, M.; ten Hoor, F. Use of the doubly labeled water technique in humans during heavy sustained exercise. J. App. Physiol. 1986, 61, 2162–2167. [Google Scholar]
- Haggarty, P.; McGaw, B.A.; Maughan, R.J.; Fenn, C. Energy expenditure of elite female athletes measured by the doubly labeled water method. Proc. Nutr. Soc. 1988, 47, 35. [Google Scholar]
- Bingham, S.A.; Gill, C.; Welch, A.; Day, K.; Cassidy, A.; Khaw, K.T.; Sneyd, M.J.; Key, T.J.A.; Roe, L.; Day, N.E. Comparison of dietary assessment methods in nutritional epidemiology: Weighed records v. 24 h recalls, food frequency questionnaires and estimated-diet records. Br. J. Nutr. 1994, 72, 619–643. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.J.; Meredith, K.; Cox, G.R.; Hopkins, W.G.; Burke, L.M. Variability in estimation of self-reported dietary intake data from elite athletes resulting from coding by different sports dietitians. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.D.; Hunt, K.M.; Burstyne Berg, M.; Slawson, D.A.; Vukadinovich, C.M.; McClanahan, B.S.; Clemens, L.H. Reliability and validity of a brief questionnaire to assess calcium intake in female collegiate athletes. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.; Boushey, C.J.; Kerr, D.; Okos, M.; Ebert, D.S.; Delp, E.J. An overview of The Technology Assisted Dietary Assessment Project at Purdue University. In Proceedings of the IEEE International Symposium on Multimedia, Ningbo, China, 29–31 October 2010; pp. 290–295. [Google Scholar]
- Stumbo, P.J. New technology in dietary assessment: A review of digital methods in improving food record accuracy. Proc. Nutr. Soc. 2013, 72, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gemming, L.; Utter, J.; Ni Mhurchu, C. Image-assisted dietary assessment: A systematic review of the evidence. J. Acad. Nutr. Diet. 2015, 115, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Boushey, C.J.; Spoden, M.; Zhu, F.M.; Delp, E.J.; Kerr, D.A. New mobile methods for dietary assessment: Review of image-assisted and image-based dietary assessment methods. Proc. Nutr. Soc. 2016, 76, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Br. Med. J. 2009, 339, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Truswell, S. Essentials of Human Nutrition, 5th ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Academy of Nutrition and Dietetics. Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process; Academy of Nutrition and Dietetics: Chicago, IL, USA, 2016. [Google Scholar]
- Serra-Majem, L.; Frost Andersen, L.; Henríque-Sánchez, P.; Doreste-Alonso, J.; Sánchez-Villegas, A.; Ortiz-Andrelluchi, A.; Negri, E.; La Vecchia, C. Evaluating the quality of dietary intake studies. Br. J. Nutr. 2009, 102, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Ebine, N.; Feng, J.-Y.; Homma, M.; Saitoh, S.; Jones, P.J.H. Total energy expenditure of elite synchronized swimmers measured by the doubly labeled water method. Eur. J. Appl. Physiol. 2000, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.E.; Lindeman, A.K.; Mikesky, A.E.; Stager, J.M. Energy balance in highly trained female endurance runners. Med. Sci. Sports Exerc. 1993, 25, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Fudge, B.W.; Westerterp, K.R.; Kiplamai, F.K.; Onywera, V.O.; Boit, M.K.; Kayser, B.; Pitsiladis, Y.P. Evidence of negative energy balance using doubly labelled water in elite Kenyan endurance runners prior to competition. Br. J. Nutr. 2006, 95, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J.; Davies, P.S.W. Short communication: The validity of a four day weighed food record for measuring energy intake in female classical ballet dancers. Eur. J. Clin. Nutr. 1999, 53, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J.; Davies, P.S. Energy intake and energy expenditure in elite lightweight female rowers. Med. Sci. Sports Exerc. 2002, 34, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.; Braun, H.; De Marées, M.; Fusch, G.; Fusch, C.; Mester, J.; Schaenzer, W. Parallel assessment of nutrition and activity in athletes: Validation against doubly labelled water, 24-h urea excretion, and indirect calorimetry. J. Sports Sci. 2010, 28, 1435–1449. [Google Scholar] [CrossRef] [PubMed]
