MicroRNA-132 Negatively Regulates Palmitate-Induced NLRP3 Inflammasome Activation through FOXO3 Down-Regulation in THP-1 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and Reagents
2.2. Cell Culture, Isolation of Peritoneal Macrophages, and PA Treatment
2.3. Quantification of miRNA and mRNA Expression
2.4. Transfection with miRNA Inhibitor and Mimic
2.5. FOXO3 and NF-κB Small Interfering RNA (siRNA) Transfection
2.6. Preparation of Whole, Nuclear, and Cytosolic Extracts and Supernatants, and Immunoblotting
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Statistical Analyses
3. Results
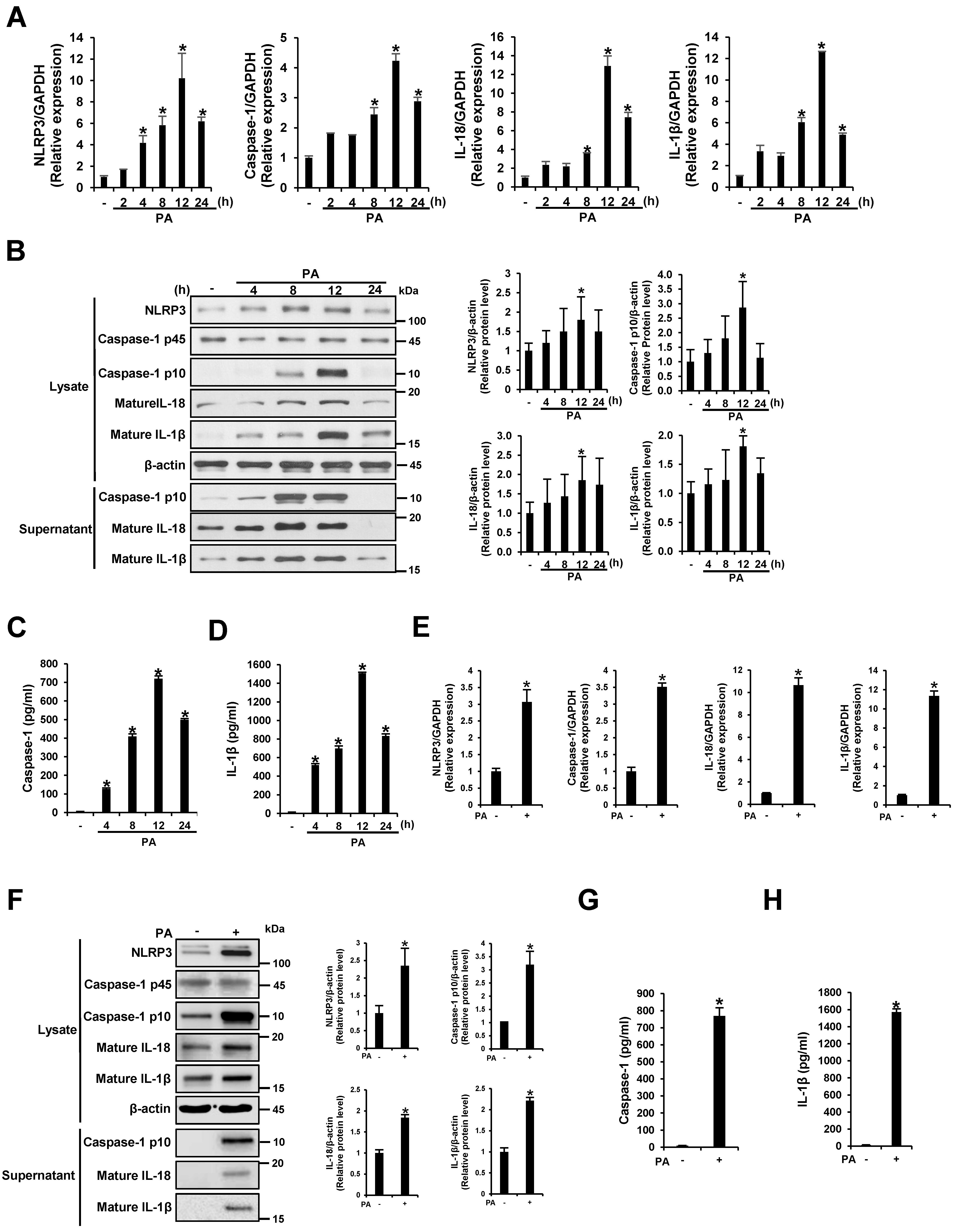
3.1. PA Induces NLRP3 Inflammasome Activation in THP-1 Cells and Primary Murine Macrophages
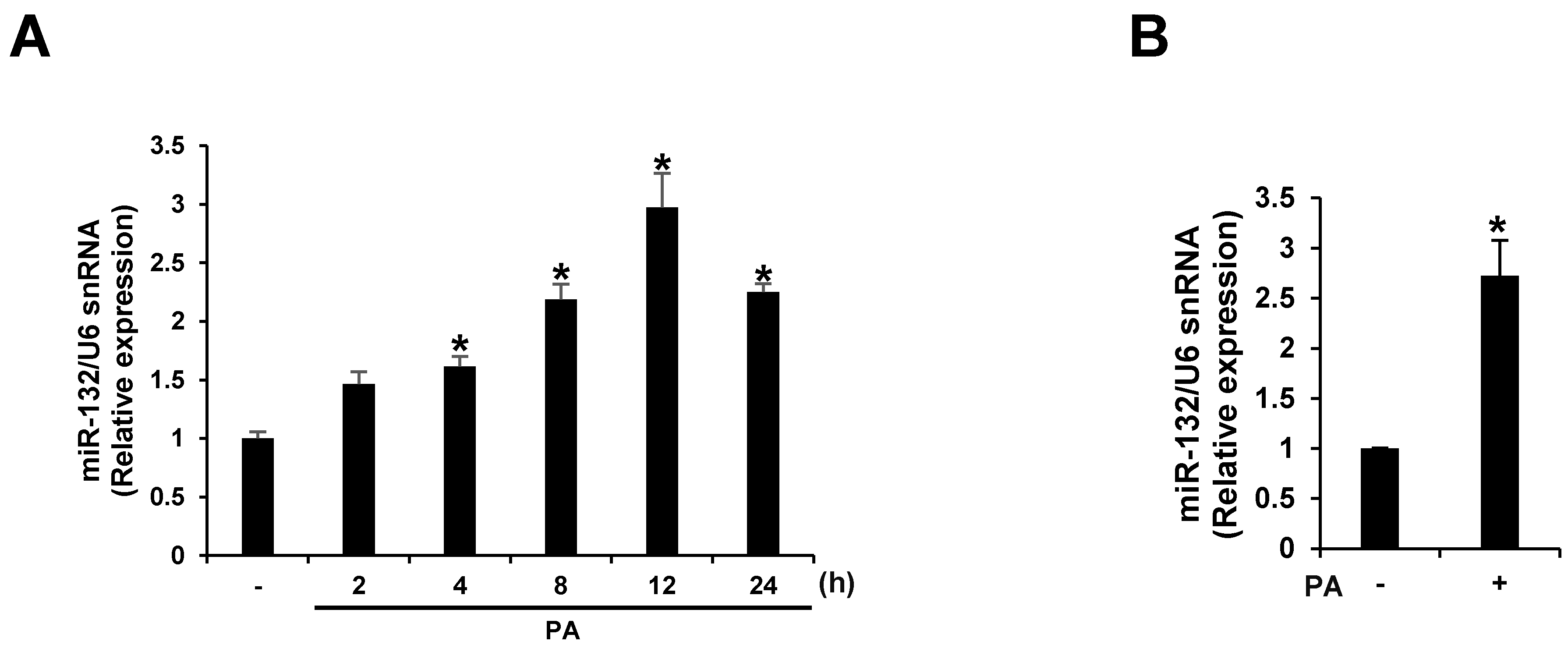
3.2. PA Up-Regulates miR-132 Expression in THP-1 Cells and Primary Murine Macrophages
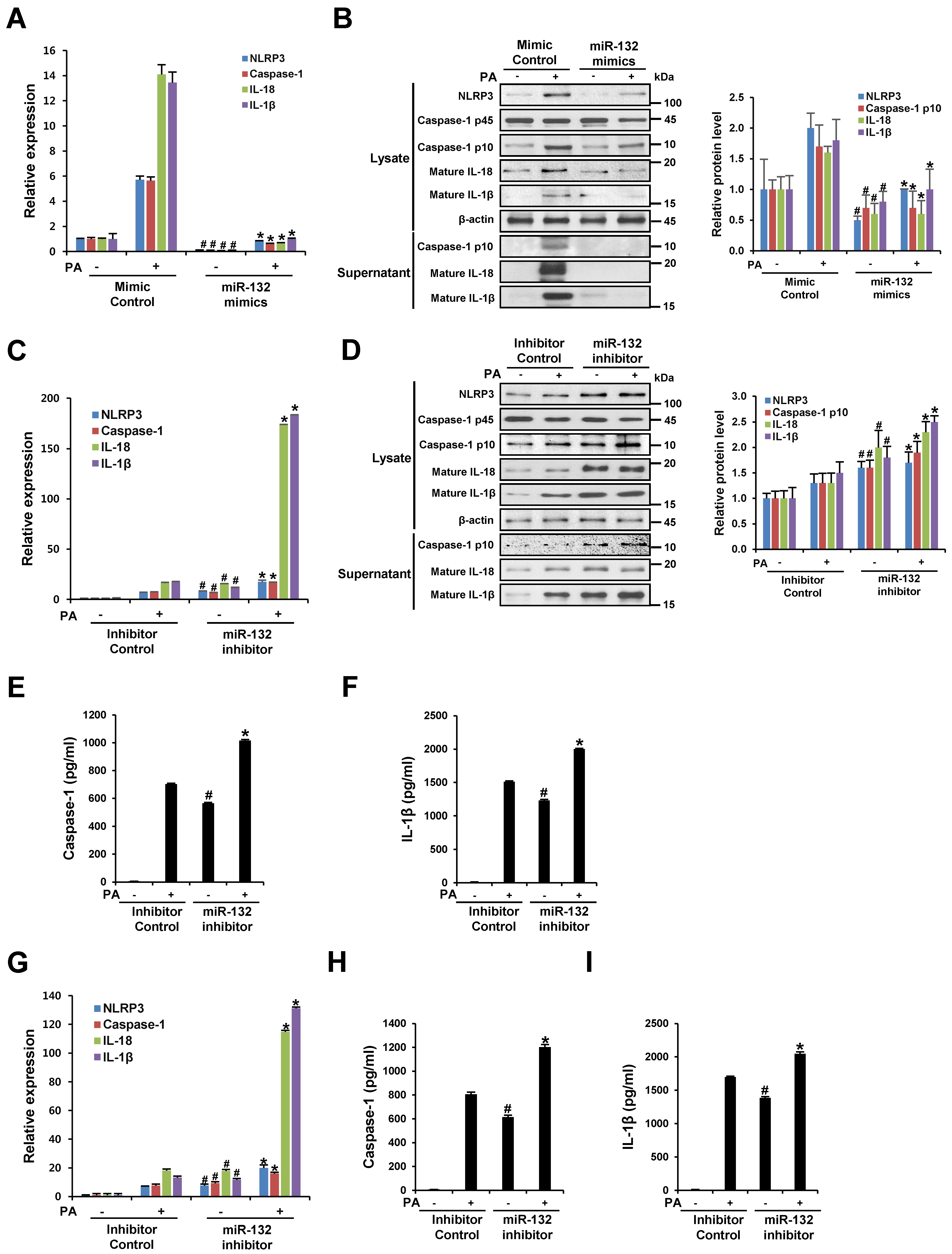
3.3. miR-132 Regulates PA Induced-NLRP3 Inflammasome Activation in THP-1 Cells and Primary Murine Macrophages
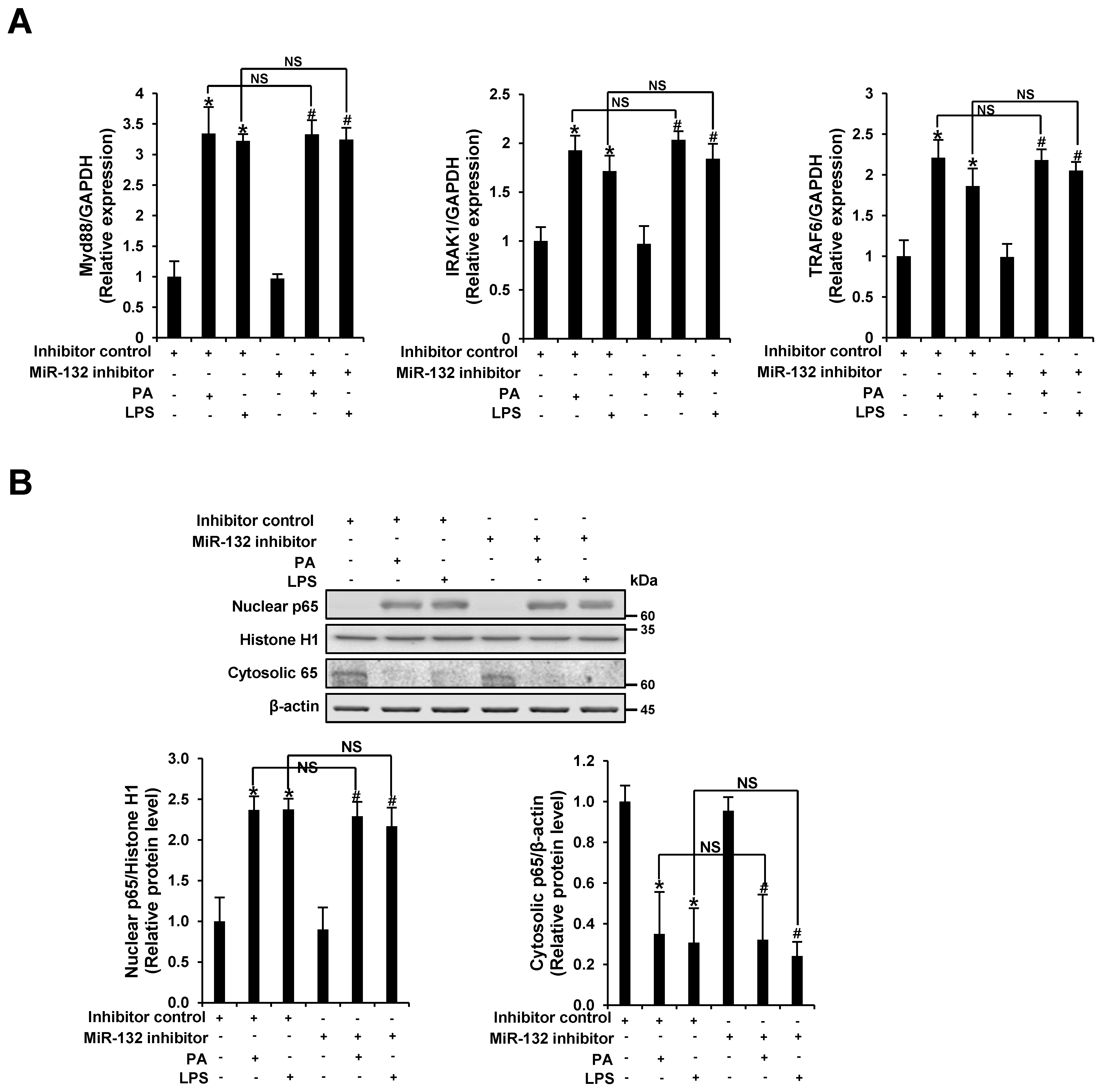
3.4. miR-132 Does Not Regulate PA-Induced TLR Signaling in THP-1 Cells
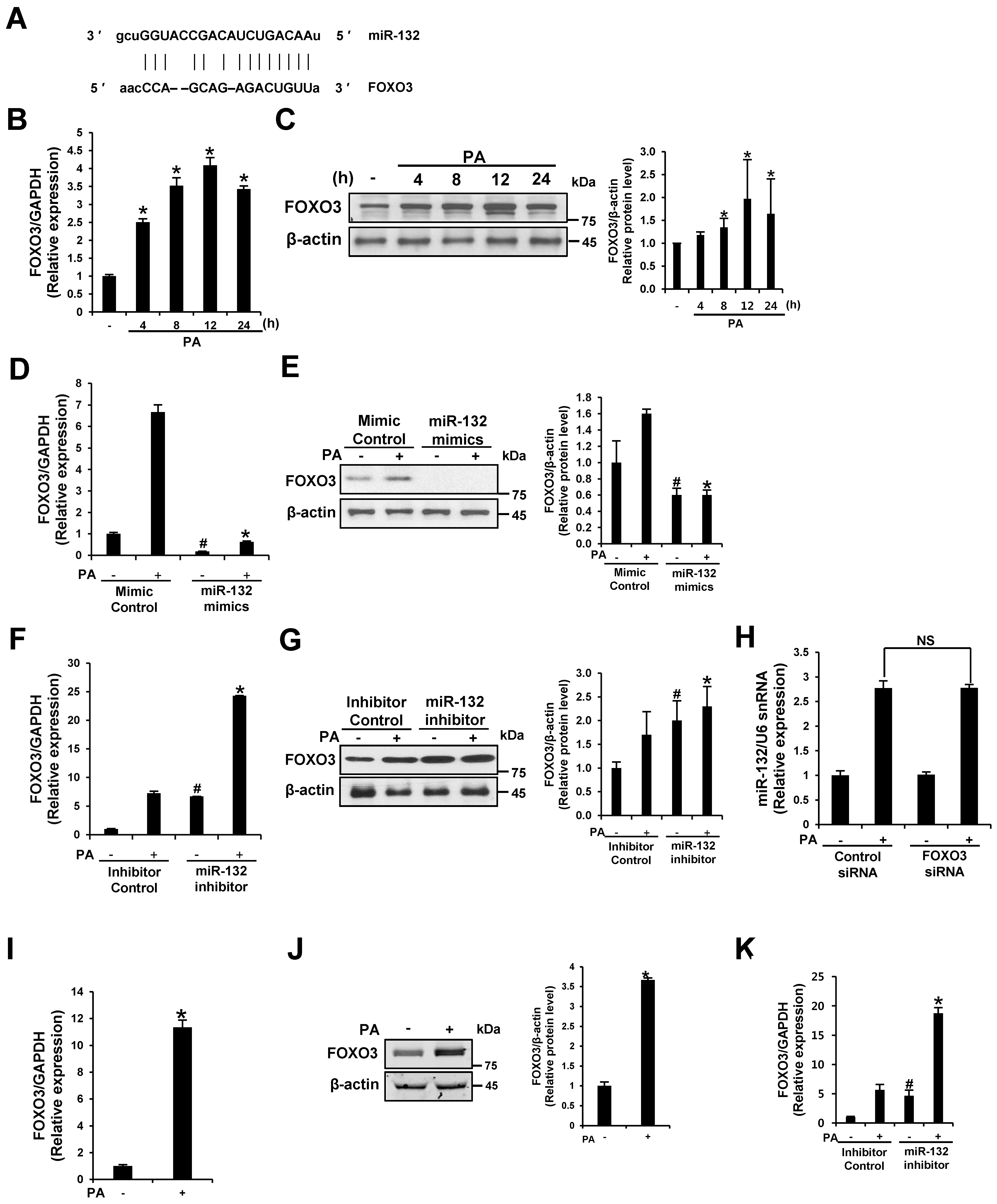
3.5. FOXO3 Is a Target of miR-132
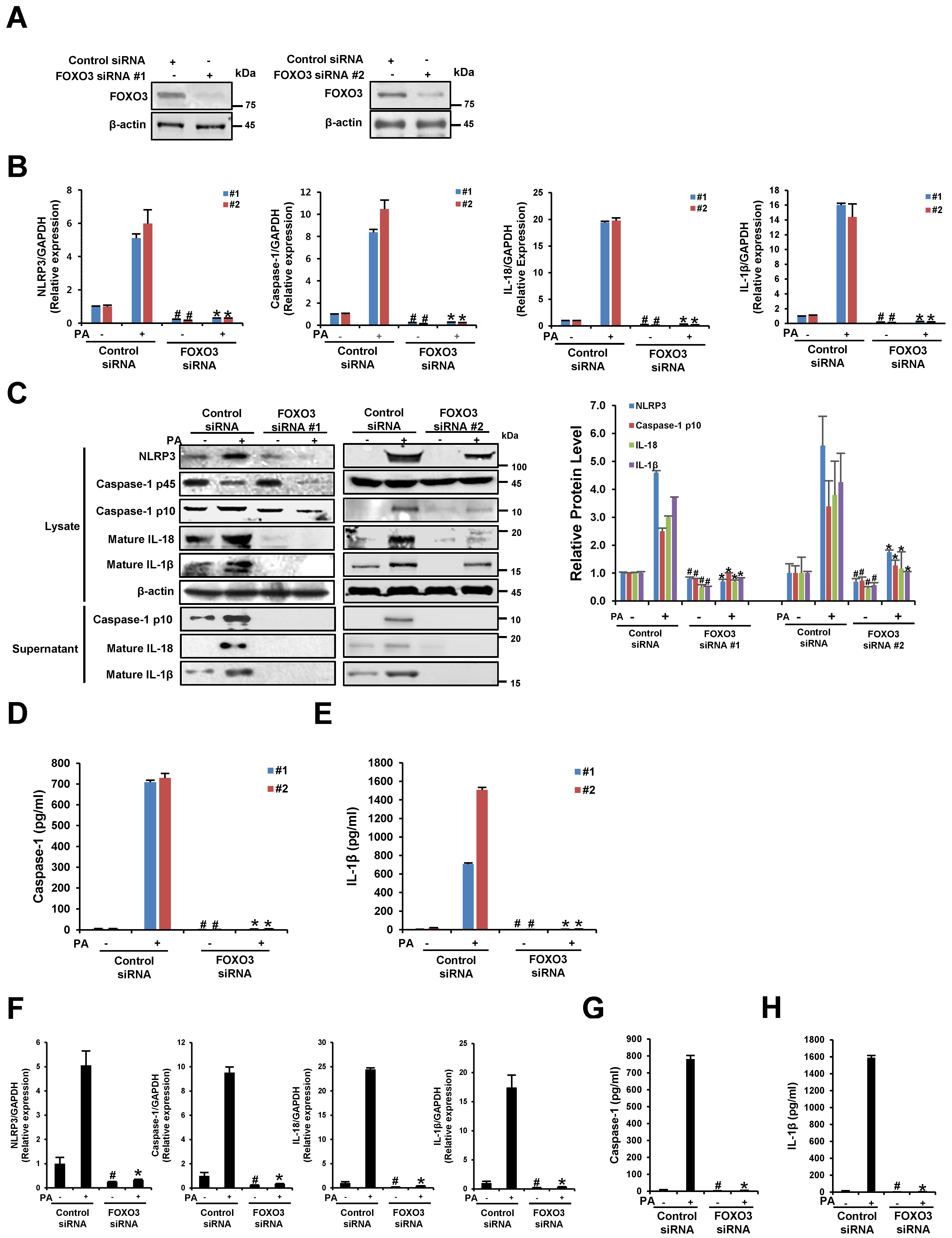
3.6. The Silencing of FOXO3 Suppresses PA-Induced NLRP3 Inflammasome Activation in THP-1 Cells and Primary Murine Macrophages
