Early Taste Experiences and Later Food Choices
Abstract
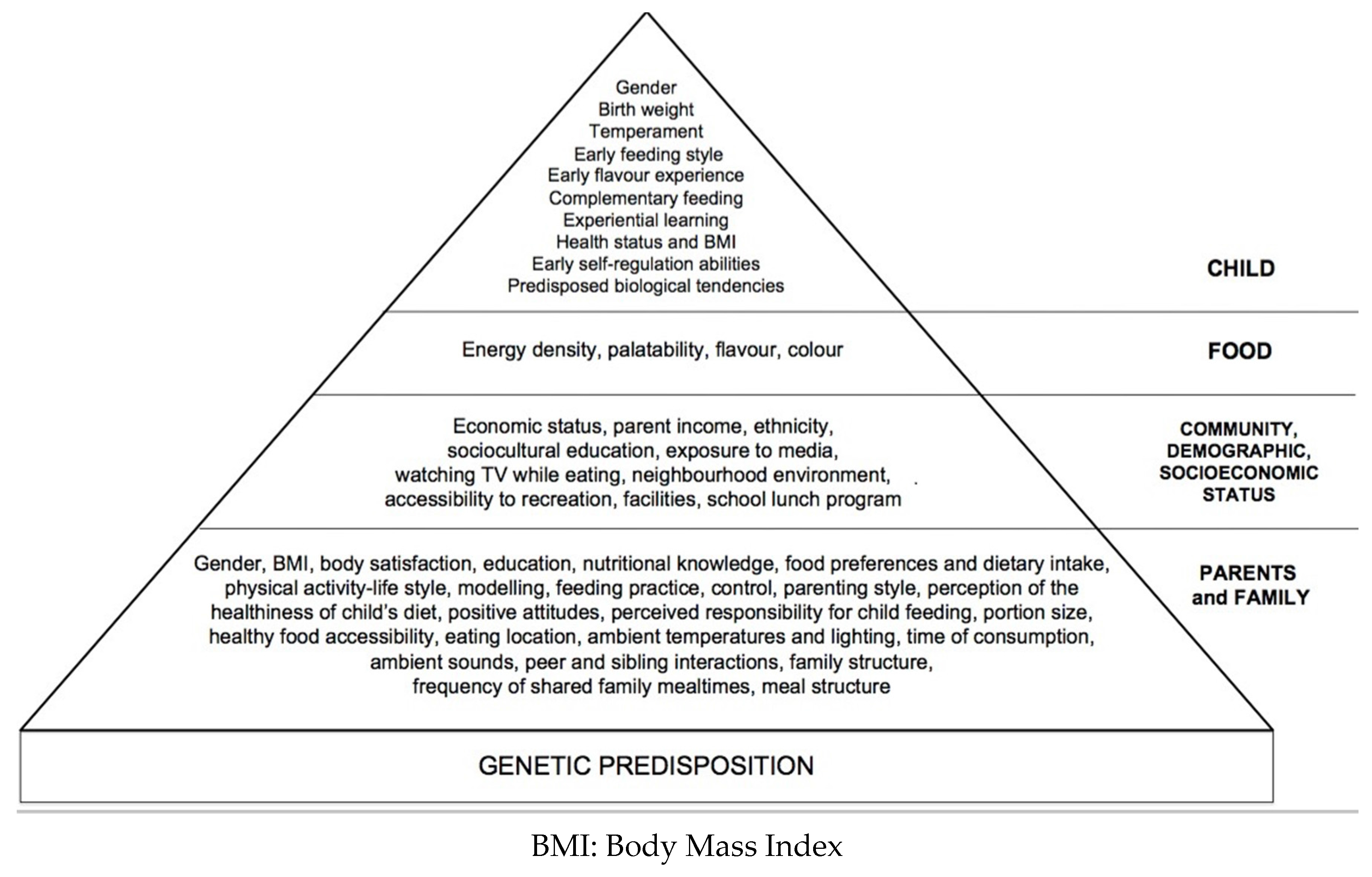
:1. Introduction
2. Methods—Literature Search Strategy
2.1. Effects of Early Taste Experiences
2.2. Amniotic Fluid and Breast Milk
2.3. Formula-Fed Infants
2.4. Complementary Feeding and Future Consumption of Fruits and Vegetables during Childhood
2.5. Sociocultural and Family Environment
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alles, M.S.; Eussen, S.R.; Van Der Beek, E.M. Nutritional challenges and opportunities during the weaning period and in young childhood. Ann. Nutr. Metab. 2014, 64, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Amanda, L.T.; Margaret, E. The critical period of infant feeding for the development of early disparities in obesity. Soc. Sci. Med. 2013. [Google Scholar] [CrossRef]
- Benyshek, D.C. The developmental origins of obesity and related health disorders–prenatal and perinatal factors. Coll. Antropol. 2007, 31, 11–17. [Google Scholar] [PubMed]
- Gillman, M.W. Developmental origins of health and disease. N. Engl. J. Med. 2005, 353, 1848–1850. [Google Scholar] [CrossRef] [PubMed]
- Bellows, L.L.; Johnson, S.L.; Davies, P.L.; Anderson, J.; Gavin, W.J.; Boles, R.E. The Colorado LEAP study: Rationale and design of a study to assess the short term longitudinal effectiveness of a preschool nutrition and physical activity program. BMC Public Health 2013. [Google Scholar] [CrossRef] [PubMed]
- Arenz, S.; Rückerl, R.; Koletzko, B.; von Kries, R. Breast-feeding and childhood obesity—A systematic review. Int. J. Obes. 2004, 28, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Smith, G.D.; Cook, D.G. Effect of infant feeding on the risk of obesity across the life course: A quantitative review of published evidence. Pediatrics 2005, 115, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Birch, L.L.; Fisher, J.O. Development of eating behaviours among children and adolescents. Pediatrics 1998, 101, 539–549. [Google Scholar] [PubMed]
- Mitchell, G.L.; Farrow, C.; Haycraft, E.; Meyer, C. Parental influences on children’s eating behaviour and characteristics of successful parent-focussed interventions. Appetite 2013, 60, 85–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, S.; Fall, C. Infant nutrition and later health: A review of current evidence. Nutrients 2012, 4, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A. Ontogeny of taste preferences: Basic biology and implications for health. Am. J. Clin. Nutr. 2014, 99, 704S–711S. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, M.M.; Schwartz, C.; Madrelle, J.; Croden, F.; Nekitsing, C.; Vereijken, C.M.J.L.; Weenen, H. A step-by-step introduction to vegetables at the beginning of complementary feeding. The effects of early and repeated exposure. Appetite 2015, 84, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.L.; Adise, S. Variation in the Ability to Taste Bitter Thiourea Compounds: Implications for Food Acceptance, Dietary Intake, and Obesity Risk in Children. Annu. Rev. Nutr. 2016, 36, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Shamir, R. Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, N.D.; Allotey, P.A.; Soyiri, I.N.; Reidpath, D.D. Complementary feeding and the early origins of obesity risk: A study protocol. BMJ Open 2016. [Google Scholar] [CrossRef] [PubMed]
