Quantity and Quality of Carbohydrate Intake during Pregnancy, Newborn Body Fatness and Cardiac Autonomic Control: Conferred Cardiovascular Risk?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Maternal Characteristics and Dietary Intake
2.3. Infant Anthropometry and Autonomic Function
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Body Composition and Maternal Carbohydrate Intake
3.3. Infant Autonomic Function and Maternal Carbohydrate Intake
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVD’s). Available online: http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed on 27 August 2017).
- Reynolds, C.M.; Gray, C.; Li, M.; Segovia, S.A.; Vickers, M.H. Early life nutrition and energy balance disorders in offspring in later life. Nutrients 2015, 7, 8090–8111. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Osmond, C.; Forsen, T.J.; Kajantie, E.; Eriksson, J.G. Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 2005, 353, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Visentin, S.; Grumolato, F.; Nardelli, G.B.; Di Camillo, B.; Grisan, E.; Cosmi, E. Early origins of adult disease: Low birth weight and vascular remodeling. Atherosclerosis 2014, 237, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Skilton, M.R.; Crispi, F. Human fetal growth restriction: A cardiovascular journey through to adolescence. J. Dev. Orig. Health Dis. 2016, 7, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Whincup, P.H.; Bredow, M.; Payne, F.; Sadler, S.; Golding, J. Size at birth and blood pressure at 3 years of age. The avon longitudinal study of pregnancy and childhood (ALSPAC). Am. J. Epidemiol. 1999, 149, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Schellong, K.; Schulz, S.; Harder, T.; Plagemann, A. Birth weight and long-term overweight risk: Systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS ONE 2012, 7, e47776. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.L.; Yadav, P.K.; Yadav, L.K.; Agrawal, K.; Sah, S.K.; Islam, M.N. Association between obesity and heart rate variability indices: An intuition toward cardiac autonomic alteration—A risk of CVD. Diabetes Metab. Syndr. 2017, 10, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Judy, W.V.; Farrell, S.K. Arterial baroreceptor reflex control of sympathetic nerve activity in the spontaneously hypertensive rat. Hypertension 1979, 1, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Judy, W.V.; Watanabe, A.M.; Henry, D.P.; Besch, H.R., Jr.; Murphy, W.R.; Hockel, G.M. Sympathetic nerve activity: Role in regulation of blood pressure in the spontaenously hypertensive rat. Circ. Res. 1976, 38, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G. Sympathetic neural activity in hypertension and related diseases. Am. J. Hypertens. 2010, 23, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Quarti-Trevano, F. The ‘neuroadrenergic hypothesis’ in hypertension: Current evidence. Exp. Physiol. 2010, 95, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Straznicky, N.; Eikelis, N.; Masuo, K.; Lambert, G.; Lambert, E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 2006, 48, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, H.; McMullan, R.; Phang, M.; Gordon, A.; Hyatt, J.; Rains-Greenow, C.; Celermajer, D.; Polson, J.; Skilton, M. Newborn body fatness and autonomic function: Identification of infants at risk of later cardiovascular disease. FASEB J. 2017, 31, 1071–1078. [Google Scholar]
- Schulz, L.C. The Dutch Hunger Winter and the developmental origins of health and disease. Proc. Natl. Acad. Sci. USA 2010, 107, 16757–16758. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.; de Rooij, S.; Painter, R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 2006, 82, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Costa-Silva, J.H.; Simoes-Alves, A.C.; Fernandes, M.P. Developmental origins of cardiometabolic diseases: Role of the maternal diet. Front. Physiol. 2016, 7, 504. [Google Scholar] [CrossRef] [PubMed]
- Kizirian, N.V.; Goletzke, J.; Brodie, S.; Atkinson, F.S.; Markovic, T.P.; Ross, G.P.; Buyken, A.; Brand-Miller, J.P. Lower glycemic load meals reduce diurnal glycemic oscillations in women with risk factors for gestational diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000351. [Google Scholar] [CrossRef] [PubMed]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.G.; Ireland, P.D. Dietary Questionnaire for Epidemiological Studies, version 2; Victorian Cancer Council: Melbourne, Australia, 1996. [Google Scholar]
- Breij, L.M.; Steegers-Theunissen, R.P.; Briceno, D.; Hokken-Koelega, A.C. Maternal and fetal determinants of neonatal body composition. Horm. Res. Paediatr. 2015, 84, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Kizirian, N.V.; Kong, Y.; Muirhead, R.; Brodie, S.; Garnett, S.P.; Petocz, P.; Sim, K.A.; Celermajer, D.S.; Louie, J.C.; Markovic, T.P.; et al. Effects of a low-glycemic index diet during pregnancy on offspring growth, body composition, and vascular health: A pilot randomized controlled trial. Am. J. Clin. Nutr. 2016, 103, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Polson, J.W.; McCallion, N.; Waki, H.; Thorne, G.; Tooley, M.A.; Paton, J.F.; Wolf, A.R. Evidence for cardiovascular autonomic dysfunction in neonates with coarctation of the aorta. Circulation 2006, 113, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar]
- NHMRC. Nutrient Reference Values for Australia and New Zealand; Australian Government Department of Health and Ageing, New Zealand Ministry of Health: Canberra, Australia, 2006.
