Prevalence and Effects of Functional Vitamin K Insufficiency: The PREVEND Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Measurements
2.3. Clinical End Points
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
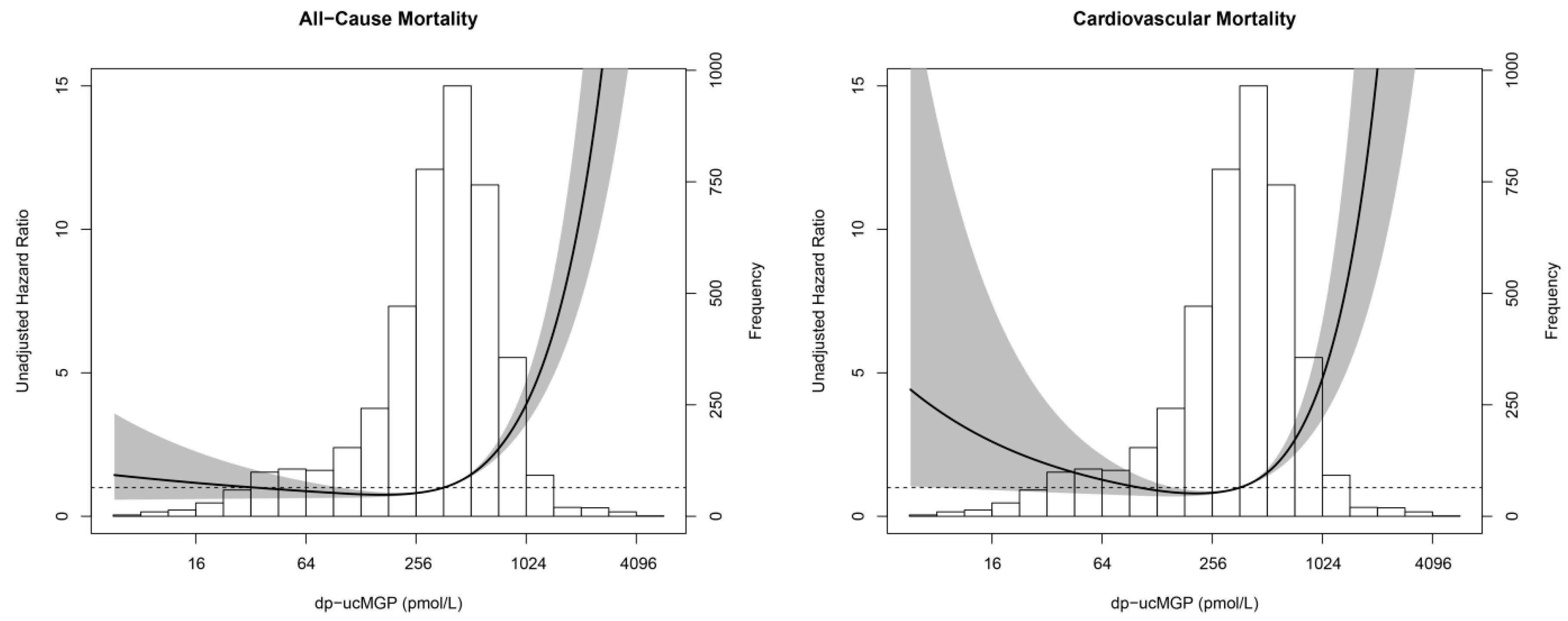
3.2. Dp-ucMGP and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart Disease and Stroke Statistics—2015 Update: A Report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Tintut, Y. Vascular Calcification: Pathobiology of a Multifaceted Disease. Circulation 2008, 117, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous Calcification of Arteries and Cartilage in Mice Lacking Matrix GLA Protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-Dependent Carboxylation of Matrix Gla-Protein: A Crucial Switch to Control Ectopic Mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Van Ballegooijen, A.J.; Beulens, J.W. The Role of Vitamin K Status in Cardiovascular Health: Evidence from Observational and Clinical Studies. Curr. Nutr. Rep. 2017, 6, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Keyzer, C.A.; Vermeer, C.; Joosten, M.M.; Knapen, M.H.; Drummen, N.E.; Navis, G.; Bakker, S.J.; de Borst, M.H. Vitamin K Status and Mortality After Kidney Transplantation: A Cohort Study. Am. J. Kidney Dis. 2015, 65, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Cranenburg, E.C.; Koos, R.; Schurgers, L.J.; Magdeleyns, E.J.; Schoonbrood, T.H.; Landewe, R.B.; Brandenburg, V.M.; Bekers, O.; Vermeer, C. Characterisation and Potential Diagnostic Value of Circulating Matrix Gla Protein (MGP) Species. Thromb. Haemost. 2010, 104, 811–822. [Google Scholar] [PubMed]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.J.; Vermeer, C.; Verschuren, W.M.; Boer, J.M.; Beulens, J.W. Matrix Gla Protein Species and Risk of Cardiovascular Events in Type 2 Diabetic Patients. Diabetes Care 2013, 36, 3766–3771. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Renard, C.; Magdeleyns, E.J.; Vermeer, C.; Choukroun, G.; Massy, Z.A. The Circulating Inactive Form of Matrix Gla Protein is a Surrogate Marker for Vascular Calcification in Chronic Kidney Disease: A Preliminary Report. Clin. J. Am. Soc. Nephrol. 2010, 5, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Schlieper, G.; Westenfeld, R.; Kruger, T.; Cranenburg, E.C.; Magdeleyns, E.J.; Brandenburg, V.M.; Djuric, Z.; Damjanovic, T.; Ketteler, M.; Vermeer, C.; et al. Circulating Nonphosphorylated Carboxylated Matrix Gla Protein Predicts Survival in ESRD. J. Am. Soc. Nephrol. 