- Forestell, C.A. The Development of Flavor Perception and Acceptance: The Roles of Nature and Nurture. Nestle Nutr. Inst. Workshop Ser. 2016, 85, 135–143. [Google Scholar] [PubMed]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Galloway, A.T.; Lee, Y.; Birch, L.L. Predictors and consequences of food neophobia and pickiness in young girls. J. Am. Diet. Assoc. 2003, 103, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.D.; Carruth, B.R.; Bounds, W.; Ziegler, P.; Reidy, K. Do food-related experiences in the first 2 years of life predict dietary variety in school-aged children? J. Nutr. Educ. Behav. 2002, 34, 310–315. [Google Scholar] [CrossRef]
- Cooke, L.J.; Wardle, J.; Gibson, E.L.; Sapochnik, M.; Sheiham, A.; Lawson, M. Demographic, familial and trait predictors of fruit and vegetable consumption by pre-school children. Public Health Nutr. 2004, 7, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Taveras, E.M.; Scanlon, K.S.; Birch, L.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Gillman, M.W. Association of breastfeeding with maternal control of infant feeding at age 1 year. Pediatrics 2004, 114, e577–e583. [Google Scholar] [CrossRef] [PubMed]
- Trabulsi, J.C.; Mennella, J.A. Diet, sensitive periods in flavour learning, and growth. Int. Rev. Psychiatry 2012, 24, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Patel, R.; Kramer, M.S.; Guthrie, L.; Vilchuck, K.; Bogdanovich, N.; Rifas-Shiman, S.L. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: A randomized trial. JAMA 2013, 309, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Sengier, A. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Ventura, A.K.; Beauchamp, G.K. Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics 2011, 127, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.K.; Beauchamp, G.K.; Mennella, J.A. Infant regulation of intake: The effect of free glutamate content in infant formulas. Am. J. Clin. Nutr. 2012, 95, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Larnkjær, A.; Bruun, S.; Pedersen, D.; Zachariassen, G.; Barkholt, V.; Agostoni, C.; Michaelsen, K.F. Free Amino Acids in Human Milk and Associations with Maternal Anthropometry and Infant Growth. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Nicklaus, S. The role of food experiences during early childhood in food pleasure learning. Appetite 2016, 104, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bouhlal, S.; Issanchou, S.; Chabanet, C.; Nicklaus, S. ‘Just a pinch of salt’. An experimental comparison of the effect of repeated exposure and flavor-flavor learning with salt or spice on vegetable acceptance in toddlers. Appetite 2014, 83, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ventura, A.K.; Worobey, J. Early influences on the development of food preferences. Curr. Biol. 2013, 23, R401–R408. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.A.; Birch, L.L. Infant dietary experience and acceptance of solid foods. Pediatrics 1994, 93, 271–277. [Google Scholar] [PubMed]
- Maier, A.; Chabanet, C.; Schaal, B.; Issanchou, S.; Leathwood, P. Effects of repeated exposure on acceptance of initially disliked vegetables in 7-month old infants. Food Qual. Preference 2007, 18, 1023–1032. [Google Scholar] [CrossRef]