- Schulz, L.O.; Alger, S.; Harper, I.; Wilmore, J.H.; Ravussin, E. Energy expenditure of elite female runners measured by respiratory chamber and doubly labeled water. J. Appl. Physiol. 1992, 72, 23–28. [Google Scholar] [PubMed]
- Silva, A.M.; Santos, D.A.; Matias, C.N.; Minderico, C.S.; Schoeller, D.A.; Sardinha, L.B. Total energy expenditure assessment in elite junior basketball players: A validation study using doubly labeled water. J. Strength Cond. Res. 2013, 27, 1920–1927. [Google Scholar] [CrossRef] [PubMed]
- Sjödin, A.M.; Andersson, A.B.; Högberg, J.M.; Westerterp, K.R. Energy balance in cross-country skiiers: A study using doubly labeled water. Med. Sci. Sports Exerc. 1994, 26, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Heaton, L.E.; Stein, K.W.; Nuccio, R.P.; Jeukendrup, A.E. Validity and relative validity of a novel digital approach for 24-h dietary recall in athletes. Nutr. J. 2014, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.J.; Hopkins, W.G.; Lowe, T.E.; Rush, E.C. Development and validation of a food-frequency questionnaire to assess short-term antioxidant intake in athletes. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, M.; Lahti-Koski, M. The validity of a food use questionnaire in assessing the nutrient intake of physically active men. Eur. J. Clin. Nutr. 1991, 45, 267–272. [Google Scholar] [PubMed]
- Scoffier, S.; Gernigon, C.; Billi, E.; d’Arripe-Longueville, F. Development and preliminary validation of a new instrument to assess eating behaviors: The virtual self-service restaurant (VSSR). Sci. Sports 2013, 28, 140–145. [Google Scholar] [CrossRef]
- Sunami, A.; Sasaki, K.; Suzuki, Y.; Oguma, N.; Ishihara, J.; Nakai, A.; Yasuda, J.; Yokoyama, Y.; Yoshizaki, T.; Tada, Y.; et al. Validity of a semi-quantitative food frequency questionnaire for collegiate athletes. J. Epidemiol. 2016, 26, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Wardenaar, F.C.; Steennis, J.; Ceelan, I.J.M.; Mensink, M.; Witkamp, R.; de Vries, J.H.M. Validation of a web-based, multiple 24-h recalls combined with nutritional supplement intake questionnaires against nitrogen excretions to determine protein intake in Dutch elite athletes. Br. J. Nutr. 2015, 114, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar] [PubMed]
- Black, A.E.; Welch, A.A.; Bingham, S.A. Validation of dietary intakes measured by diet history against 24 h urinary nitrogen excretion and energy expenditure measured by the doubly-labelled water method in middle aged women. Br. J. Nutr. 2000, 83, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Barnard, J.; Tapsell, L.C.; Davies, P.; Storlien, S.H. Relationship of high energy expenditure and variation in dietary intake with reporting accuracy on 7 day food records and diet histories in a group of healthy adult volunteers. Eur. J. Clin. Nutr. 2002, 56, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, D.A. Measurement of energy expenditure in free-living humans by using doubly labeled water. J. Nutr. 1988, 118, 1278–1289. [Google Scholar] [PubMed]