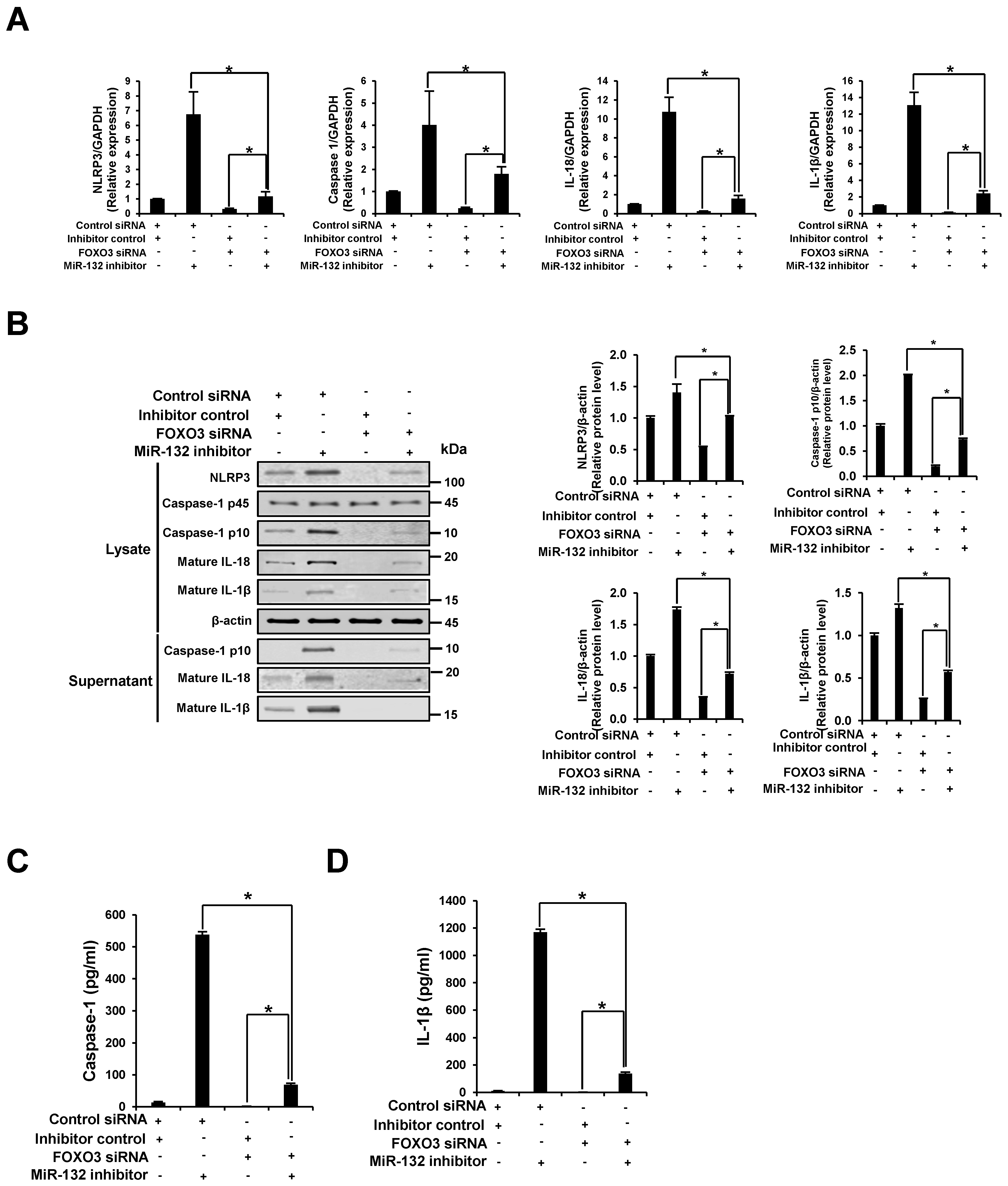
3.7. NLRP3 Inflammasome Activation through miR-132 Inhibitor Is Attenuated by the Knockdown of FOXO3
3.8. Schematic Diagram of the Proposed Model Showing How miR-132 Regulates PA-Induced NLRP3 Inflammasome Activation
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Romeo, G.R.; Lee, J.; Shoelson, S.E. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Kanneganti, T.D.; Ozoren, N.; Body-Malapel, M.; Amer, A.; Park, J.H.; Franchi, L.; Whitfield, J.; Barchet, W.; Colonna, M.; Vandenabeele, P.; et al. Bacterial rna and small antiviral compounds activate caspase-1 through cryopyrin/nalp3. Nature 2006, 440, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Ogura, Y.; Szczepanik, M.; Lara-Tejero, M.; Lichtenberger, G.S.; Grant, E.P.; Bertin, J.; Coyle, A.J.; Galan, J.E.; Askenase, P.W.; et al. Critical role for nalp3/cias1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 2006, 24, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Tack, C.J.; Kanneganti, T.D.; Joosten, L.A.; Netea, M.G. The inflammasome puts obesity in the danger zone. Cell Metab. 2012, 15, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Legrand-Poels, S.; Esser, N.; L’Homme, L.; Scheen, A.; Paquot, N.; Piette, J. Free fatty acids as modulators of the nlrp3 inflammasome in obesity/type 2 diabetes. Biochem. Pharmacol. 