- Carruth, B.R.; Ziegler, P.J.; Gordon, A.; Barr, S.I. Prevalence of picky eaters among infants and toddlers and their caregivers’ decisions about offering a new food. J. Am. Diet. Assoc. 2004, 104, S57–S64. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Chabanet, C.; Schaal, B.; Leathwood, P.; Issanchou, S. Food-related sensory experience from birth through weaning: Contrasted patterns in two nearby European regions. Appetite 2007, 49, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Nicklaus, S. Complementary Feeding Strategies to Facilitate Acceptance of Fruits and Vegetables: A Narrative Review of the Literature. Int. J. Environ. Res. Public Health 2016. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Visalli, M.; Jacob, S.; Chabanet, C.; Schlich, P.; Nicklaus, S. Maternal feeding practices during the first year and their impact on infants’ acceptance of complementary food. Food Qual. Preference 2013, 29, 89–98. [Google Scholar] [CrossRef]
- De Lauzon-Guillain, B.; Jones, L.; Oliveira, A.; Moschonis, G.; Betoko, A.; Lopes, C.; Charles, M.A. The influence of early feeding practices on fruit and vegetable intake among preschool children in 4 European birth cohorts. Am. J. Clin. Nutr. 2013, 98, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Wadhera, D.; Phillips, E.D.C.; Wilkie, L.M. Teaching children to like and eat vegetables. Appetite 2015, 93, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Chabanet, C.; Lange, C.; Issanchou, S.; Nicklaus, S. The role of taste in food acceptance at the beginning of complementary feeding. Physiol. Behav. 2011, 104, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.A.; Mallan, K.M.; Nicholson, J.M.; Thorpe, K.; Nambiar, S.; Mauch, C.E.; Magarey, A. An early feeding practices intervention for obesity prevention. Pediatrics 2015, 136, e40–e49. [Google Scholar] [CrossRef] [PubMed]
- Northstone, K.; Emmett, P.M. Are dietary patterns stable throughout early and mid-childhood? A birth cohort study. Br. J. Nutr. 2008, 100, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.D.; Carruth, B.R.; Bounds, W.; Ziegler, P.J. Children’s food preferences: A longitudinal analysis. J. Am. Diet. Assoc. 2002, 102, 1638–1647. [Google Scholar] [CrossRef]
- Singer, M.R.; Moore, L.L.; Garrahie, E.J.; Ellison, R.C. The tracking of nutrient intake in young children: The Framingham Children’s Study. Am. J. Public Health 1995, 85, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Kral, T.V.; Rauh, E.M. Eating behaviors of children in the context of their family environment. Physiol. Behav. 2010, 100, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.S.; Fisher, J.O.; Birch, L.L. Parental influence on eating behavior: Conception to adolescence. J. Law Med. Ethics 2007, 35, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Kennedy, J.M.; Beauchamp, G.K. Vegetable acceptance by infants: Effects of formula flavors. Early Hum. Dev. 2006, 82, 463–468. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Cosmi, V.; Scaglioni, S.; Agostoni, C. Early Taste Experiences and Later Food Choices. Nutrients 2017, 9, 107. https://doi.org/10.3390/nu9020107
De Cosmi V, Scaglioni S, Agostoni C. Early Taste Experiences and Later Food Choices. Nutrients. 2017; 9(2):107. https://doi.org/10.3390/nu9020107
Chicago/Turabian StyleDe Cosmi, Valentina, Silvia Scaglioni, and Carlo Agostoni. 2017. "Early Taste Experiences and Later Food Choices" Nutrients 9, no. 2: 107. https://doi.org/10.3390/nu9020107