- Prentice, A.M.; Coward, A.; Cole, T.; Schoeller, D.; Haggarty, P. Practical recommendations and worked examples. In The Doubly-Labelled Water Method for Measuring Energy Expenditure: Technical Recommendations for Use in Humans, A Consensus Report by the International Dietary Energy Consulting Group (IDECG) Working Group; Cole, T.J., Coward, W.A., Elia, M., Fjeld, C.J., Franklin, M., Goran, M.I., Eds.; International Atomic Energy Agency: Vienna, Austria, 1990; pp. 212–247. [Google Scholar]
- Black, A.E.; Prentice, A.M.; Coward, W.A. Use of food quotients to predict respiratory quotients for the doubly labelled water method for measuring energy expenditure. Hum. Nutr. Clin. Nutr. 1986, 40C, 381–391. [Google Scholar]
- Basiotis, P.P.; Welsh, S.O.; Cronin, F.J.; Kelsay, J.L.; Mertz, W. Number of days of food intake records required to estimate individual and group nutrient intakes with defined confidence. J. Nutr. 1987, 117, 1638–1641. [Google Scholar] [PubMed]
- Willett, W. Nutritional Epidemiology, 3rd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Livingstone, M.B.; Black, A.E. Markers of the validity of reported energy intake. J. Nutr. 2003, 133, 895S–920S. [Google Scholar] [PubMed]
- Westerterp, K.R.; Goris, A.H.C. Validity of the assessment of dietary intake: Problems of misreporting. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Bradley, W.J.; Cavanagh, B.; Douglas, W.; Donovan, T.F.; Twist, C.; Morton, J.P.; Close, G.L. Energy intake and expenditure assessed ‘in-season’ in an elite European rugby union squad. Eur. J. Sport Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Howatson, G.; Quin, E.; Redding, E.; Stevenson, E.J. Energy intake and energy expenditure of pre-professional female contemporary dancers. PLoS ONE 2017. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, D.A. How accurate is self-reported dietary energy intake? Nutr. Rev. 1990, 48, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Bandini, L.G.; Schoeller, D.A.; Cyr, H.N.; Dietz, W.H. Validity of reported energy intake in obese and nonobese adolescents. Am. J. Clin. Nutr. 1990, 52, 421–425. [Google Scholar] [PubMed]
- Johannson, G.; Wikman, Å.; Åhrén, A.; Hallmans, G.; Johannson, I. Underreporting of energy intake in repeated 24-hour recalls related to gender, age, weight status, day of interview, educational level, reported food intake, smoking habits and area of living. Public Health Nutr. 2001, 4, 919–927. [Google Scholar] [CrossRef]
- Heitmann, B.L. The influence of fatness, weight change, slimming history and other lifestyle variables on diet reporting in Danish men and women aged 35–65 years. Int. J. Obes. 1993, 17, 329–336. [Google Scholar]
- Lafay, L.; Basdevant, A.; Charles, M.-A.; Vray, M.; Balkau, B.; Borys, J.M.; Eschwège, E.; Romon, M. Determinants and nature of dietary under-reporting in a free-living population: The Feurbaix Laventie Ville Sante (FLVS) study. Int. J. Obes. 1997, 21, 567–573. [Google Scholar] [CrossRef]
- Bathalon, G.P.; Tucker, K.L.; Hays, N.P.; Vinken, A.G.; Greenberg, A.S.; McCrory, M.A.; Roberts, S.B. Psychological measures of eating behavior and the accuracy of 3 common dietary assessment methods in healthy postmenopausal women. Am. J. Clin. Nutr. 2000, 71, 739–745. [Google Scholar] [PubMed]
- Schoeller, D.A.; Bandini, L.G.; Dietz, W.H. Inaccuracies in self-reported intake identified by comparison with the doubly labelled water method. Can. J. Physiol. Pharmacol. 1990, 68, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Bandini, L.G.; Cyr, H.; Must, A.; Dietz, W.H. Validity of reported energy intake in preadolescent girls. Am. J. Clin. Nutr. 1997, 65, 1138S–1141S. [Google Scholar] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Bingham, S.A.; Cassidy, A.; Cole, T.; Welch, A.; Runswick, S.A.; Black, A.E.; Thurnham, D.; Bates, C.; Khaw, K.T.; Day, N.E. Validation of weighed records and other methods of dietary assessment using the 24h urine nitrogen technique and other biological markers. Br. J. Nutr. 1995, 73, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Gersovitz, M.; Madden, J.P.; Smiciklas-Wright, H. Validity of the 24-hr dietary recall and seven day record for group comparisons. J. Am. Diet. Assoc. 