A Novel Prebiotic Blend Product Prevents Irritable Bowel Syndrome in Mice by Improving Gut Microbiota and Modulating Immune Response
Abstract
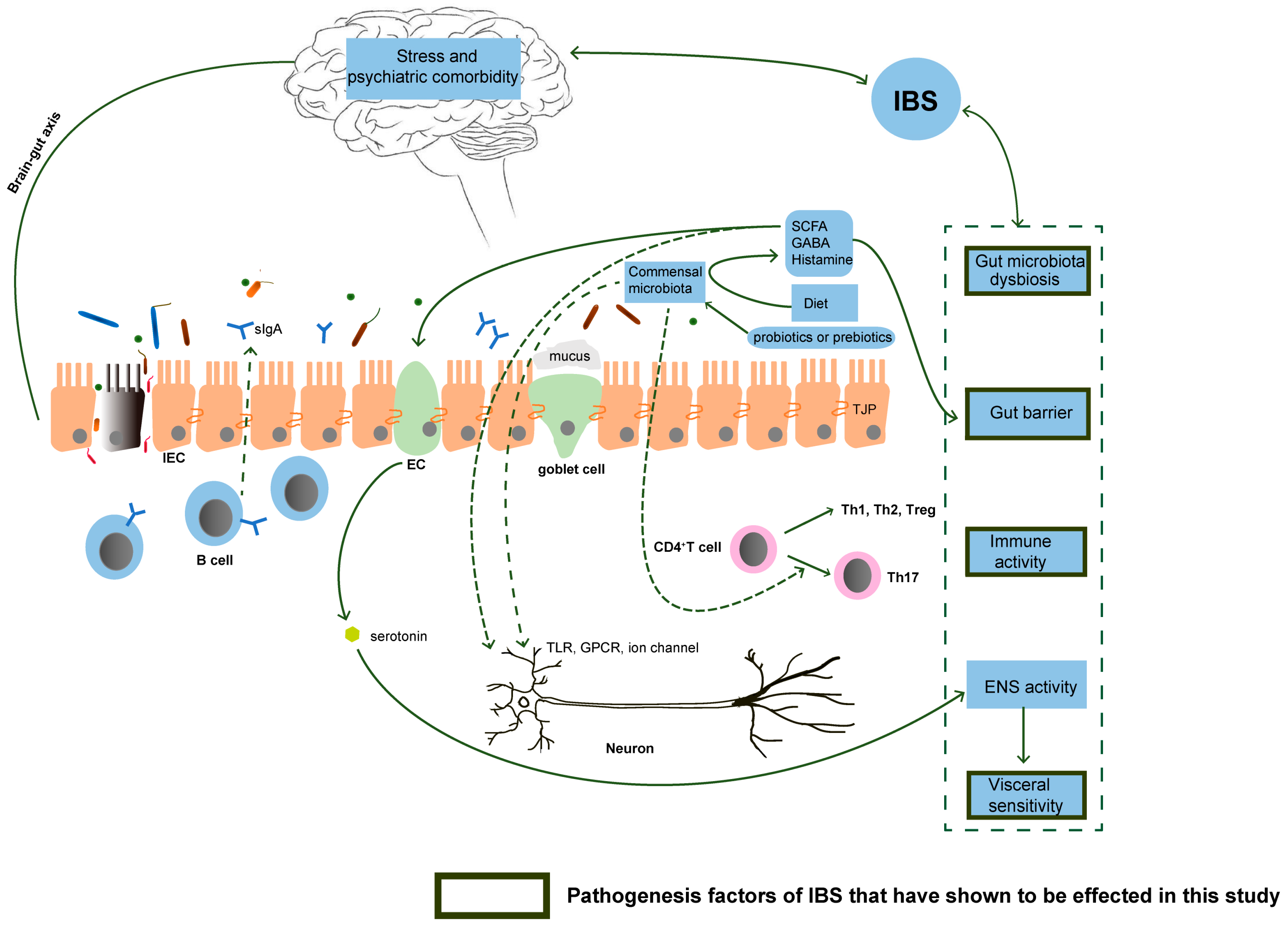
:1. Introduction
2. Materials and Methods
2.1. Bacteria Cultures
2.2. Prebiotic-Containing Product
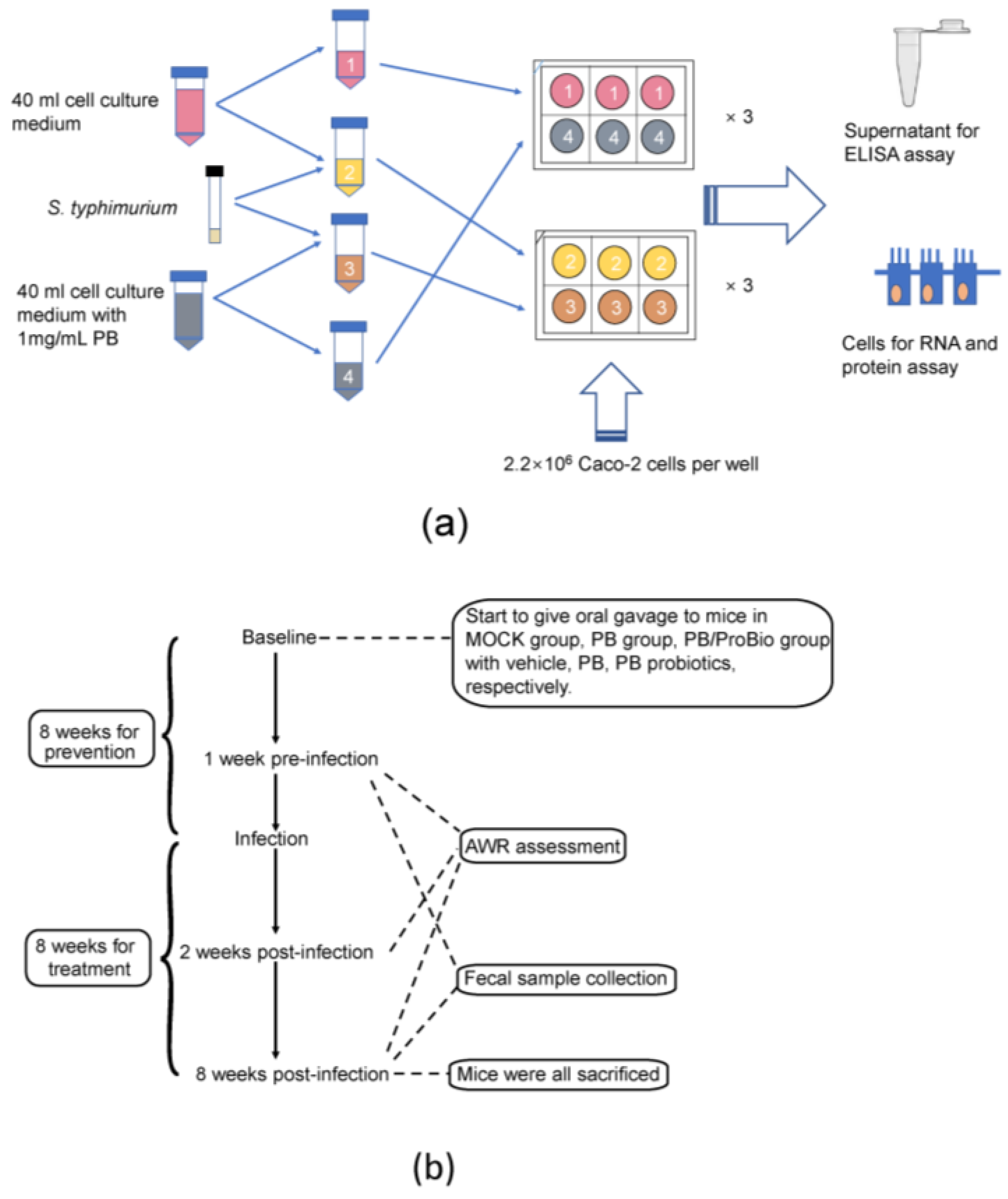
2.3. Caco-2 Cell Treatment
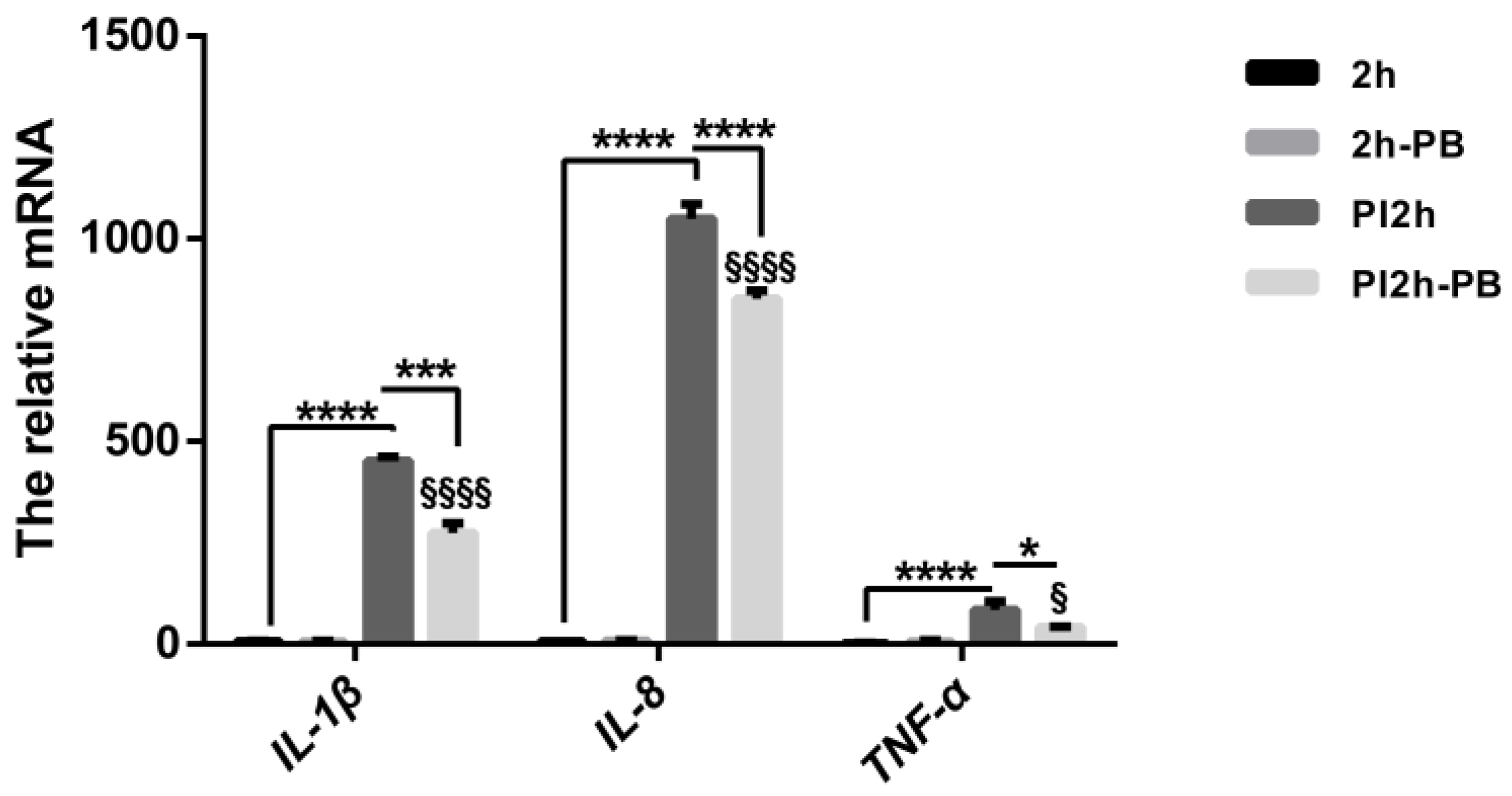
2.4. Animal Treatment and Experimental Diets
2.5. Trichinella Spiralis Infection
2.6. Abdominal Withdrawal Reflex (AWR) Scores Assessment
2.7. Sample Collection and Processing
2.8. Histological Analysis
2.9. Immunohistochemistry (IHC) Detection
2.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assay
2.11. Western Blot Analysis
2.12. Fecal Microbiota Analysis
2.13. Proteomics Analysis
2.14. Statistical Analysis
3. Results
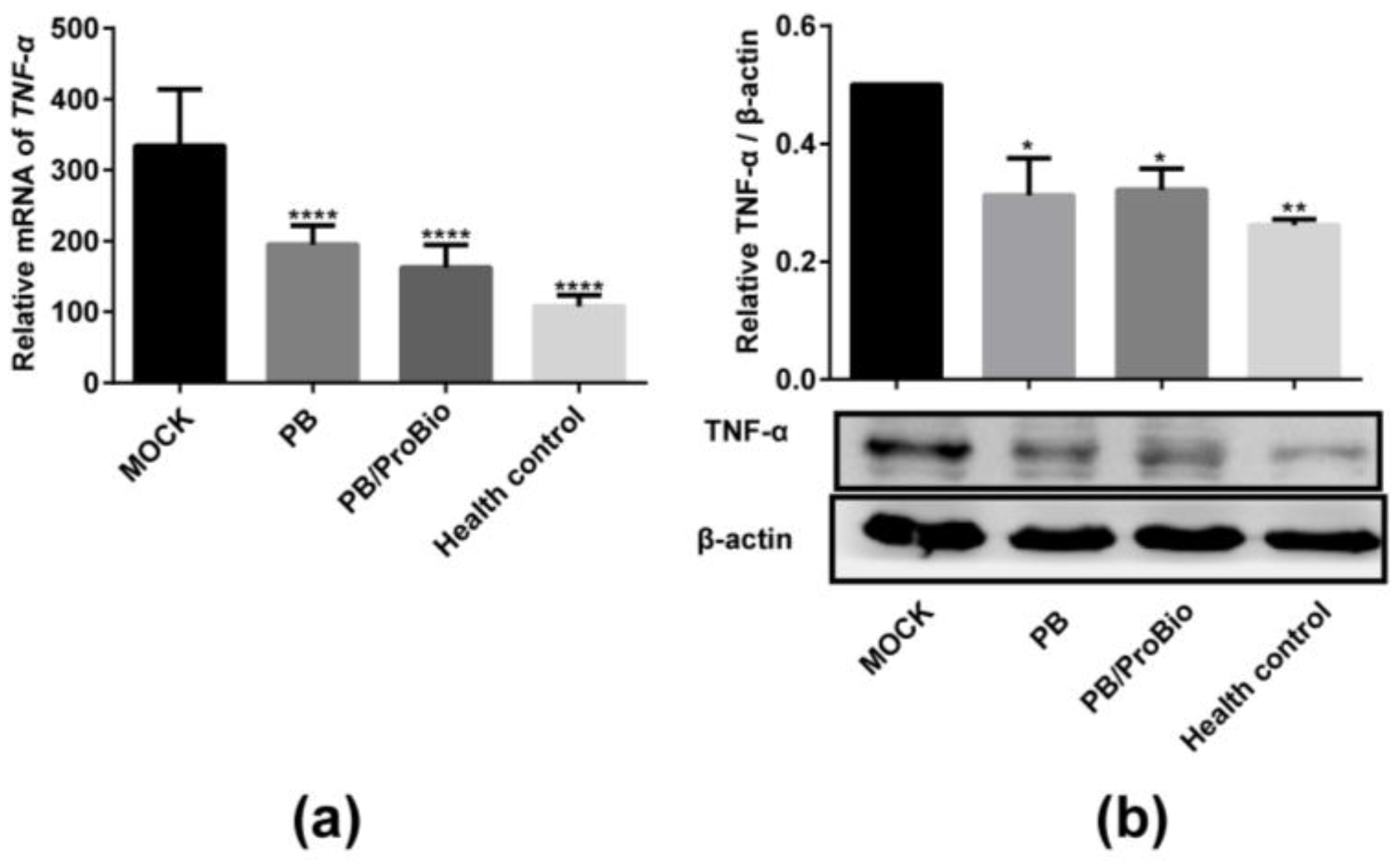
3.1. Pro-Inflammatory Cytokine Profiles in Caco-2 Cells and Animal Models
3.2. Symptoms in PI-IBS Model
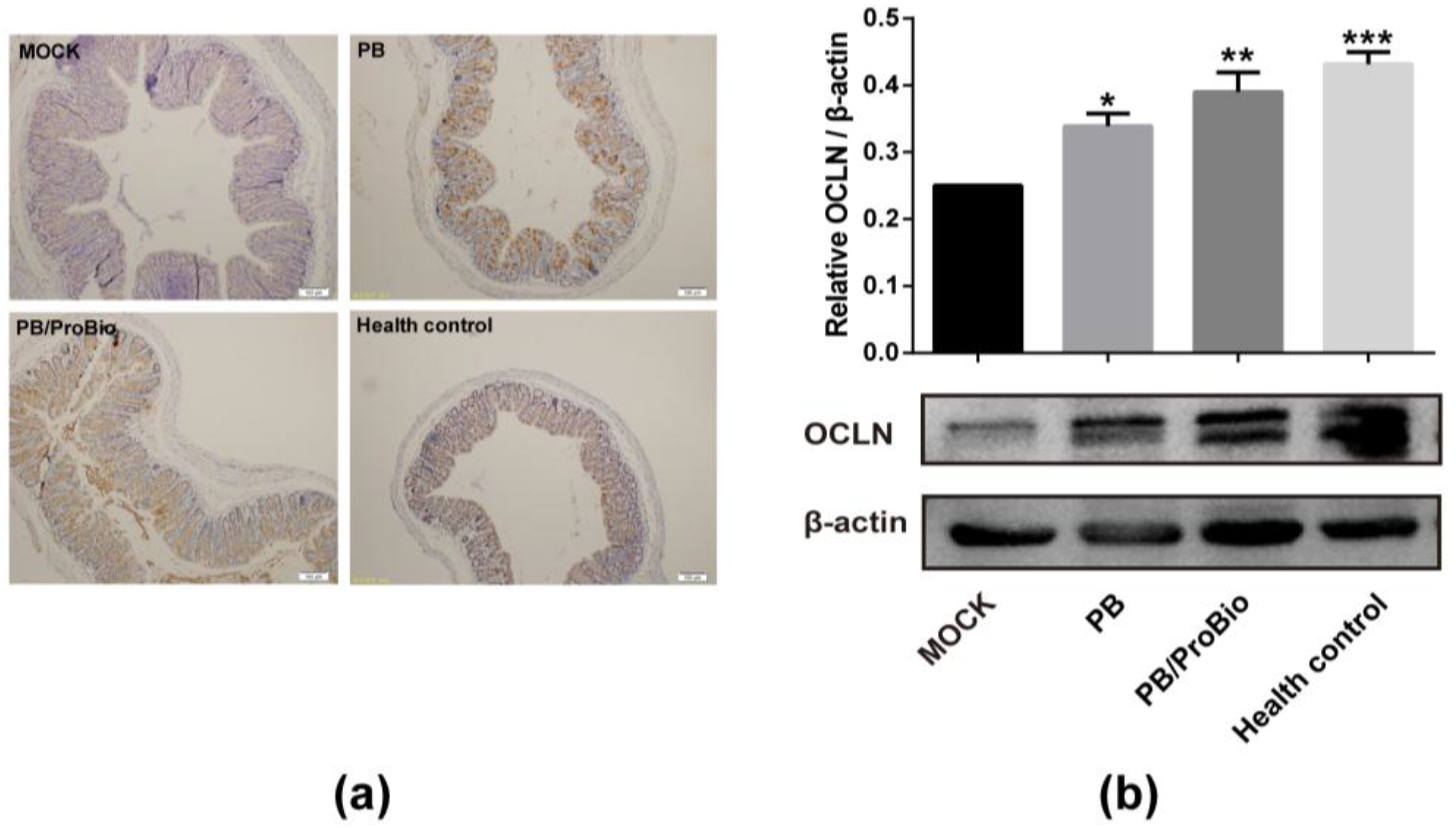
3.3. Tight Junction Protein in PI-IBS Mice
3.4. PPARγ Expression
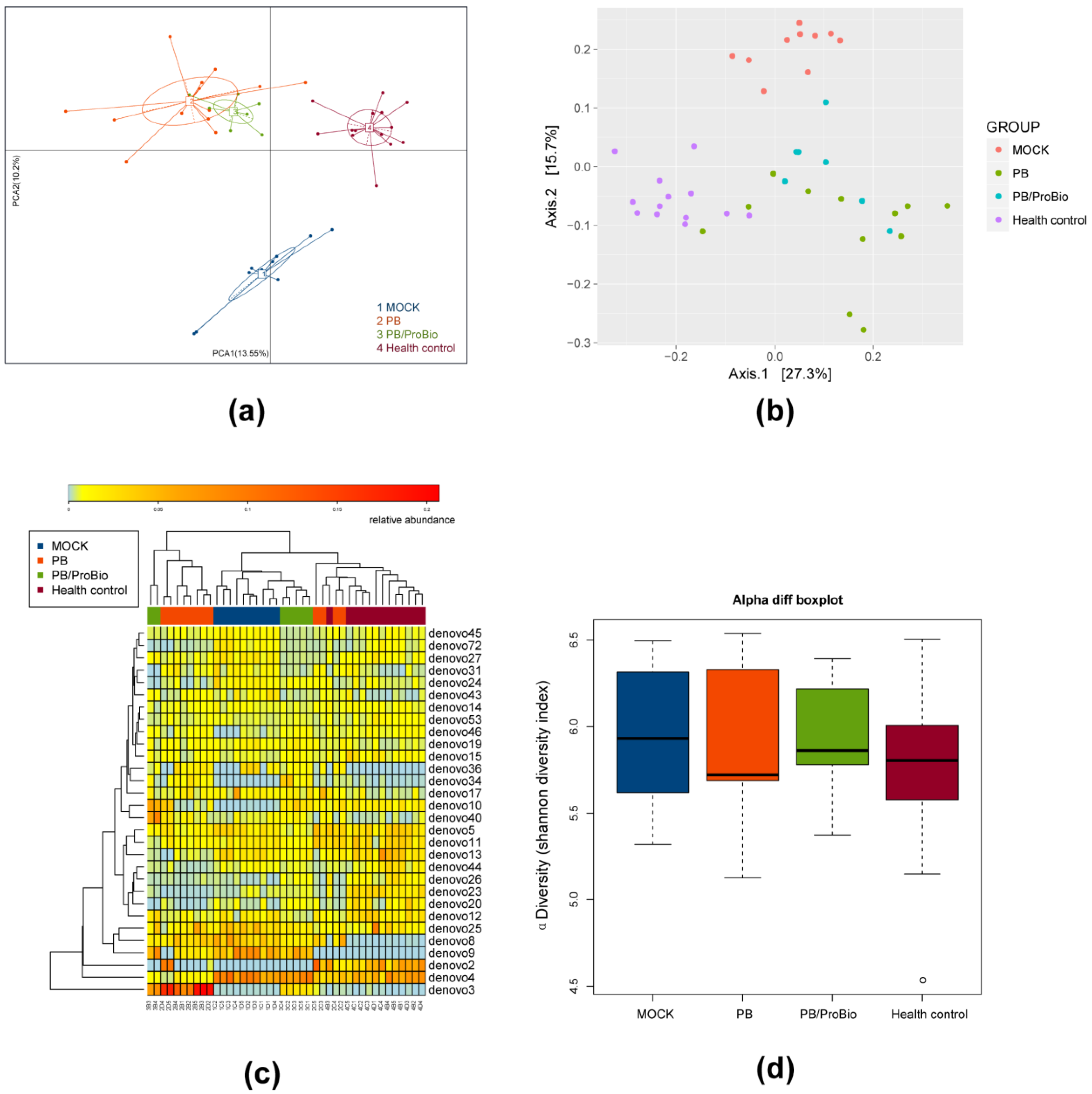
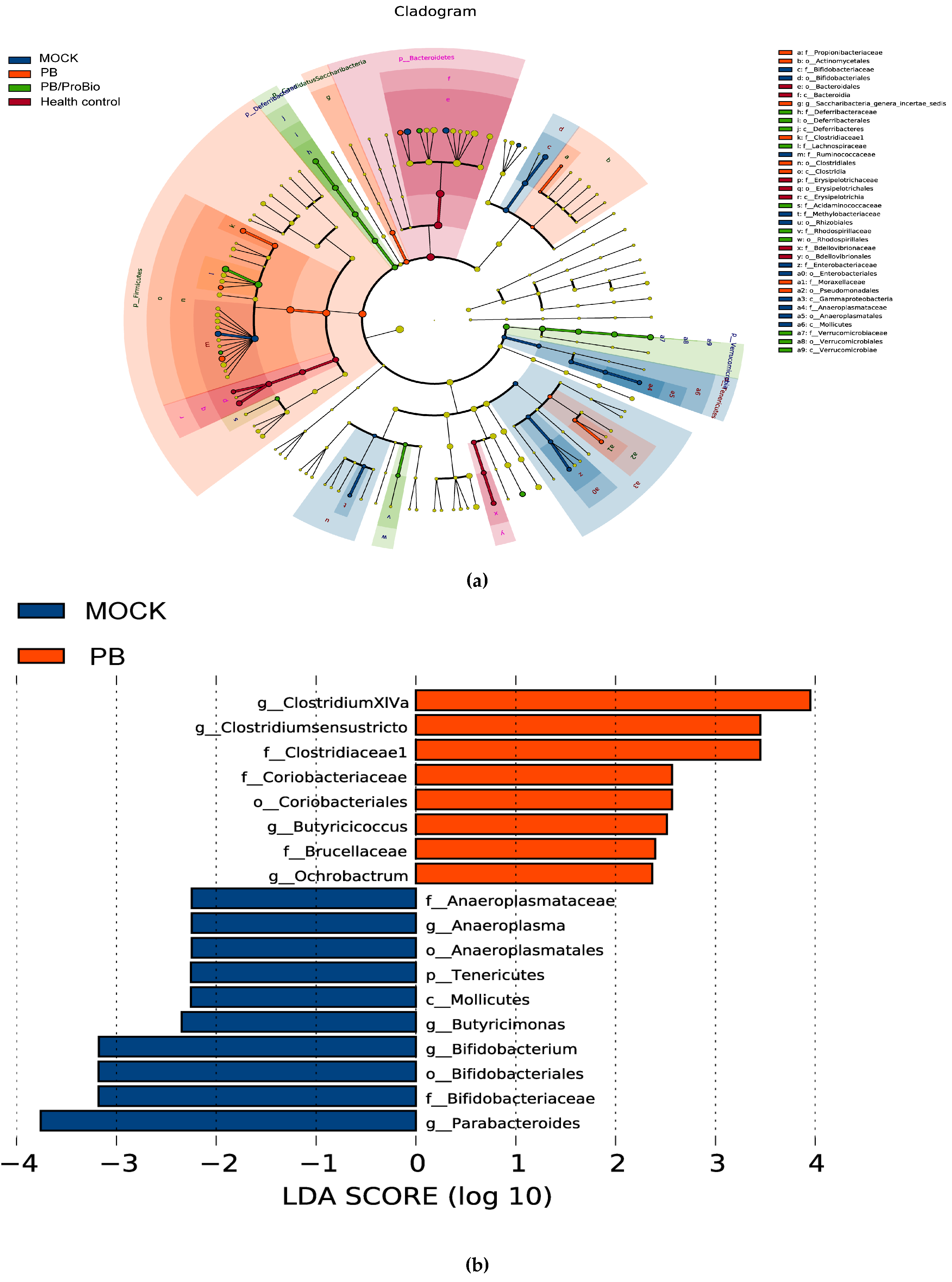
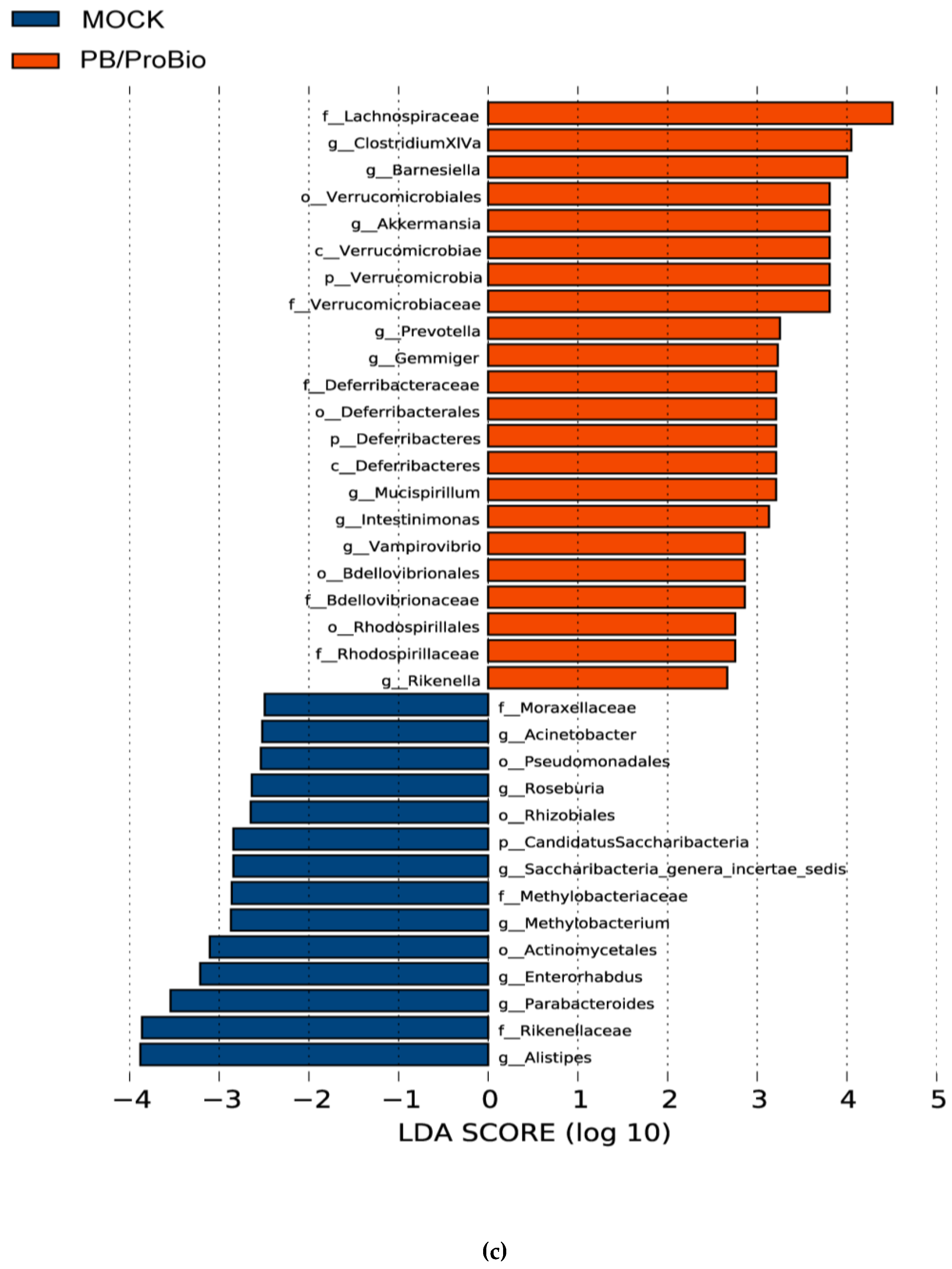
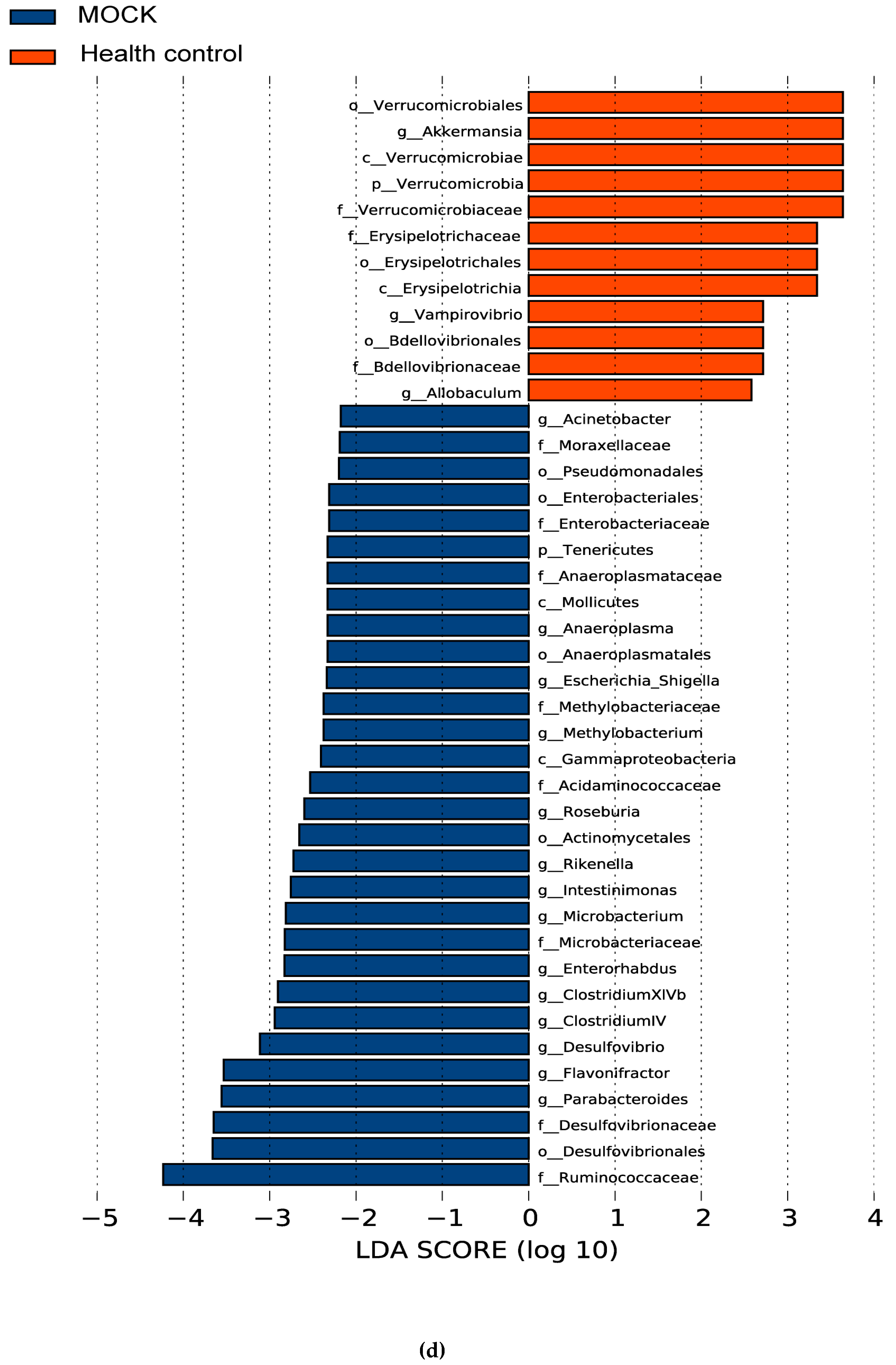
3.5. The Alteration of Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cenac, N.; Andrews, C.N.; Holzhausen, M.; Chapman, K.; Cottrell, G.; Andrade-Gordon, P.; Steinhoff, M.; Barbara, G.; Beck, P.; Bunnett, N.W.; et al. Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Investig. 2007, 117, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Drossman, D.A. Functional gastrointestinal disorders: History, pathophysiology, clinical features and Rome IV. Gastroenterology 2016. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Canavan, C.; West, J.; Card, T. The epidemiology of irritable bowel syndrome. Clin. Epidemiol. 2014, 6, 71–80. [Google Scholar] [PubMed]
- Thabane, M.; Marshall, J.K. Post-infectious irritable bowel syndrome. World J. Gastroenterol. 2009, 15, 3591–3596. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, H.A.; Schlett, C.D.; Riddle, M.S. Postinfectious irritable bowel syndrome—A meta-analysis. Am. J. Gastroenterol. 2006, 101, 1894–1899. [Google Scholar] [CrossRef] [PubMed]