- Willett, K.; Stampfer, M. Implications of Total Energy Intake for Epidemiologic Analyses; Oxford University Press: New York, NY, USA, 1998; pp. 1273–1301. [Google Scholar]
- Australian Government. Healthy Eating During your Pregnancy; Health, D.O., Ed.; National Medical Health and Medical Research Council: Canberra, Australia, 2013.
- Lown, B.; Verrier, R.L. Neural activity and ventricular fibrillation. N. Engl. J. Med. 1976, 294, 1165–1170. [Google Scholar] [PubMed]
- Thayer, J.F.; Lane, R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007, 74, 224–242. [Google Scholar] [CrossRef] [PubMed]
- Seravalle, G.; Mancia, G.; Grassi, G. Role of the sympathetic nervous system in hypertension and hypertension-related cardiovascular disease. High Blood Press. Cardiovasc. Prev. 2014, 21, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Lombardi, F.; Guzzetti, S.; Rimoldi, O.; Furlan, R.; Pizzinelli, P.; Sandrone, G.; Malfatto, G.; Dell’Orto, S.; Piccaluga, E.; et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 1986, 59, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.M.; Varigos, G.A.; Hunt, D.; Sloman, J.G. Sinus arrhythmia in acute myocardial infarction. Med. J. Aust. 1978, 2, 52–53. [Google Scholar] [PubMed]
- Appel, M.L.; Berger, R.D.; Saul, J.P.; Smith, J.M.; Cohen, R.J. Beat to beat variability in cardiovascular variables: Noise or music? J. Am. Coll. Cardiol. 1989, 14, 1139–1148. [Google Scholar] [CrossRef]
- Montano, N.; Ruscone, T.G.; Porta, A.; Lombardi, F.; Pagani, M.; Malliani, A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994, 90, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.V.; Fallen, E.L. Power spectral analysis of heart rate variability: A noninvasive signature of cardiac autonomic function. Crit. Rev. Biomed. Eng. 1993, 21, 245–311. [Google Scholar] [PubMed]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Tzanetakou, I.P.; Mikhailidis, D.P.; Perrea, D.N. Nutrition during pregnancy and the effect of carbohydrates on the offspring’s metabolic profile: In search of the “Perfect Maternal Diet”. Open Cardiovasc. Med. J. 2011, 5, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Australian Bereau of Statistics. Australian Health Survey. Available online: http://www.abs.gov.au/ausstats/[email protected]/Lookup/by%20Subject/4364.0.55.007~2011-12~Main%20Features~Carbohydrate~705 (accessed on 16 November 2017).