1978, 73, 48–55. [Google Scholar] [PubMed]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy availability in athletes. J. Sports Sci. 2011, 29, S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Kant, A.K. Indexes of overall diet quality: A review. J. Am. Diet. Assoc. 1996, 96, 785–791. [Google Scholar] [CrossRef]
- Wirt, A.; Collins, C.E. Diet quality—What is it and does it matter? Public Health Nutr. 2009, 2, 2473–2492. [Google Scholar] [CrossRef] [PubMed]
- Spronk, I.; Heaney, S.E.; Prvan, T.; O’Connor, H.T. Relationship between general nutrition knowledge and dietary quality in elite athletes. Int. J. Sports Nutr. Exerc. Metab. 2015, 25, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.L.; Martin, R.J.; Collins, C.E. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J. Am. Diet. Assoc. 2010, 110, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.B.; Trommelen, J.; Wardenaar, F.C.; Brinkmans, N.Y.; Versteegen, J.J.; Jonvik, K.L.; Kapp, C.; de Vries, J.; van den Borne, J.J.; Gibala, M.J.; et al. Dietary protein intake and distribution patterns of well-trained Dutch athletes. Int. J. Sports Nutr. Exerc. Metab. 2017, 27, 105–114. [Google Scholar] [CrossRef] [PubMed]
- El-Chab, A.; Simpson, C.; Lightowler, H. The reproducibility of a diet using three different dietary standardisation techniques in athletes. Eur. J. Clin. Nutr. 2016, 70, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.B.E.; Robson, P.J. Measurement of dietary intake in children. Proc. Nutr. Soc. 2000, 59, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Rumbold, P.L.; St Clair Gibson, A.; Stevenson, E.; Dodd-Reynolds, C.J. Agreement between two methods of dietary data collection in female adolescent netball players. Appetite 2011, 57, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.A.; Rumbold, P.L.S.; Cockburn, E.; Russell, M.; Stevenson, E.J. Agreement between two methods of dietary data collection in male adolescent academy-level soccer players. Nutrients 2015, 7, 5948–5960. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.; Gemming, L.; Baker, D.; Braakhuis, A. Do image-assisted mobile applications improve dietary habits, knowledge, and behaviours in elite athletes? A pilot study. Sports 2017, 5, 60. [Google Scholar] [CrossRef]

| Reference, Country | Group (n) | Sport, Calibre | Age (Years) | Body Mass (kg) | Stature (cm) | BMI (kg/m2) | Body Fat (%) | Comments |
|---|---|---|---|---|---|---|---|---|
| Baker et al. (2014), USA [49] | 56 (41 M, 15 F) | Mixed sports 1, competitive, tertiary | 16 ± 2 (14–20) | 69.4 ± 14.3 | 174.3 ± 9.4 | n = 12 excluded (non-adherence) | ||
| Braakhuis et al. (2011), USA [50] | 113 (56 M, 57 F) | Rowers, national competitive | 22 ± 3 (17–36) | 78.0 ± 11.0 | NR | n = 2 excluded (EI < 1.39 * RMR) | ||
| Ebine et al. (2000), Japan [40] | 9 F | Synchronised swimmers, national | 19.8 ± 2.8 (16–23) | 52.5 ± 2.7 | 159.0 ± 3.0 | 20.7 ± 0.7 | DLW | |
| Ebine et al. (2002), Japan [21] | 7 M | Soccer, professional | 22.1 ± 1.9 | 69.8 ± 4.7 | 175.0 ± 5.0 | 13.4 ± 3.6 | DLW | |
| Edwards et al. (1993), USA [41] | 9 F | Cross-country runners, highly trained, tertiary | NR # | 55.3 ± 6.2 | 169.1 ± 5.5 | 19.3 ± 1.7 | 13.0 ± 3.2 | DLW |
| Fogelholm & Lahti-Koski (1991), Finland [51] | 84 M | Mixed sports 2, recreational | 24 ± 4 | 72.0 ± 7.0 | 180.0 ± 6.0 | n = 12 excluded (incomplete data) | ||
| Fudge et al. (2006), Kenya [42] | 9 M | Middle & distance runners, elite | 21 ± 2 | 56.0 ± 3.4 | 174.0 ± 2.9 | 18.3 ± 1.3 | 7.1 ± 2.5 (BIA) | DLW |