- Schwille-Kiuntke, J.; Frick, J.S.; Zanger, P.; Enck, P. Post-infectious irritable bowel syndrome—A review of the literature. Z. Gastroenterol. 2011, 49, 997–1003. [Google Scholar] [CrossRef]
- Dunlop, S.P.; Hebden, J.; Campbell, E.; Naesdal, J.; Olbe, L.; Perkins, A.C.; Spiller, R.C. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am. J. Gastroenterol. 2006, 101, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable bowel syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef] [PubMed]
- Ohman, L.; Tornblom, H.; Simren, M. Crosstalk at the mucosal border: Importance of the gut microenvironment in ibs. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, K.; Choi, J.N.; Kim, J.; Lee, S.Y.; Lee, C.H. Microbial community and metabolomic comparison of irritable bowel syndrome faeces. J. Med. Microbiol. 2011, 60, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; de Vos, W.M.; Palva, A. Gastrointestinal microbiota in irritable bowel syndrome: Present state and perspectives. Microbiology 2010, 156, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; Quigley, E.M.; Ohman, L.; Simren, M.; O’Toole, P.W. The microbiota link to irritable bowel syndrome: An emerging story. Gut Microbes 2012, 3, 572–576. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.M. A role for the gut microbiota in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Neal, K.; Barker, L.; Spiller, R. Prognosis in post-infective irritable bowel syndrome: A six year follow up study. Gut 2002, 51, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Distrutti, E.; Monaldi, L.; Ricci, P.; Fiorucci, S. Gut microbiota role in irritable bowel syndrome: New therapeutic strategies. World J. Gastroenterol. 2016, 22, 2219–2241. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Ford, A.C.; Talley, N.J.; Cremonini, F.; Foxx-Orenstein, A.E.; Brandt, L.J.; Quigley, E.M. The efficacy of probiotics in the treatment of irritable bowel syndrome: A systematic review. Gut 2010, 59, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Cryan, J.F.; Dinan, T.G.; Quigley, E.M. Review article: Probiotics for the treatment of irritable bowel syndrome—Focus on lactic acid bacteria. Aliment. Pharmacol. Ther. 2012, 35, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.M.; Moeller, M.J.; Chey, W.D.; Schoenfeld, P.S. The utility of probiotics in the treatment of irritable bowel syndrome: A systematic review. Am. J. Gastroenterol. 