- Walsh, J.M.; McAuliffe, F.M. Impact of maternal nutrition on pregnancy outcome—Does it matter what pregnant women eat? Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Swain, J.; Goldfine, A.B.; Rifai, N.; Ludwig, D.S. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA 2004, 292, 2482–2490. [Google Scholar] [CrossRef] [PubMed]
- Ebbeling, C.B.; Leidig, M.M.; Feldman, H.A.; Lovesky, M.M.; Ludwig, D.S. Effects of a low-glycemic load vs low-fat diet in obese young adults: A randomized trial. JAMA 2007, 297, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, T.; Ninomiya, T.; Wang, A.; Neal, B.; Jun, M.; Wong, M.G.; Jardine, M.; Hillis, G.S.; Perkovic, V. Effects of the mediterranean diet on cardiovascular outcomes—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0159252. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.M.; McGowan, C.A.; Mahony, R.; Foley, M.E.; McAuliffe, F.M. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): Randomised control trial. BMJ 2012, 345, e5605. [Google Scholar] [CrossRef] [PubMed]
- Horan, M.K.; McGowan, C.A.; Gibney, E.R.; Donnelly, J.M.; McAuliffe, F.M. Maternal low glycaemic index diet, fat intake and postprandial glucose influences neonatal adiposity—Secondary analysis from the ROLO study. Nutr. J. 2014, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Louie, J.C.; Markovic, T.P.; Perera, N.; Foote, D.; Petocz, P.; Ross, G.P.; Brand-Miller, J.C. A randomized controlled trial investigating the effects of a low-glycemic index diet on pregnancy outcomes in gestational diabetes mellitus. Diabetes Care 2011, 34, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.P.; Hourihane, J.O.; Kenny, L.C.; Irvine, A.D.; Kiely, M.; Murray, D.M. Gender- and gestational age-specific body fat percentage at birth. Pediatrics 2011, 128, E645–E651. [Google Scholar] [CrossRef] [PubMed]
- Kizirian, N.V.; Markovic, T.P.; Muirhead, R.; Brodie, S.; Garnett, S.P.; Louie, J.C.; Petocz, P.; Ross, G.P.; Brand-Miller, J.C. Macronutrient balance and dietary glycemic index in pregnancy predict neonatal body composition. Nutrients 2016, 8, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhle, S.; Maguire, B.; Ata, N.; MacInnis, N.; Dodds, L. Birth weight for gestational age, anthropometric measures, and cardiovascular disease markers in children. J. Pediatr. 2017, 182, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Skilton, M.R.; Marks, G.B.; Ayer, J.G.; Garden, F.L.; Garnett, S.P.; Harmer, J.A.; Leeder, S.R.; Toelle, B.G.; Webb, K.; Baur, L.A.; et al. Weight gain in infancy and vascular risk factors in later childhood. Pediatrics 2013, 131, e1821–e1828. [Google Scholar] [CrossRef] [PubMed]