| Hill and Davies (1999), Australia [43] | 12 F | Ballet dancers, tertiary | 18.7 ± 1.2 | 57.3 ± 3.8 | 169.1 ± 4.6 | DLW | ||
| Hill and Davies (2002), Australia [44] | 7 F | Lightweight rowers, elite | 20.0 ± 1.1 | 60.9 ± 2.3 | 168.8 ± 4.7 | 22.8 ± 5.1 | DLW n = 1 excluded (incomplete data) | |
| Jones and Leitch (1993), Canada [22] | 8 (5 M, 3 F) | Swimmers, tertiary | 20.1 ± 1.7 | 74.1 ± 9.3 | 186.0 ± 11.0 | 16.0 ± 6.4 | DLW n = 1 excluded (disagreement between measures) | |
| Koehler et al. (2010), Germany [45] | 12 M | Triathletes, well-trained a | 30.4 ± 6.2 | 80.6 ± 6.5 | 186.0 ± 8.0 | 23.2 ± 1.4 | 11.2 ± 2.1(BIA) | DLW n = 2 excluded (change BW > 3%; EI < 1.39 * RMR) |
| Schulz et al. (1992), USA [46] | 9 F | Distance runners, elite, national | 26.0 ± 3.3 | 52.4 ± 4.1 | 163.0 ± 7.0 | 12 ± 3 (UWW) 17 ± 3 (BIA) | DLW | |
| Scoffier et al. (2013), France ^ [52] | 22 (13 F, 9 M) 20 (12 F, 8 M) | Mixed sports a Adolescent, weight sports Adolescent, other sports | 25.3 ± 4.7 26.4 ± 5.7 | 59.7 ± 15.7 * 62.1 ± 10.7 * | 169.8 ± 2.1 * 168.8 ± 2.7 * | |||
| Silva et al. (2013), Portugal [47] | 19 (12 M, 7 F) | Basketball, junior national, elite | 17.0 ± 0.7 (M) 16.9 ± 0.7 (F) (16–18) | 74.7 ± 10.8 | 185.4 ± 11.0 | 21.7 ± 1.9 | DLW | |
| Sjodin et al. (1994), Sweden [48] | 8 (4 M, 4 F) | Cross-country skiers, elite, national | 26 ± 2 (M) 25 ± 2 (F) | 75.1 ± 4.9 (M) 54.4 ± 5.1 (F) | 180.0 ± 6.0 (M) 166.0 ± 2.0 (F) | DLW | ||
| Sunami et al. (2016), Japan [53] | 156 (92 M, 64 F) | Mixed sports 3, tertiary | NR# | 68.7 ± 8.4 (M) 56.1 ± 5.9 (F) | 174.7 ± 6.5 (M) 163.4 ± 5.8 (F) | 22.5 ± 2.1 (M) 21.0 ± 1.5 (F) | ||
| Ward et al. (2004), USA [29] | 76 F | Mixed sports 4, Division I and III NCAA, tertiary | 18.8 ± 1.1 (17–21) | 59.8 ± 7.1 * | 165.6 ± 6.1 * | 21.7 ± 2.3 | ||
| Wardenaar et al. (2015), Holland [54] | 47 (31 M, 16 F) | Mixed sports 5, elite, Olympic | 21.2 ± 3.9 (18–35) | 74.3 ± 10.3 | 179.3 ± 7.2 | 21.6 ± 4.1 (17.5–31) | n = 1 excluded (incomplete data) |
| Reference | Dietary Method (Other Methods) | EI (kJ/day) | CHO (%) | Pro (%) | Fat (%) | TEE DLW (kJ/day) | REE (kJ/day) | (TEE–EI)/TEE * 100 (%) (Mean TEE–EI) | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Ebine et al. [40] | 7-day FR | 8900 ± 1700 * | 11,500 ± 2800 * (6-day DLW) | 5200 ± 300 | 23.3% * (−2600 ± 3200 kJ) | r = −0.854, p < 0.05 No change in BW r = 0.808, p < 0.01 (BW & TEE) r = 0.856, p < 0.01 (BW & REE) r = 0.715, p < 0.05 (BW & PAL) | |||
| Ebine et al. [21] | 7-day FR | 13,000 ± 2400 ** | 14,800 ± 1700 ** (7-day DLW) | 7000 ± 300 (eqn.) | 12%** (−1800 ± 1200 kJ) | r = 0.893, p < 0.01 No change in BW | |||
| Edwards et al. [41] | 7-day FR Food Attitude Scale (30 items) AR | 8527 ± 1246 ** | 12,516 ± 1737 ** (7-day DLW) | 32% ** (−3989 ± 2855 kJ) | r = −0.83, p < 0.01 (i.e., higher TEE, lower reported EI) No change in BW r = 0.82, p < 0.01 (BW & TEE) (i.e., heavier expended more energy) r = −0.74, p < 0.01 (BW & EI) (i.e., heavier reported lower EI) r = −0.78, p < 0.01 (BW & food attitude) (i.e., heavier women reported lower body image scores) | ||||
| Fudge et al. [42] | 7-day FR (W) ActiGraph™ activity monitor | 13,241 ± 1330 * | 67.3 ± 7.8% (9.8 g/kg) | 15.3 ± 4.0% (2.2 g/kg) | 17.4 ± 3.9% (1.1 g/kg) | 14,611 ± 1043 * (7-day DLW) | 6408 ± 222 (eqn.) | 13% * (−24 to 9%) (−1370 ± 1738 kJ) | no correlation between EI & TEE r = −0.071, p < 0.855, No change in BW |
| Hill and Davies [43] | 4-day FR (W) | 10,192 ± 2268 | 12,498 ± 1117 (14-day DLW) Adj. TEE 12,983 ± 2268 | 7150 ± 757 | 21% (−2791 kJ) | n = 8 increased BW (0.3 kg) while reportedly consuming less than real EI. Underreporting of EI not related to % body fat (r = 0.11) | |||