2009, 104, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Ford, A.C. The effect of fiber supplementation on irritable bowel syndrome: A systematic review and meta-analysis. Am. J. Gastroenterol. 2014, 109, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Snook, J.; Shepherd, H.A. Bran supplementation in the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 1994, 8, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Kelly, E.C.; Lembo, A.J. Current gut-directed therapies for irritable bowel syndrome. Curr. Treat. Options Gastroenterol. 2006, 9, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.J.; Muir, J.G.; Barrett, J.S.; Gibson, P.R. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols: Role in irritable bowel syndrome. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 819–834. [Google Scholar] [CrossRef]
- Rogha, M.; Esfahani, M.Z.; Zargarzadeh, A.H. The efficacy of a synbiotic containing bacillus coagulans in treatment of irritable bowel syndrome: A randomized placebo-controlled trial. Gastroenterol. Hepatol. Bed Bench 2014, 7, 156–163. [Google Scholar] [PubMed]
- Quigley, E.M. Therapies aimed at the gut microbiota and inflammation: Antibiotics, prebiotics, probiotics, synbiotics, anti-inflammatory therapies. Gastroenterol. Clin. N. Am. 2011, 40, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, O.F.; Akbar, A. Microbiome, antibiotics and irritable bowel syndrome. Br. Med. Bull. 2016, 120, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Chang, C.; Chua, K.S.; Mirocha, J.; DiBaise, J.; Rao, S.; Amichai, M. Antibiotic treatment of constipation-predominant irritable bowel syndrome. Dig. Dis. Sci. 2014, 59, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhou, X.; Lan, C. Changes of cytokine levels in a mouse model of post-infectious irritable bowel syndrome. BMC Gastroenterol. 2015, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, J.; Wang, W.; Long, Y.; Fu, X.; Fu, Y.; Qian, W.; Hou, X. Are there any different effects of bifidobacterium, lactobacillus and streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS ONE 2014, 9, e90153. [Google Scholar] [CrossRef] [PubMed]