2014, 92, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. Tlr4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Favelyukis, S.; Nguyen, A.K.; Reichart, D.; Scott, P.A.; Jenn, A.; Liu-Bryan, R.; Glass, C.K.; Neels, J.G.; Olefsky, J.M. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptors 2 and 4 and jnk-dependent pathways. J. Biol. Chem. 2007, 282, 35279–35292. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The nlrp3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.; Brickey, W.J.; Ting, J.P. Fatty acid-induced nlrp3-asc inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Micrornas: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Baltimore, D.; Boldin, M.P.; O’Connell, R.M.; Rao, D.S.; Taganov, K.D. Micrornas: New regulators of immune cell development and function. Nat. Immunol. 2008, 9, 839–845. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Sheedy, F.J.; McCoy, C.E. Micrornas: The fine-tuners of toll-like receptor signalling. Nat. Rev. Immunol. 2011, 11, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. Nf-kappab-dependent induction of microrna mir-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Lagos, D.; Pollara, G.; Henderson, S.; Gratrix, F.; Fabani, M.; Milne, R.S.; Gotch, F.; Boshoff, C. Mir-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat. Cell Biol. 2010, 12, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.E.; Jung, I.R.; Lee, Y.J.; Lee, S.J.; Lee, J.H.; Kim, Y.; Jun, H.S.; Lee, K.W.; Park, C.B.; Kang, Y. Stimulation of lipogenesis as well as fatty acid oxidation protects against palmitate-induced ins-1 beta-cell death. Endocrinology 2011, 152, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Rhee, D.K.; Pyo, S. Involvement of protein kinase c and tyrosin kinase in tumoricidal activation of macrophage induced by streptococcus pneumoniae type ii capsular polysaccharide. Int. Immunopharmacol. 2002, 2, 129–137. [Google Scholar] [CrossRef]
- Wong, H.K.; Veremeyko, T.; Patel, N.; Lemere, C.A.; Walsh, D.M.; Esau, C.; Vanderburg, C.; Krichevsky, A.M. De-repression of foxo3a death axis by microrna-132 and -212 causes neuronal apoptosis in alzheimer’s disease. Hum. Mol. Genet. 2013, 22, 3077–3092. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Zhao, J.L.; Sinha, N.; Marinov, G.K.; Mann, M.; Kowalczyk, M.S.; Galimidi, R.P.; Du, X.; Erikci, E.; Regev, A.; et al. The microrna-132 and microrna-212 cluster regulates hematopoietic stem cell maintenance and survival with age by buffering foxo3 expression. Immunity 2015, 42, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; van Diepen, J.A.; Tack, C.J.; Zaki, M.H.; van de Veerdonk, F.L.; Perera, D.; Neale, G.A.; Hooiveld, G.J.; Hijmans, A.; Vroegrijk, I.; et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 15324–15329. [Google Scholar] [CrossRef] [PubMed]