| Hill and Davies [44] | 4-day FR (W) | 9263 ± 1309 ** | 16,556 ± 5100 ** (14-day DLW) Adj. TEE 14,008 ± 5560 | 5812 ± 142 (eqn.) | 34% ** (−7293 ± 6075 kJ **) Adj. TEE-EI −4740 ± 6439 kJ ** | r = −0.93, p < 0.01 Mean TEE adjusted for BW change (−1.2 ± 1.2 kg) Adjusted r = −0.93, p < 0.01 Adjusted (95% LOA—17,619 to 8134 kJ) | |||
| Jones and Leitch [22] | 10-day test diet (32% fat, 15% pro, 48% CHO) AR | 16,297 ± 2598 Adjusted EI 14,410 ± 3870 | 48% | 15% | 32% | 14,502 ± 4151 (10-day DLW) Adj. TEE 14,878 ± 4289 | 5% (−468 kJ) | No change in BW EI from test diet increased by 10% (n = 1); and decreased by 15% (n = 1) due to fluctuations in BW and complaint of large portion sizes, respectively. n = 1 removed due to disagreement between measures (35%) | |
| Koehler et al. [45] | 7-day FR (198 items) 7-day AR (25 items) 24-h N+ excretion | 14,786 ± 1682 | 1.38 ± 0.55 g/kg (Ex.) 1.51 ± 0.70 g/kg (Ex. free) | 15,196 ± 3598 (7-day DLW) | 2.7% (−410 kJ) | Weak association EI & TEE (r = 0.48) Removal n = 2 (TEE < 1.39 × REE) Adjusted correlation (r = 0.69, p < 0.05) Bland-Altman comparison indicates bias towards underestimating high EI (p < 0.01) (95% LOA—5736 to 4912 kJ/day) (−39% to 33% mean EI) No change in BW (n = 1 excluded BW change > 3%) Bland-Altman TEE DLW & AR—151 kJ/day (95% LOA—3356 to 3054 kJ/day) r = 0.83, p < 0.01 (Pro & 24-h urea N+) Bland-Altman mean difference Pro & urinary N− 0.01 g/kg/day (95% LOA—0.65 to 0.67 g/kg/day) r = 0.95, SEE = 816 kJ/day | |||
| Schulz et al. [46] | 6-day FR (AR) | 9175 ± 1950 (6560–13,359) | 59% (333 g/day) (216–612 g/day) | 13% (73 g/day) (50–104 g/day) | 27% (66 g/day) (49–100 g/day) | 11,824 ± 1305 (9832–13,874) (6-day DLW) | 7037 ± 351 | 22% (−925 ± 2301 kJ) | No relationship (r = 0.063) No significant change in BW but most lost mass (−84 ± 71 g/day) |
| Silva et al. [47] | 7-day FR # | 11,274 ± 2567 * | 50.2 ± 3.5% (338.8 ± 82.7 g/day) | 18.6 ± 2.6% (125.7 ± 30.5 g/day) | 29.4 ± 2.6% (88.1 ± 22.0 g/day) | 17,598 ± 3298 * (7-day DLW) | 6199 ± 1007 ^ | 34% (−6837 kJ) | No relationship (r = 0.58, p = 0.057) |
| Sjodin et al. [48] | 5-day FR (W) (F) 4-day FR (W) (M) (AR) | 18,200 ± 1900 (F) (5700–20,200) 30,200 ± 4600 (M) (5400–34,900) | 58% | 13% | 28% | 18,300 ± 2200 (F) (7-day DLW) 30,200 ± 4200 (M) (6-day DLW) | 5500 ± 300 (F) 7600 ± 300 (M) (eqn.) | 1.1 ± 15.7% (F) 0.6 ± 3.3% (M) (−100 ± 1900 kJ) | r = 0.96, p = 0.0001 No change in BW |
| Reference | n | M | F | EI (kJ) (±SD) | TEE (kJ) (±SD) | Difference (%) (TEE–EI kJ) | Weighed Mean Difference (%) |
|---|---|---|---|---|---|---|---|
| Ebine et al. [40] | 9 | 9 | 8900 (1700) | 11,500 (2800) | 22.6 (2600 kJ) | 203 | |
| Ebine et al. [21] | 7 | 7 | 13,000 (2400) | 14,800 (1700) | 12.2 (1800 kJ) | 85 | |
| Edwards et al. [41] | 9 | 9 | 8527 (1246) | 12,516 (1737) | 31.9 (3989 kJ) | 287 | |
| Fudge et al. [42] | 9 | 9 | 13,241 (1330) | 14,611 (1043) | 9.4 (1370 kJ) | 84 | |
| Hill and Davies [43] | 12 | 12 | 10,192 (2268) | 12,983 (2268) | 21.5 (2791 kJ) | 238 | |
| Hill and Davies [44] | 7 | 7 | 9263 (1309) | 14,008 (5560) | 33.9 (4745 kJ) | 237 | |
| Jones and Leitch [22] | 8 | 5 | 3 | 14,410 (3870) | 14,878 (4289) | 3.1 (469 kJ) | 25 |
| Koehler et al. [45] | 12 | 12 | 14,786 (1682) | 15,196 (3598) | 2.7 (410 kJ) | 32 | |
| Schulz et al. [46] | 9 | 9 | 9176 (1950) | 11,824 (1305) | 22.4 (2648 kJ) | 202 | |
| Silva et al. [47] | 19 | 12 | 7 | 11,274 (2567) | 17,598 (3298) | 35.9 (6324 kJ) | 683 |
| Sjodin et al. [48] | 8 | 4 | 4 | 24,200 (3250) | 24,250 (3200) | 0.4 (100 kJ) | 3 |
| ∑ athletes | 109 | 49 | 60 | ||||
| Mean | 12,452 kJ (2143) | 14,924 kJ (2800) | 18% (2477 kJ) | 19.1% |
| Reference | Dietary Method | Reference Method(s) | EI (kJ/day) | CHO (g) | Pro (g) | Fat (g) | Main Findings |
|---|---|---|---|---|---|---|---|