- Zanini, B.; Ricci, C.; Bandera, F.; Caselani, F.; Magni, A.; Laronga, A.M.; Lanzini, A. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am. J. Gastroenterol. 2012, 107, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.K.; Thabane, M.; Garg, A.X.; Clark, W.F.; Salvadori, M.; Collins, S.M. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology 2006, 131, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.A.; Ruigomez, A. Increased risk of irritable bowel syndrome after bacterial gastroenteritis: Cohort study. BMJ Clin. Res. Ed. 1999, 318, 565–566. [Google Scholar] [CrossRef]
- Spiller, R.; Garsed, K. Postinfectious irritable bowel syndrome. Gastroenterology 2009, 136, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Compare, D.; Rocco, A.; Coccoli, P.; Angrisani, D.; Sgamato, C.; Iovine, B.; Salvatore, U.; Nardone, G. Lactobacillus casei dg and its postbiotic reduce the inflammatory mucosal response: An ex-vivo organ culture model of post-infectious irritable bowel syndrome. BMC Gastroenterol. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Gibson, P.R. Fructose malabsorption and symptoms of irritable bowel syndrome: Guidelines for effective dietary management. J. Am. Diet. Assoc. 2006, 106, 1631–1639. [Google Scholar] [PubMed]
- Shepherd, S.J.; Parker, F.C.; Muir, J.G.; Gibson, P.R. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: Randomized placebo-controlled evidence. Clin. Gastroenterol. Hepatol. 2008, 6, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients 2017, 9, E555. [Google Scholar] [CrossRef] [PubMed]
- Hod, K.; Sperber, A.D.; Ron, Y.; Boaz, M.; Dickman, R.; Berliner, S.; Halpern, Z.; Maharshak, N.; Dekel, R. A double-blind, placebo-controlled study to assess the effect of a probiotic mixture on symptoms and inflammatory markers in women with diarrhea-predominant ibs. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.A.; de Rosso, V.V.; Estadella, D.; Pisani, L.P. Anthocyanins as inflammatory modulators and the role of the gut microbiota. J. Nutr. Biochem. 2016, 33, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Klimis-Zacas, D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-kappab and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, N.; Yamazaki, S. Probiotics and safety. Am. J. Clin. Nutr. 2001, 73, 465S–470S. [Google Scholar] [PubMed]