- L’Homme, L.; Esser, N.; Riva, L.; Scheen, A.; Paquot, N.; Piette, J.; Legrand-Poels, S. Unsaturated fatty acids prevent activation of nlrp3 inflammasome in human monocytes/macrophages. J. Lipid Res. 2013, 54, 2998–3008. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.M.; Kim, J.J.; Kim, H.J.; Shong, M.; Ku, B.J.; Jo, E.K. Upregulated nlrp3 inflammasome activation in patients with type 2 diabetes. Diabetes 2013, 62, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.G.; Huang, S.; Choi, I.W.; Rutledge, J.C.; Hwang, D.H. Inflammasome-mediated secretion of il-1beta in human monocytes through tlr2 activation; modulation by dietary fatty acids. J. Immunol. 2013, 191, 4337–4347. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Y.; Shao, F. Non-canonical activation of inflammatory caspases by cytosolic lps in innate immunity. Curr. Opin. Immunol. 2015, 32, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Lee, S.; Park, M.A.; Siempos, II; Haslip, M.; Lee, P.J.; Yun, M.; Kim, C.K.; Howrylak, J.; Ryter, S.W.; et al. Ucp2-induced fatty acid synthase promotes nlrp3 inflammasome activation during sepsis. J. Clin. Investig. 2015, 125, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.; Klein, M.E.; Varlamova, O.; Keller, D.M.; Yamamoto, T.; Goodman, R.H.; Impey, S. A camp-response element binding protein-induced microrna regulates neuronal morphogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 16426–16431. [Google Scholar] [CrossRef] [PubMed]
- Wayman, G.A.; Davare, M.; Ando, H.; Fortin, D.; Varlamova, O.; Cheng, H.Y.; Marks, D.; Obrietan, K.; Soderling, T.R.; Goodman, R.H.; et al. An activity-regulated microrna controls dendritic plasticity by down-regulating p250gap. Proc. Natl. Acad. Sci. USA 2008, 105, 9093–9098. [Google Scholar] [CrossRef] [PubMed]
- Nahid, M.A.; Yao, B.; Dominguez-Gutierrez, P.R.; Kesavalu, L.; Satoh, M.; Chan, E.K. Regulation of tlr2-mediated tolerance and cross-tolerance through irak4 modulation by mir-132 and mir-212. J. Immunol. 2013, 190, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- Pauley, K.M.; Satoh, M.; Chan, A.L.; Bubb, M.R.; Reeves, W.H.; Chan, E.K. Upregulated mir-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res. Ther. 2008, 10, R101. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Yoshitomi, H.; Tanida, S.; Ishikawa, M.; Nishitani, K.; Ito, H.; Nakamura, T. Plasma and synovial fluid micrornas as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2010, 