- Hull, H.R.; Dinger, M.K.; Knehans, A.W.; Thompson, D.M.; Fields, D.A. Impact of maternal body mass index on neonate birthweight and body composition. Am. J. Obstet. Gynecol. 2008, 198, e411–e416. [Google Scholar] [CrossRef] [PubMed]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, validation and utilisation of food-frequency questionnaires—A review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef] [PubMed]
- Subar, A.F.; Ziegler, R.G.; Thompson, F.E.; Johnson, C.C.; Weissfeld, J.L.; Reding, D.; Kavounis, K.H.; Hayes, R.B.; Colorectal and Ovarian Cancer Screening Trial Investigators. Is shorter always better? Relative importance of questionnaire length and cognitive ease on response rates and data quality for two dietary questionnaires. Am. J. Epidemiol. 2001, 153, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Yiallourou, S.R.; Sands, S.A.; Walker, A.M.; Horne, R.S.C. Maturation of heart rate and blood pressure variability during sleep in term-born infants. Sleep 2012, 35, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Kleinman, K.P.; Belfort, M.B.; Hammitt, J.K.; Gillman, M.W. Associations of gestational weight gain with short- and longer-term maternal and child health outcomes. Am. J. Epidemiol. 2009, 170, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Stotland, N.E.; Cheng, Y.W.; Hopkins, L.M.; Caughey, A.B. Gestational weight gain and adverse neonatal outcome among term infants. Obstet. Gynecol. 2006, 108, 635–643. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | ||
|---|---|---|
| Maternalcharacteristics | Cardiac autonomic function subgroup | |
| Maternal age, years | 33.3 ± 4.4 | 33.2 ± 4.6 |
| Maternal height, m | 165.3 ± 6.5 | 165.0 ± 6.6 |
| Pre-pregnancy BMI, kg/m2 | 22.0 ± 3.6 | 22.1 ± 4.1 |
| Total energy intake, kJ/day | 7854 ± 3756 | 7478 ± 3464 |
| Total fat intake, % energy | 37.7 ± 4.6 | 37.9 ± 4.3 |
| Protein intake, % energy | 19.4 ± 2.8 | 19.0 ± 3.3 |
| Carbohydrate, % energy | 41.0 ± 5.0 | 41.0 ± 4.8 |
| Fibre, g/day | 22.8 ± 9.8 | 21.9 ± 8.7 |
| Glycaemic Index | 49.8 ± 5.1 | 49.4 ± 0. |
| Glycaemic Load | 89.3 ± 44.8 | 96.3 ± 5.3 |
| Newborncharacteristics | ||
| Female, n% | 83 (58%) | 58 (57%) |
| Gestation, week | 39.3 ± 1.2 | 39.2 ± 1.1 |
| Birth Weight, g | 3426 ± 521 | 3436 ± 558 |
| Birth length, cm | 49.9 ± 2.3 | 49.9 ± 2.5 |