- Zhou, Q.; Zhang, B.; Verne, G.N. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009, 146, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Van Der Veek, P.P.; Van Den Berg, M.; de Kroon, Y.E.; Verspaget, H.W.; Masclee, A.A. Role of tumor necrosis factor-α and interleukin-10 gene polymorphisms in irritable bowel syndrome. Am. J. Gastroenterol. 2005, 100, 2510–2516. [Google Scholar] [CrossRef] [PubMed]
- Kindt, S.; Van Oudenhove, L.; Broekaert, D.; Kasran, A.; Ceuppens, J.; Bossuyt, X.; Fischler, B.; Tack, J. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol. Motil. 2009, 21, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Elsenbruch, S.; Holtmann, G.; Oezcan, D.; Lysson, A.; Janssen, O.; Goebel, M.U.; Schedlowski, M. Are there alterations of neuroendocrine and cellular immune responses to nutrients in women with irritable bowel syndrome? Am. J. Gastroenterol. 2004, 99, 703–710. [Google Scholar] [CrossRef]
- Liebregts, T.; Adam, B.; Bredack, C.; Roth, A.; Heinzel, S.; Lester, S.; Downie-Doyle, S.; Smith, E.; Drew, P.; Talley, N.J.; et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007, 132, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gonzalez, M.; Ocon, B.; Romero-Calvo, I.; Anzola, A.; Guadix, E.; Zarzuelo, A.; Suarez, M.D.; Sanchez de Medina, F.; Martinez-Augustin, O. Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFκB. Mol. Nutr. Food Res. 2014, 58, 384–393. [Google Scholar] [PubMed]
- Wu, R.Y.; Maattanen, P.; Napper, S.; Scruten, E.; Li, B.; Koike, Y.; Johnson-Henry, K.C.; Pierro, A.; Rossi, L.; Botts, S.R.; et al. Non-digestible oligosaccharides directly regulate host kinome to modulate host inflammatory responses without alterations in the gut microbiota. Microbiome 2017, 5, 135. [Google Scholar] [CrossRef] [PubMed]
- Scaldaferri, F.; Pizzoferrato, M.; Gerardi, V.; Lopetuso, L.; Gasbarrini, A. The gut barrier: New acquisitions and therapeutic approaches. J. Clin. Gastroenterol. 2012, 46, S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Vivinus-Nébot, M.; Dainese, R.; Anty, R.; Saint-Paul, M.; Nano, J.; Gonthier, N.; Marjoux, S.; Frin-Mathy, G.; Bernard, G.; Hébuterne, X. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: Role of barrier defects and mast cells. Am. J. Gastroenterol. 2012, 107, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Correa-Matos, N.J.; Donovan, S.M.; Isaacson, R.E.; Gaskins, H.R.; White, B.A.; Tappenden, K.A. Fermentable fiber reduces recovery time and improves intestinal function in piglets following salmonella typhimurium infection. J. Nutr. 2003, 133, 1845–1852. [Google Scholar] [PubMed]