12, R86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calin, G.A.; Liu, C.G.; Sevignani, C.; Ferracin, M.; Felli, N.; Dumitru, C.D.; Shimizu, M.; Cimmino, A.; Zupo, S.; Dono, M.; et al. Microrna profiling reveals distinct signatures in b cell chronic lymphocytic leukemias. Proc. Natl. Acad. Sci. USA 2004, 101, 11755–11760. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Na, H.S.; Song, Y.R.; Shin, S.Y.; Kim, Y.M.; Chung, J. Activation of nlrp3 and aim2 inflammasomes by porphyromonas gingivalis infection. Infect. Immun. 2014, 82, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Park, E.; Kim, H.J.; Na, H.S.; Chung, J. Porphyromonas gingivalis-induced mir-132 regulates tnfalpha expression in thp-1 derived macrophages. SpringerPlus 2016, 5, 761. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence 5′ to 3′ | |
|---|---|---|
| Human | NLRP3 | F: ACAGCCACCTCACTTCCAG, R: CCAACCACAATCTCCGAATG |
| Caspase-1 | F: GCACAAGACCTCTGACAGCA, R: TTGGGCAGTTCTTGGTATTC | |
| IL-18 | F: GCCTAGAGGTATGGCTGTAA, R: GCGTCACTACACTCAGCTAA | |
| IL-1β | F: GCCCTAAACAGATGAAGTGCTC, R: GAACCAGCATCTTCCTCAG | |
| FOXO3 | F: CGGACAAACGGCTCACTCT, R: GGACCCGCATGAATCGACTAT | |
| MyD88 | F: GAGCGTTTCGATGCTTCAT, R: CGGATCATCTCCTGCACAAA | |
| IRAK1 | F: ACTGGCCCTTGGCAGCTC, R: GGCCAGCTTCTGGACCATC | |
| TRAF6 | F: CCTTTGGCAAATGTCATCTGTG, R: CTCTGCATCTTTTCATGGCAAC | |
