The Effects of Myo-Inositol and B and D Vitamin Supplementation in the db/+ Mouse Model of Gestational Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Genotyping
2.2. Experimental Groups
2.3. Oral Glucose Tolerance Test
2.4. Tissue Collection
2.5. Plasma Assays
2.6. Gene Expression Analysis
2.7. Statistics
3. Results
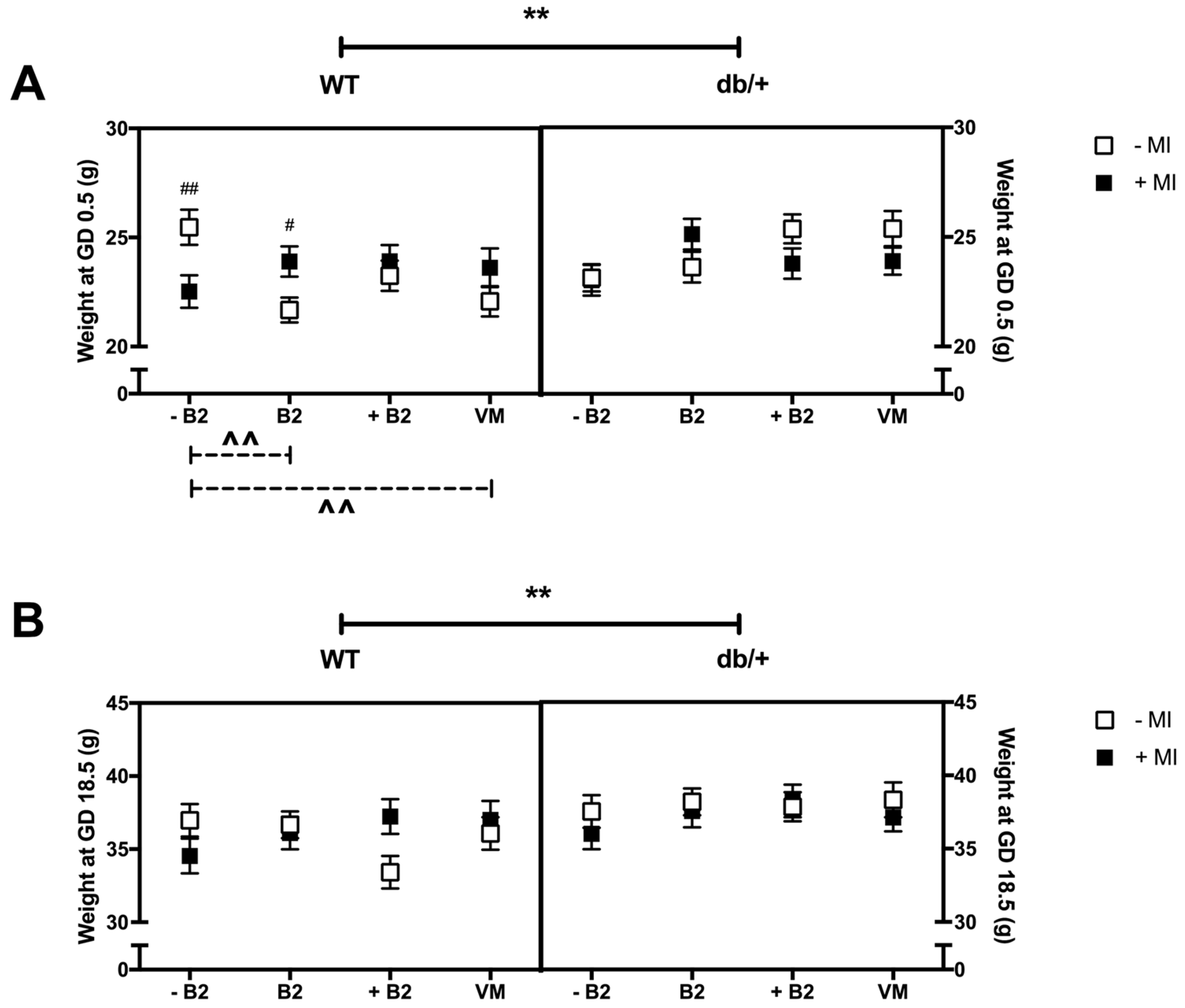
3.1. Body Weight at GD0.5 and GD18.5