- Zhu, L.; Qin, S.; Zhai, S.; Gao, Y.; Li, L. Inulin with different degrees of polymerization modulates composition of intestinal microbiota in mice. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Sabater-Molina, M.; Larque, E.; Torrella, F.; Zamora, S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Lam, V.; Salzman, N.; Huang, Y.W.; Yu, J.; Zhang, J.; Wang, L.S. Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F-344 rats. Nutr. Cancer 2017, 69, 43–951. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, C.; Bucci, V.; Caballero, S.; Djukovic, A.; Toussaint, N.C.; Equinda, M.; Lipuma, L.; Ling, L.; Gobourne, A.; No, D.; et al. Intestinal microbiota containing Barnesiella species cures vancomycin-resistant Enterococcus faecium colonization. Infect. Immun. 2013, 81, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Presley, L.L.; Wei, B.; Braun, J.; Borneman, J. Bacteria associated with immunoregulatory cells in mice. Appl. Environ. Microbiol. 2010, 76, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Luckey, D.; Yeoman, C.J.; Marietta, E.V.; Berg Miller, M.E.; Murray, J.A.; White, B.A.; Taneja, V. Loss of sex and age driven differences in the gut microbiome characterize arthritis-susceptible 0401 mice but not arthritis-resistant 0402 mice. PLoS ONE 2012, 7, e36095. [Google Scholar]
- Ye, J.; Lee, J.W.; Presley, L.L.; Bent, E.; Wei, B.; Braun, J.; Schiller, N.L.; Straus, D.S.; Borneman, J. Bacteria and bacterial rRNA genes associated with the development of colitis in IL-10−/− mice. Inflamm. Bowel Dis. 2008, 14, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal clostridia: Leading players in the maintenance of gut homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Segain, J.; de La Blétiere, D.R.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottiere, H.; Galmiche, J. Butyrate inhibits inflammatory responses through NFκB inhibition: Implications for crohn‘s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Lührs, H.; Gerke, T.; Schauber, J.; Dusel, G.; Melcher, R.; Scheppach, W.; Menzel, T. Cytokine-activated degradation of inhibitory κB protein alpha is inhibited by the short-chain fatty acid butyrate. Int. J. Colorectal Dis. 2001, 16, 195–201. [Google Scholar] [PubMed]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Umesaki, Y.; Setoyama, H.; Matsumoto, S.; Imaoka, A.; Itoh, K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect. Immun. 