| Baker et al. (2014) [49] | DATA ipad administered modified multiple pass 24-h DR (n = 56) (pre-test DATA, n = 19) | INTERVIEW 24-h DR (n = 56) OBSERVATION RD observed 24-h (n = 26) | DATA 14,636 ± 5945 kJ * OBS. 12,728 ± 5280 kJ * DATA 13,870 ± 6117 kJ INTERVIEW 14,041 ± 6627 kJ | DATA 475 ± 190 OBS. 426 ± 159 DATA 449 ± 205 INTERVIEW 449 ± 216 | DATA 151 ± 59 OBS. 139 ± 63 DATA 140 ± 62 INTERVIEW 147 ± 77 | DATA 116 ± 65 * OBS. 91 ± 53 * DATA 112 ± 73 INTERVIEW 112 ± 61 | Significant difference between DATA & OBS. for energy, CHO, Pro, fat, water, sodium, iron, calcium (ICC 0.78–0.91) NS between DATA & INTERVIEW for energy, CHO, Pro or fat (ICC 0.75–0.91) 95% CI between DATA & OBS. NS for CHO 10.1% (−1.2–22.7%) or Pro 14.1% (−3.2–34.5%) but significantly greater for EI * 14.4% (1.2–29.3%) and fat * 26.4% (6.9–49.6%). Additional findings: TEE (DATA + OBS.) 14,836 ± 4012 kJ TEE (DATA + INTERVIEW) 13,117 ± 4305 kJ NS between TEE & EI from DATA, INTERVIEW or OBS. however a tendency for TEE be greater than EI from OBS. method (p = 0.104). Good relative validity for DATA for group level comparisons, but large variations of estimates for individual dietary intake, especially athletes with higher intakes (i.e., EI, CHO, Pro). |
| Braakhuis et al. (2011) [50] | FFQ (70 items) | FR (7d, W) (n = 81 FFQ & FR) Biomarker (FRAP) (n = 96 FFQ & FRAP) (n = 63 FR & FRAP) | 14,500 ± 5700 (7500–25,900) | 470 ± 190 (53.5 ± 6.8%) | 150 ± 80 (17.9 ± 5.8%) | 114 ± 54 (28.0 ± 6.2%) | Modest correlation between FR and FFQ antioxidant intake (r = 0.38 ± 0.14, 90% CI) Small correlation between biomarker and FFQ (r = 0.28 ± 0.15) Additional findings: Correlation highest for antioxidants from cereals (r = 0.55 ± 0.11), coffee and tea (r = 0.51 ± 0.15); and moderate for vegetables (r = 0.34 ± 0.16) and fruit (r = 0.31 ± 0.16). FFQ overestimated intake by 42% for those with low intake, and FFQ underestimated by 73% for those with high antioxidant intakes. |
| Fogelholm & Lahti-Koski (1991) [51] | FUQ (122 items) (FUQ1 participant reported portion size; FUQ2 medium portion sizes) | FR (7d, W) | 13,000 ± 2800 | 397 ± 123 | 122 ± 31 | 114 ± 30 | Close agreement EI between FR & FUQ1 & FUQ2 EI FR & FUQ1 (95% CI −1.7 to 0.1 ± 4.3) EI FR & FUQ2 (95% CI −0.1 to 1.7 ± 4.0) Additional findings: Mean intake CHO, Pro vit C, calcium, magnesium, iron and zinc overestimated in FUQ1. Mean intake CHO, vit C, calcium, magnesium, iron and zinc from FUQ2 did not differ from FR; however Pro & fat were underestimated. Most food group correlations above r = 0.24, except vegetable oils, some other fats, milk, pork, beef and poultry. |
| Scoffier et al. (2013) ^ [52] | VSSR | FR (1d) | Adolescent athletes, weight sports VSSR 7978 ± 2513 kJ FR 7491 ± 2116 kJ Adolescent athletes, other sports VSSR 7190 ± 2004 kJ FR 7081 ± 1785 kJ | NS between VSSR & FR (adolescent athletes, weight sports) (p < 0.11); or between VSSR & FR (adolescent athletes, other sports) (p = 0.56). | |||
| Sunami et al. (2016) [53] | FFQ (138 food, 20 beverage, 14 seasoning items) | 24-h DR (3d, non-consecutive) | 24-hDR 13,332 ± 3933 kJ (M) 8962 ± 2117 kJ (F) FFQ 12,159 ± 4996 kJ (M) 8029 ± 2519 kJ (F) | 24-hDR 486.6 ± 152.9 (M) 319.9 ± 81.8 (F) FFQ 452.4 ± 182.8 (M) 286.2 ± 76.0 (F) | 24-hDR 100.1 ± 32.5 (M) 65.5 ± 16.4 (F) FFQ 82.0 ± 35.5 (M) 59.0 ± 22.8 (F) | 24-hDR 83.7 ± 34.2 (M) 64.0 ± 18.6 (F) FFQ 76.7 ± 40.6 (M) 57.1 ± 28.6 (F) | FFQ underestimated EI by 9% M and 10% F Majority nutrients within ± 20% range; largest difference for retinol (77% M, 32% F) Additional findings: For 35 nutrients median deattenuated CC was 0.30 (0.10 to 0.57 (M) and 0.32 (−0.08 to 0.62) (F) For 19 food groups median deattenuated CC was 0.32 (0.17 to 0.72) (M) and 0.34 (−0.11 to 0.58) (F) Lower difference was noted for: cereals, vegetables, fungi food groups; while greater differences noted for: sugar, beverages, seasonings and spices. |