| Head circumference, cm | 34.8 ± 1.4 | 34.8 ± 1.4 |
| Body Fatness, % | 11.1 ± 5.1 | 11.3 ± 5.4 |
| Fat Free Mass, % | 88.9 ± 5.1 | 88.7 ± 5.4 |
| HRV – TP, ms2 | - | 1124 ± 1113 |
| HRV – LF, ms2 | - | 285 ± 286 |
| HRV – HF, ms2 | - | 122 ± 168 |
| Birth Weight (g) | Body Fatness (%) | |||
|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | |
| Carbohydrate (%) | 43 (−23, 108) | 0.20 | 0.01 (−0.18, 0.16) | 0.90 |
| Q1 | Reference | Reference | ||
| Q2 | 222 (−33, 478) | 0.09 | 2.18 (−0.30, 4.67) | 0.09 |
| Q3 | 143 (−109, 396) | 0.26 | 0.35 (−2.11, 2.80) | 0.78 |
| Q4 | 124 (−124, 372) | 0.33 | 1.44 (−0.98, 3.85) | 0.24 |
| Glycaemic Index | 291 (−970, 1553) | 0.65 | 1.73 (−10.59, 14.04) | 0.78 |
| Q1 | Reference | Reference | ||
| Q2 | −13 (−259, 233) | 0.91 | −0.58 (−3.00, 1.83) | 0.63 |
| Q3 | 122 (−129, 372) | 0.34 | 0.37 (−2.08, 2.82) | 0.77 |
| Q4 | 32 (−218, 282) | 0.80 | 0.26 (−2.19, 2.71) | 0.83 |
| Glycaemic Load | 265 (−138, 667) | 0.20 | 0.88 (−3.07, 4.83) | 0.66 |
| Q1 | Reference | Reference | ||
| Q2 | −236 (−478, 6) | 0.06 | −1.79 (−4.20, 0.61) | 0.14 |
| Q3 | 125 (−114, 363) | 0.30 | 0.55 (−1.82, 2.93) | 0.65 |
| Q4 | −56 (−294, 181) | 0.64 | −0.28 (−2.64, 2.08) | 0.81 |
| Fibre (g) | 45 (−51, 141) | 0.36 | 0.01 (−0.15, 0.16) | 0.95 |
| Q1 | Reference | Reference | ||
| Q2 | −98 (−340, 144) | 0.43 | −0.11 (−2.42, 2.20) | 0.93 |
| Q3 | 97 (−147, 341) | 0.43 | 2.56 (0.23, 4.90) | 0.03 |
| Q4 | −30 (−273, 214) | 0.81 | −0.39 (−2.72, 1.94) | 0.75 |
| TP *, ms2 | LF *, ms2 | HF *, ms2 | LF:HF * | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | |
| Carbohydrate, % | 0.01 (−0.04, 0.03) | 0.75 | 0.00 (−0.03, 0.03) | 0.97 | 0.00 (−0.04, 0.05) | 0.96 | 0.00 (−0.03, 0.03) | 0.85 |
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | −0.03 (−0.50, 0.44) | 0.90 | −0.20 (−0.70, 0.30) | 0.42 | −0.32 (−0.96, 0.32) | 0.32 | 0.23 (−0.22, 0.68) | 0.31 |
| Q3 | 0.17 (0.26, 0.60) | 0.44 | −0.11 (−0.34, 0.57) | 0.62 | 0.22 (−0.37, 0.81) | 0.46 | −0.01 (−0.42, 0.40) | 0.96 |
| Q4 | −0.03 (−0.46, 0.41) | 0.91 | −0.01 (−0.47, 0.45) | 0.97 | −0.09 (−0.67, 0.50) | 0.76 | 0.12 (−0.29, 0.53) | 0.57 |
| Glycaemic Index * | −1.37 (−3.43, 0.70) | 0.19 | −1.94 (−4.12, 0.24) | 0.08 | −1.59 (−4.46, 1.27) | 0.27 | −0.10 (−2.09, 1.89) | 0.92 |
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | −0.11 (−0.51, 0.30) | 0.61 | −0.21 (−0.64, 0.21) | 0.32 | −0.28 (−0.84, 0.27) | 0.32 | 0.10 (−0.29, 0.49) | 0.60 |