3.1.1. Weight at the Beginning of Pregnancy (GD0.5)
3.1.2. Weight at GD18.5
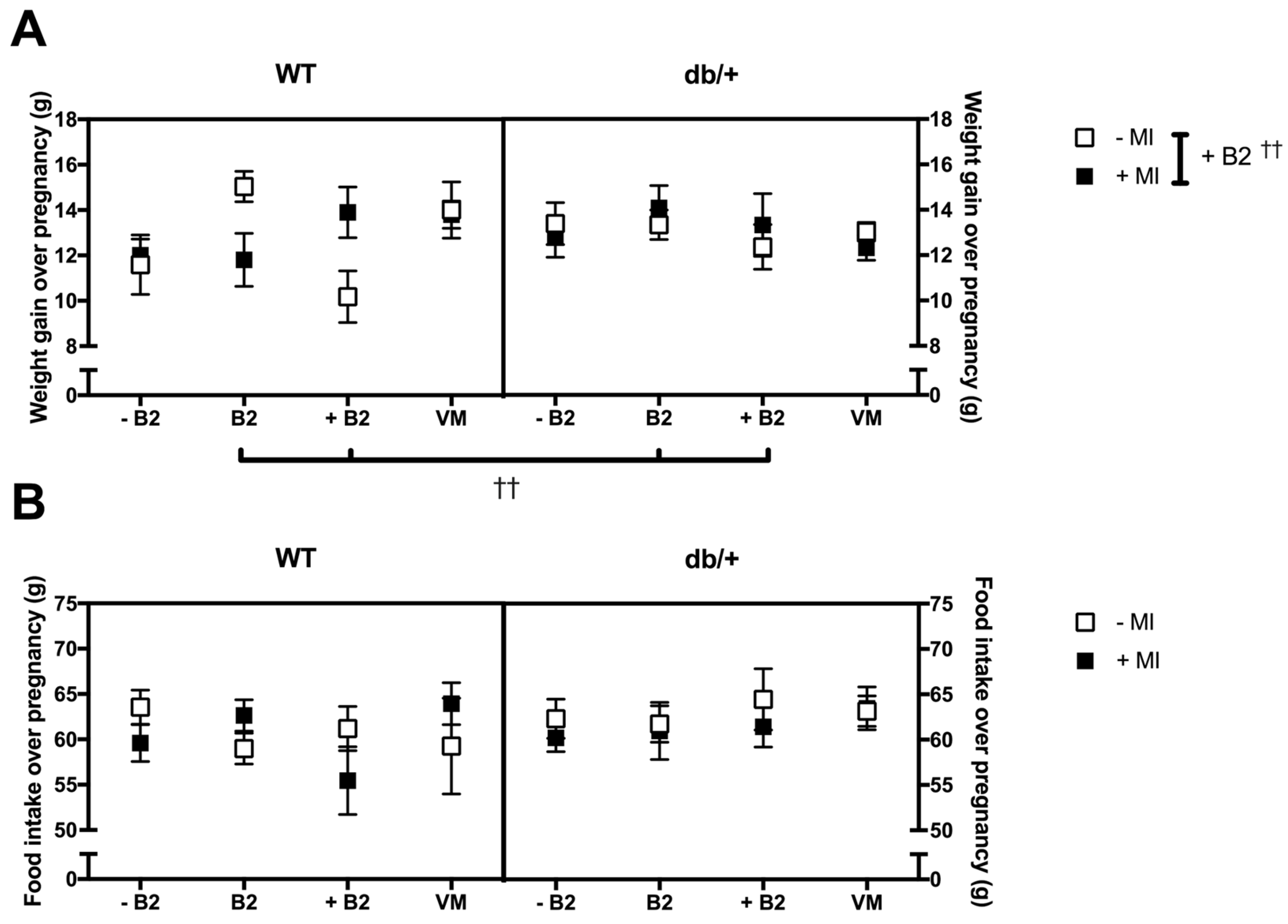
3.2. Pregnancy Weight Gain, Food Intake, and Litter Size