2011, 22, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Gullestad, L.; Dahl, C.P.; Aukrust, P.; Aakhus, S.; Solberg, O.G.; Vermeer, C.; Schurgers, L.J. Undercarboxylated Matrix Gla Protein is Associated with Indices of Heart Failure and Mortality in Symptomatic Aortic Stenosis. J. Intern. Med. 2010, 268, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O., Jr.; Seidlerova, J.; Bruthans, J.; Filipovsky, J.; Timoracka, K.; Vanek, J.; Cerna, L.; Wohlfahrt, P.; Cifkova, R.; Theuwissen, E.; et al. Desphospho-Uncarboxylated Matrix Gla-Protein is Associated with Mortality Risk in Patients with Chronic Stable Vascular Disease. Atherosclerosis 2014, 235, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Gu, Y.M.; Thijs, L.; Knapen, M.H.; Salvi, E.; Citterio, L.; Petit, T.; Carpini, S.D.; Zhang, Z.; Jacobs, L.; et al. Inactive Matrix Gla Protein is Causally Related to Adverse Health Outcomes: A Mendelian Randomization Study in a Flemish Population. Hypertension 2015, 65, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hillege, H.L.; Janssen, W.M.; Bak, A.A.; Diercks, G.F.; Grobbee, D.E.; Crijns, H.J.; Van Gilst, W.H.; De Zeeuw, D.; De Jong, P.E.; Prevend Study Group. Microalbuminuria is Common, also in a Nondiabetic, Nonhypertensive Population, and an Independent Indicator of Cardiovascular Risk Factors and Cardiovascular Morbidity. J. Intern. Med. 2001, 249, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Koning, S.H.; Gansevoort, R.T.; Mukamal, K.J.; Rimm, E.B.; Bakker, S.J.; Joosten, M.M.; Prevend Study Group. Alcohol Consumption is Inversely Associated with the Risk of Developing Chronic Kidney Disease. Kidney Int. 2015, 87, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Visser, S.T.; Schuiling-Veninga, C.C.; Bos, J.H.; de Jong-van den Berg, L.T.; Postma, M.J. The Population-Based Prescription Database IADB.Nl: Its Development, Usefulness in Outcomes Research and Challenges. Expert Rev. Pharmacoecon. Outcomes Res. 2013, 13, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- De Goeij, M.C.; van Diepen, M.; Jager, K.J.; Tripepi, G.; Zoccali, C.; Dekker, F.W. Multiple Imputation: Dealing with Missing Data. Nephrol. Dial. Transplant. 2013, 28, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple Imputation for Missing Data in Epidemiological and Clinical Research: Potential and Pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef] [PubMed]
- Harel, O.; Zhou, X.H. Multiple Imputation: Review of Theory, Implementation and Software. Stat. Med. 2007, 26, 3057–3077. [Google Scholar] [CrossRef] [PubMed]
- Liu, X. Classification Accuracy and Cut Point Selection. Stat. Med. 2012, 31, 2676–2686. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Booth, S.L.; Broe, K.E.; Gagnon, D.R.; Tucker, K.L.; Hannan, M.T.; McLean, R.R.; Dawson-Hughes, B.; Wilson, P.W.; Cupples, L.A.; Kiel, D.P. Vitamin K Intake and Bone Mineral Density in Women and Men. Am. J. Clin. Nutr. 2003, 77, 512–516. [Google Scholar] [PubMed]