| Q3 | 0.00 (−0.46, 0.46) | 0.99 | −0.13 (−0.62, 0.35) | 0.58 | 0.00 (−0.64, 0.63) | 1.00 | 0.07 (−0.37, 0.51) | 0.76 |
| Q4 | −0.31 (−0.73, 0.12) | 0.16 | −0.44 (−0.89, 0.01) | 0.05 | −0.42 (−1.01, 0.17) | 0.16 | 0.06 (−0.35, 0.47) | 0.77 |
| Glycaemic load * | 0.38 (−0.34, 1.09) | 0.30 | 0.19 (−0.57, 0.95) | 0.62 | 0.34 (−0.65, 1.33) | 0.49 | −0.15 (−0.83, 0.54) | 0.67 |
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 0.19 (−0.32, 0.62) | 0.39 | 0.20 (−0.25, 0.65) | 0.38 | 0.22 (−0.37, 0.82) | 0.46 | 0.08 (−0.34, 0.49) | 0.71 |
| Q3 | −0.05 (−0.48, 0.37) | 0.80 | −0.21 (−0.66, 0.23) | 0.34 | −0.11 (−0.69, 0.47) | 0.71 | 0.05 (−0.36, 0.45) | 0.82 |
| Q4 | −0.02 (−0.46, 0.42) | 0.92 | 0.00 (−0.47, 0.46) | 1.00 | −0.15 (−0.76, 0.46) | 0.62 | 0.14 (−0.29, 0.56) | 0.52 |
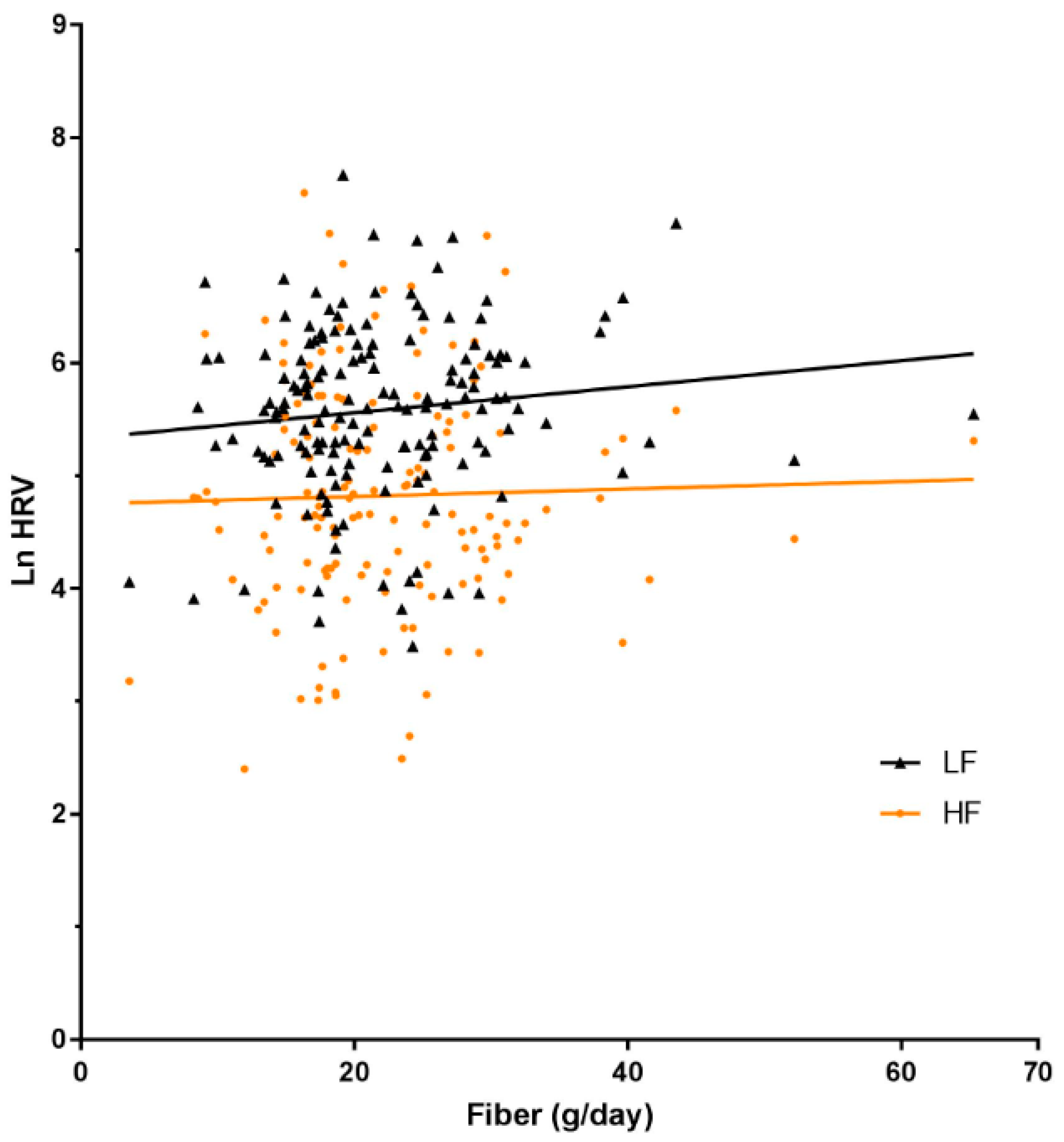
| Fibre, g | 0.02 (−0.01, 0.05) | 0.14 | 0.03 (−0.00, 0.06) | 0.06 | 0.01 (−0.04, 0.05) | 0.79 | 0.03 (−0.00, 0.06) | 0.06 |
| Q1 | Reference | Reference | Reference | Reference | ||||
| Q2 | 0.23 (−0.19, 0.65) | 0.29 | 0.45 (−0.01, 0.89) | 0.05 | 0.28 (−0.31, 0.86) | 0.35 | 0.19 (−0.21, 0.59) | 0.34 |
| Q3 | 0.24 (−0.19, 0.66) | 0.28 | 0.21 (−0.24, 0.65) | 0.36 | 0.09 (−0.51, 0.68) | 0.78 | 0.26 (−0.15, 0.66) | 0.22 |
| Q4 | 0.27 (−0.16, 0.70) | 0.22 | 0.40 (−0.05, 0.85) | 0.08 | 0.15 (−0.45, 0.75) | 0.62 | 0.26 (−0.15, 0.67) | 0.21 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mckenzie, K.M.; Dissanayake, H.U.; McMullan, R.; Caterson, I.D.; Celermajer, D.S.; Gordon, A.; Hyett, J.; Meroni, A.; Phang, M.; Raynes-Greenow, C.; et al. Quantity and Quality of Carbohydrate Intake during Pregnancy, Newborn Body Fatness and Cardiac Autonomic Control: Conferred Cardiovascular Risk? Nutrients 2017, 9, 1375. https://doi.org/10.3390/nu9121375
Mckenzie KM, Dissanayake HU, McMullan R, Caterson ID, Celermajer DS, Gordon A, Hyett J, Meroni A, Phang M, Raynes-Greenow C, et al. Quantity and Quality of Carbohydrate Intake during Pregnancy, Newborn Body Fatness and Cardiac Autonomic Control: Conferred Cardiovascular Risk? Nutrients. 2017; 9(12):1375. https://doi.org/10.3390/nu9121375
Chicago/Turabian StyleMckenzie, Kirsty M., Hasthi U. Dissanayake, Rowena McMullan, Ian D. Caterson, David S. Celermajer, Adrienne Gordon, Jonathan Hyett, Alice Meroni, Melinda Phang, Camille Raynes-Greenow, and et al. 2017. "Quantity and Quality of Carbohydrate Intake during Pregnancy, Newborn Body Fatness and Cardiac Autonomic Control: Conferred Cardiovascular Risk?" Nutrients 9, no. 12: 1375. https://doi.org/10.3390/nu9121375