| Ward et al. (2004) [29] | RAM calcium checklist (54 items) | FR (6d) | Mean calcium (mg/day) RAM 822 ± 331 FR 823 ± 387 | Test-retest reliability of RAM was moderate ICC = 0.54 (p < 0.0001, r = 0.58) RAM had moderate agreement with FR ICC = 0.41 (p < 0.0067, r = 0.42). RAM correctly identified 84% with low calcium intake based on FR. | |||
| Wardenaar et al. (2015) [54] | Compl-eat™ 24-h DR (3d, non-consecutive) | 24-h urinary N+ excretion; Q (training load, sports foods, dietary supplements) | 16,900 ± 4200 (8540–26,600) | 24-hDR * 109.6 ± 33 (1.49 ± 0.35 g/kg/day) 24-h N+ excretion * 141.3 ± 38.2 (1.9 ± 0.39 g/kg/day) | Significant mean difference of 25.5 ± 21.3% (−31.7 ± 30 g/day) between 24-hDR and 24-h N+ excretion (p < 0.001) (r = 0.65; 95% CI 0.45–0.79) Additional findings: Underestimation of Pro related to amount of protein intake (r = −0.20; 95% CI −0.46 to 0.09) Mean FIL 1.6 ± 0.4 with 78.7% athletes FIL < 1.75 indicating possible under-reporting. Underreporting greater in individuals with higher protein intakes than with lower intakes. |
| References | 1. Hypothesis Stated | 2. Outcomes Described | 3. Subject Characteristics Described | 4. Principal Confounders Described | 5. Main Findings Described | 6. Random Variability provided | 7. Actual p Value Reported | 8. Clinical Significance Reported | 9. Biases and Limitations Discussed | 10. Representative of Population | 11. Participating Subjects Representative | 12. Attempt Made to Blind Main Outcome of Intervention | 13. Data Dredging Reported | 14. Statistical Tests Appropriate | 15. Compliance to Intervention Method | 16. Measures Used Accurate (Valid And Reliable) | 17. Funding and Affiliations Described | 18. Recruited over Same Time | 19. Adjustment for Confounding | 20. Participant Losses Accounted | 21. Power | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baker et al. [49] | ||||||||||||||||||||||
| Braakhuis et al. [50] | ||||||||||||||||||||||
| Ebine et al. [40] | ||||||||||||||||||||||
| Ebine et al. [21] | ||||||||||||||||||||||
| Edwards et al. [41] | ||||||||||||||||||||||
| Fogelholm et al. [51] | ||||||||||||||||||||||
| Fudge et al. [42] | ||||||||||||||||||||||
| Hill and Davies [43] | ||||||||||||||||||||||
| Hill and Davies [44] | ||||||||||||||||||||||
| Jones and Leitch [22] | ||||||||||||||||||||||
| Koehler et al. [45] | ||||||||||||||||||||||
| Schulz et al. [46] | ||||||||||||||||||||||
| Scoffier et al. [52] | ||||||||||||||||||||||
| Silva et al. [47] | ||||||||||||||||||||||
| Sjodin et al. [48] | ||||||||||||||||||||||
| Sunami et al. [53] | ||||||||||||||||||||||
| Ward et al. [29] | ||||||||||||||||||||||
| Wardenaar et al. [54] | ||||||||||||||||||||||
| Yes | ||||||||||||||||||||||
| No | ||||||||||||||||||||||
| Unsure/unable to determine | ||||||||||||||||||||||
| N/A due to DLW methodology | ||||||||||||||||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capling, L.; Beck, K.L.; Gifford, J.A.; Slater, G.; Flood, V.M.; O’Connor, H. Validity of Dietary Assessment in Athletes: A Systematic Review. Nutrients 2017, 9, 1313. https://doi.org/10.3390/nu9121313
Capling L, Beck KL, Gifford JA, Slater G, Flood VM, O’Connor H. Validity of Dietary Assessment in Athletes: A Systematic Review. Nutrients. 2017; 9(12):1313. https://doi.org/10.3390/nu9121313
Chicago/Turabian StyleCapling, Louise, Kathryn L. Beck, Janelle A. Gifford, Gary Slater, Victoria M. Flood, and Helen O’Connor. 2017. "Validity of Dietary Assessment in Athletes: A Systematic Review" Nutrients 9, no. 12: 1313. https://doi.org/10.3390/nu9121313