- Cheung, C.L.; Sahni, S.; Cheung, B.M.; Sing, C.W.; Wong, I.C. Vitamin K Intake and Mortality in People with Chronic Kidney Disease from NHANES III. Clin. Nutr. 2015, 34, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; D’Alessandro, C.; Noale, M.; Tripepi, G.; Plebani, M.; Veronese, N.; Iervasi, G.; Giannini, S.; Rossini, M.; Tarroni, G.; et al. Low Vitamin K1 Intake in Haemodialysis Patients. Clin. Nutr. 2017, 36, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.; Booth, S.L.; van den Heuvel, E.G.; Stoecklin, E.; Baka, A.; Vermeer, C. The Role of Menaquinones (Vitamin K2) in Human Health. Br. J. Nutr. 2013, 110, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Gallieni, M.; Rizzo, M.A.; Stucchi, A.; Delanaye, P.; Cavalier, E.; Moyses, R.M.A.; Jorgetti, V.; Iervasi, G.; Giannini, S.; et al. Vitamin K Plasma Levels Determination in Human Health. Clin. Chem. Lab. Med. 2017, 55, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Ramotar, K.; Conly, J.M.; Chubb, H.; Louie, T.J. Production of Menaquinones by Intestinal Anaerobes. J. Infect. Dis. 1984, 150, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kaesler, N.; Magdeleyns, E.; Herfs, M.; Schettgen, T.; Brandenburg, V.; Fliser, D.; Vermeer, C.; Floege, J.; Schlieper, G.; Kruger, T. Impaired Vitamin K Recycling in Uremia is Rescued by Vitamin K Supplementation. Kidney Int. 2014, 86, 286–293. [Google Scholar] [CrossRef] [PubMed]
- McCabe, K.M.; Booth, S.L.; Fu, X.; Ward, E.; Adams, M.A.; Holden, R.M. Vitamin K Metabolism in a Rat Model of Chronic Kidney Disease. Am. J. Nephrol. 2017, 45, 4–13. [Google Scholar] [CrossRef] [PubMed]

| All Subjects (n = 4275) | Tertiles of dp-ucMGP | p-Value | |||
|---|---|---|---|---|---|
| Tertile 1 (n = 1425) | Tertile 2 (n = 1425) | Tertile 3 (n = 1425) | |||
| dp-ucMGP (pmol/L) | 372 (221–552) | <275 | 275–479 | ≥480 | - |
| Demographics | |||||
| Male gender (n, %) | 1966 (46.0) | 570 (40.0) | 669 (46.9) | 727 (51.0) | <0.001 |
| Age (years) | 53 ± 12 | 49 ± 11 | 52 ± 11 | 59 ± 12 | <0.001 |
| Race | 0.03 | ||||
| Caucasian (n, %) | 4041 (94.5) | 1333 (93.5) | 1343 (94.2) | 1365 (95.8) | |
| Black (n, %) | 42 (1.0) | 21 (1.5) | 13 (0.9) | 8 (0.6) | |
| Asian (n, %) | 100 (2.3) | 36 (2.5) | 36 (2.5) | 28 (2.0) | |
| Other (n, %) | 59 (1.4) | 27 (1.9) | 21 (1.5) | 11 (0.8) | |
| Education | <0.001 | ||||
| High (n, %) | 1431 (33.5) | 566 (39.7) | 504 (35.4) | 361 (25.3) | |
| Middle (n, %) | 1015 (23.7) | 366 (25.7) | 340 (23.9) | 309 (21.7) | |
| Low (n, %) | 1814 (42.4) | 489 (34.3) | 576 (40.4) | 749 (52.6) | |
| Smoking (n, %) | 1206 (28.2) | 472 (33.1) | 448 (31.4) | 286 (20.1) | <0.001 |
| Type 2 diabetes (n, %) | 84 (2.0) | 16 (1.1) | 19 (1.3) | 49 (3.4) | <0.001 |
| History of CVD (n, %) | 308 (7.2) | 47 (3.3) | 86 (6.0) | 175 (12.3) | <0.001 |
| Clinical measurements | |||||
| BMI (kg/m2) | 26.7 ± 4.3 | 25.5 ± 3.9 | 26.4 ± 4.0 | 28.1 ± 4.5 | <0.001 |
| SBP (mmHg) | 126 ± 19 | 121 ± 17 | 124 ± 18 | 133 ± 21 | <0.001 |
| DBP (mmHg) | 73 ± 9 | 71 ± 9 | 73 ± 9 | 75 ± 9 | <0.001 |