1999, 67, 3504–3511. [Google Scholar] [PubMed]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y. Induction of colonic regulatory t cells by indigenous clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Belzer, C.; de Vos, W.M. Microbes inside—From diversity to function: The case of Akkermansia. ISME J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Png, C.W.; Linden, S.K.; Gilshenan, K.S.; Zoetendal, E.G.; McSweeney, C.S.; Sly, L.I.; McGuckin, M.A.; Florin, T.H. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am. J. Gastroenterol. 2010, 105, 2420–2428. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Dorffel, Y.; Loening-Baucke, V.; Theissig, F.; Ruckert, J.C.; Ismail, M.; Rau, W.A.; Gaschler, D.; Weizenegger, M.; Kuhn, S.; et al. Acute appendicitis is characterised by local invasion with fusobacterium nucleatum/necrophorum. Gut 2011, 60, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Si, J.M.; Yu, Y.C.; Fan, Y.J.; Chen, S.J. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J. Gastroenterol. 2004, 10, 1802–1805. [Google Scholar] [CrossRef] [PubMed]
- Kerckhoffs, A.P.; Samsom, M.; van der Rest, M.E.; de Vogel, J.; Knol, J.; Ben-Amor, K.; Akkermans, L.M. Lower bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J. Gastroenterol. 2009, 15, 2887–2892. [Google Scholar] [CrossRef] [PubMed]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. Ppargamma signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Wahli, W.; Michalik, L. Ppars at the crossroads of lipid signaling and inflammation. Trends Endocrinol. Metab. 2012, 23, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ting, A.T.; Seed, B. Ppar-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 1998, 391, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Xiao, J.; Jin, Z.; Zheng, P. Peroxisome proliferator-activated receptor-gamma is downregulated in ulcerative colitis and is involved in experimental colitis-associated neoplasia. Oncol. Lett. 2015, 10, 1259–1266. [Google Scholar] [PubMed]