| GAPDH | F: AAAATCAAGTGGGGCGATGC, R: AGGAGGCATTGCTGATGATCT | |
| Mouse | NLRP3 | F: CGAGACCTCTGGGAAAAAGCT, R: GCATACCATAGAGGAATGTGATGTACA |
| Caspase-1 | F: GATGGCACATTTCCAGGACTGA, R: TGTTGCAGATAATGAGGGCAAGAC | |
| IL-18 | F: GTGAACCCCAGACCAGACTG, R: CCTGGAACACGTTTCTGAAAGA | |
| IL-1β | F: GAAATGCCACCTTTTGACAGTG, R: TGGATGCTCTCATCAGGACAG | |
| FOXO3 | F: AGCCGTGTACTGTGGAGCTT, R: TCTTGGCGGTATATGGGAAG | |
| GAPDH | F: TGACCTCAACTACATGGTCTACA, R: CTTCCCATTCTCGGCCTTG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byeon, H.-E.; Jeon, J.Y.; Kim, H.J.; Kim, D.J.; Lee, K.-W.; Kang, Y.; Han, S.J. MicroRNA-132 Negatively Regulates Palmitate-Induced NLRP3 Inflammasome Activation through FOXO3 Down-Regulation in THP-1 Cells. Nutrients 2017, 9, 1370. https://doi.org/10.3390/nu9121370
Byeon H-E, Jeon JY, Kim HJ, Kim DJ, Lee K-W, Kang Y, Han SJ. MicroRNA-132 Negatively Regulates Palmitate-Induced NLRP3 Inflammasome Activation through FOXO3 Down-Regulation in THP-1 Cells. Nutrients. 2017; 9(12):1370. https://doi.org/10.3390/nu9121370
Chicago/Turabian StyleByeon, Hye-Eun, Ja Young Jeon, Hae Jin Kim, Dae Jung Kim, Kwan-Woo Lee, Yup Kang, and Seung Jin Han. 2017. "MicroRNA-132 Negatively Regulates Palmitate-Induced NLRP3 Inflammasome Activation through FOXO3 Down-Regulation in THP-1 Cells" Nutrients 9, no. 12: 1370. https://doi.org/10.3390/nu9121370