| Laboratory parameters | |||||
| Total cholesterol (mmol/L) | 5.4 ± 1.1 | 5.3 ± 1.0 | 5.5 ± 1.1 | 5.5 ± 1.1 | <0.001 |
| HDL cholesterol (mmol/L) | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.2 ± 0.3 | <0.001 |
| Total cholesterol-HDL ratio | 4.5 ± 1.3 | 4.3 ± 1.3 | 4.5 ± 1.3 | 4.7 ± 1.2 | <0.001 |
| Triglycerides (mmol/L) | 1.1 (0.8–1.6) | 1.0 (0.8–1.5) | 1.1 (0.8–1.5) | 1.2 (0.9–1.7) | <0.001 |
| hs-CRP (mg/L) | 1.4 (0.6–3.1) | 1.1 (0.5–2.9) | 1.2 (0.6–2.7) | 1.8 (0.9–3.6) | <0.001 |
| UAE (mg/day) | 8.1 (5.9–13.4) | 7.6 (5.7–11.4) | 7.8 (5.8–12.0) | 9.3 (6.3–17.9) | <0.001 |
| Serum creatinine (µmol/L) | 85 ± 22 | 81 ± 13 | 83 ± 14 | 90 ± 31 | <0.001 |
| eGFR (mL/min/1.73 m2) | 85 ± 16 | 90 ± 14 | 87 ± 14 | 76 ± 17 | <0.001 |
| Medication | |||||
| Vitamin K antagonists (n, %) | 106 (2.5) | 5 (0.4) | 6 (0.4) | 95 (6.7) | <0.001 |
| Antihypertensive drugs (n, %) | 990 (23.2) | 228 (16.0) | 252 (17.7) | 510 (35.8) | <0.001 |
| Lipid-lowering drugs (n, %) | 459 (10.7) | 98 (6.9) | 130 (9.1) | 231 (16.2) | <0.001 |
| All-Cause Mortality (nevents/ntotal = 279/4275) | Cardiovascular Mortality (nevents/ntotal = 74/4275) | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Model 1 | ||||
| Linear term | 0.20 (0.12–0.33) | <0.001 | 0.12 (0.05–0.27) | <0.001 |
| Squared term | 1.14 (1.10–1.17) | <0.001 | 1.17 (1.11–1.23) | <0.001 |
| Model 2 | ||||
| Linear term | 0.27 (0.15–0.47) | <0.001 | 0.14 (0.06–0.38) | <0.001 |
| Squared term | 1.10 (1.06–1.13) | <0.001 | 1.13 (1.07–1.20) | <0.001 |
| Model 3 | ||||
| Linear term | 0.36 (0.18–0.72) | 0.004 | 0.15 (0.04–0.48) | 0.002 |
| Squared term | 1.07 (1.03–1.12) | 0.002 | 1.13 (1.04–1.22) | 0.003 |
| Model 4 | ||||
| Linear term | 0.33 (0.17–0.66) | 0.002 | 0.17 (0.05–0.58) | 0.004 |
| Squared term | 1.08 (1.03–1.13) | 0.001 | 1.11 (1.03–1.20) | 0.009 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riphagen, I.J.; Keyzer, C.A.; Drummen, N.E.A.; De Borst, M.H.; Beulens, J.W.J.; Gansevoort, R.T.; Geleijnse, J.M.; Muskiet, F.A.J.; Navis, G.; Visser, S.T.; et al. Prevalence and Effects of Functional Vitamin K Insufficiency: The PREVEND Study. Nutrients 2017, 9, 1334. https://doi.org/10.3390/nu9121334
Riphagen IJ, Keyzer CA, Drummen NEA, De Borst MH, Beulens JWJ, Gansevoort RT, Geleijnse JM, Muskiet FAJ, Navis G, Visser ST, et al. Prevalence and Effects of Functional Vitamin K Insufficiency: The PREVEND Study. Nutrients. 2017; 9(12):1334. https://doi.org/10.3390/nu9121334
Chicago/Turabian StyleRiphagen, Ineke J., Charlotte A. Keyzer, Nadja E. A. Drummen, Martin H. De Borst, Joline W. J. Beulens, Ron T. Gansevoort, Johanna M. Geleijnse, Frits A. J. Muskiet, Gerjan Navis, Sipke T. Visser, and et al. 2017. "Prevalence and Effects of Functional Vitamin K Insufficiency: The PREVEND Study" Nutrients 9, no. 12: 1334. https://doi.org/10.3390/nu9121334