- Zenhom, M.; Hyder, A.; de Vrese, M.; Heller, K.J.; Roeder, T.; Schrezenmeir, J. Prebiotic oligosaccharides reduce proinflammatory cytokines in intestinal Caco-2 cells via activation of PPARγ and peptidoglycan recognition protein 3. J. Nutr. 2011, 141, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. 2015, 60 (Suppl. 2), S129–S134. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R. Probiotics and prebiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008, 28, 385–396. [Google Scholar] [CrossRef]
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.P.; Cryan, J.F. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev. Biol. 2016, 417, 182–187. [Google Scholar] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]


| Genes | Sequences (5′-3′) | NCBI Gene ID | |
|---|---|---|---|
| β-actin (mouse) | Forward | AGTGTGACGTTGACATCCGT | 11461 |
| Reverse | TGCTAGGAGCCAGAGCAGTA | ||
| TNF-α (mouse) | Forward | GAGGCCAAGCCCTGGTATG | 21926 |
| Reverse | CGGGCCGATTGATCTCAGC | ||
| PPAR-γ (mouse) | Forward | GGAAGACCACTCGCATTCCTT | 19016 |
| Reverse | GTAATCAGCAACCATTGGGTCA | ||
| GAPDH (human) | Forward | ATGGGGAAGGTGAAGGTCG | 2597 |
| Reverse | GGGTCATTGATGGCAACAATATC | ||
| IL-1B (human) | Forward | GAAATGCCACCTTTTGACAGTG | 3553 |
| Reverse | TGGATGCTCATCAGGACAT | ||
| IL-8 (human) | Forward | GACCACACTGCGCCAACAC | 3576 |
| Reverse | CTTCTCCACAACCCTCTGCAC | ||
| TNF-α (human) | Forward | GAGGCCAAGCCCTGGTATG | 7124 |
| Reverse | CGGGCCGATTGATCTCAGC |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Ren, Y.; Lu, J.; Bartlett, M.; Chen, L.; Zhang, Y.; Guo, X.; Liu, C. A Novel Prebiotic Blend Product Prevents Irritable Bowel Syndrome in Mice by Improving Gut Microbiota and Modulating Immune Response. Nutrients 2017, 9, 1341. https://doi.org/10.3390/nu9121341
Chen Q, Ren Y, Lu J, Bartlett M, Chen L, Zhang Y, Guo X, Liu C. A Novel Prebiotic Blend Product Prevents Irritable Bowel Syndrome in Mice by Improving Gut Microbiota and Modulating Immune Response. Nutrients. 2017; 9(12):1341. https://doi.org/10.3390/nu9121341
Chicago/Turabian StyleChen, Qian, Yiping Ren, Jihong Lu, Mark Bartlett, Lei Chen, Yan Zhang, Xiaokui Guo, and Chang Liu. 2017. "A Novel Prebiotic Blend Product Prevents Irritable Bowel Syndrome in Mice by Improving Gut Microbiota and Modulating Immune Response" Nutrients 9, no. 12: 1341. https://doi.org/10.3390/nu9121341