3.2.1. Weight Gain over Pregnancy
3.2.2. Food Intake over Pregnancy
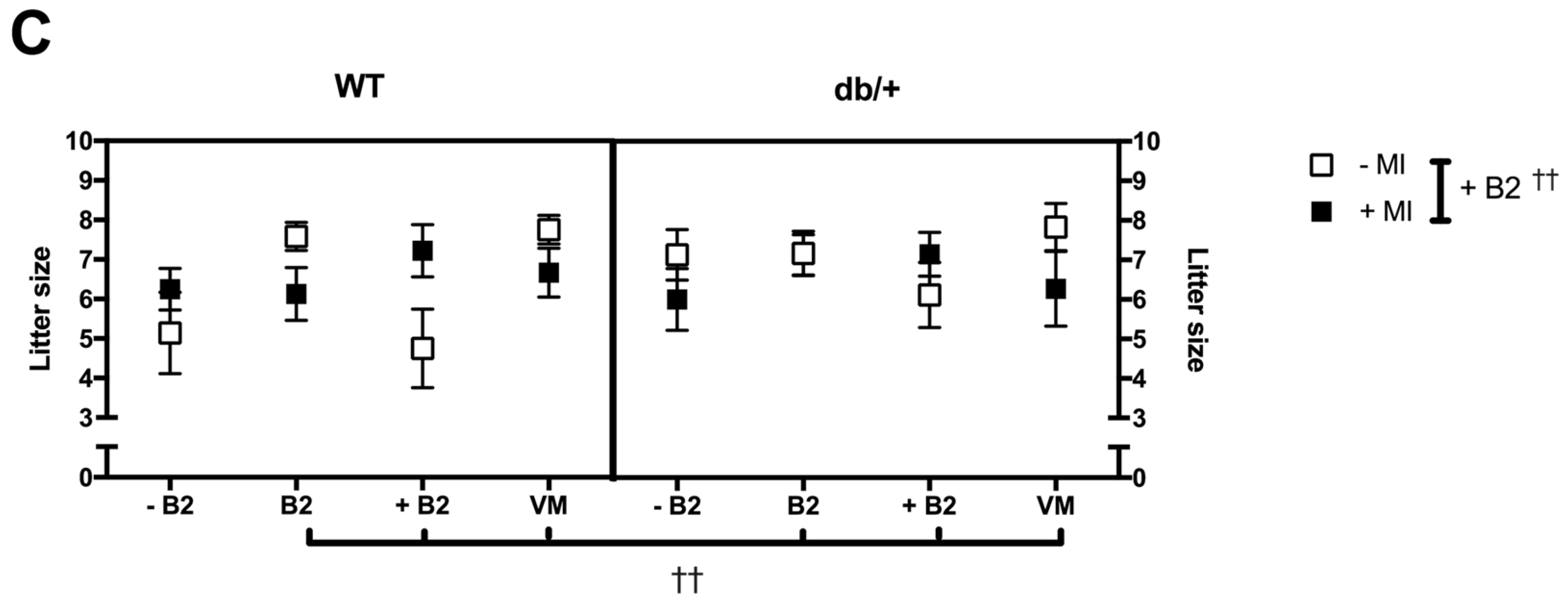
3.2.3. Litter Size
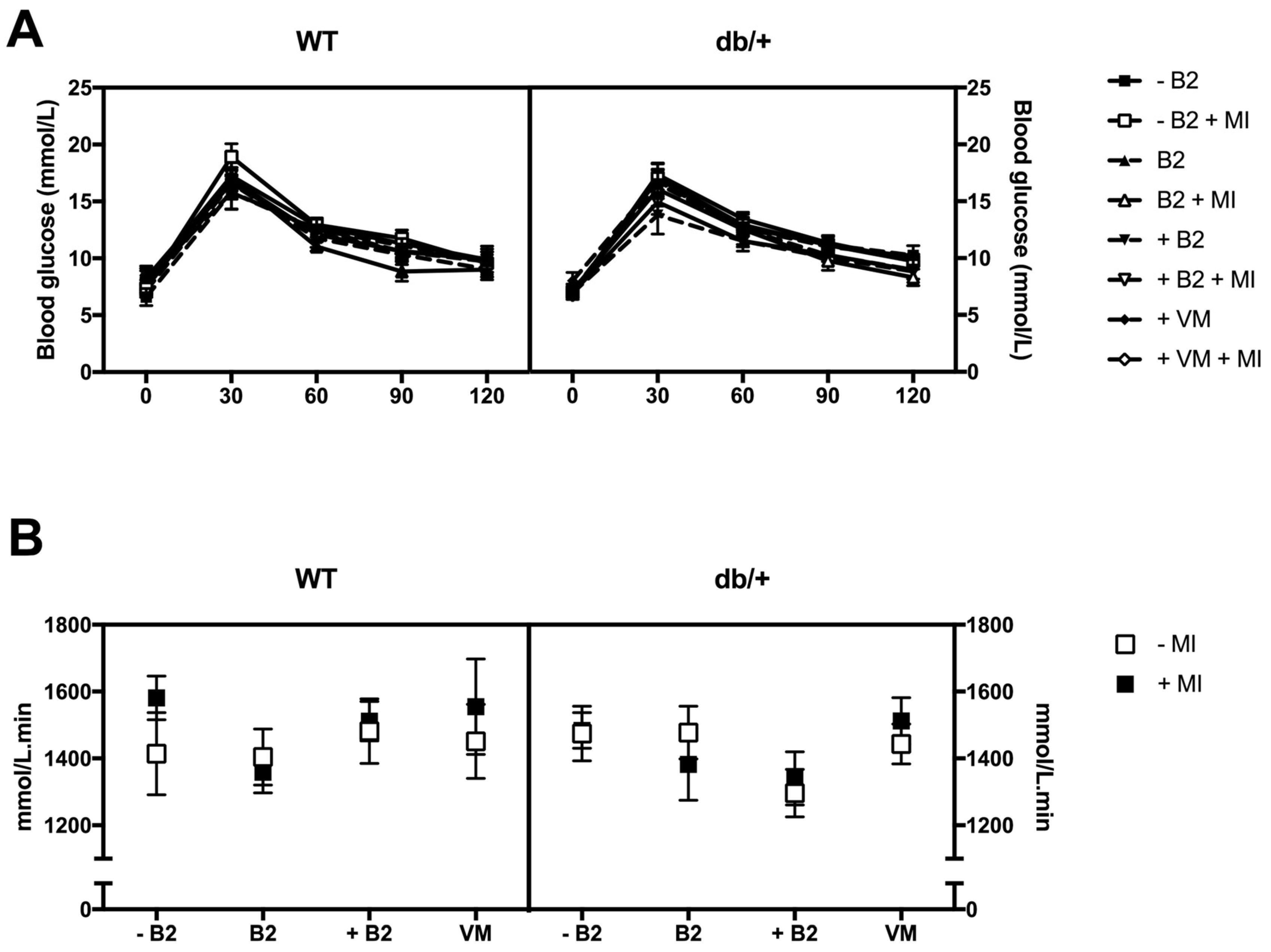
3.3. Glucose Tolerance during Pregnancy
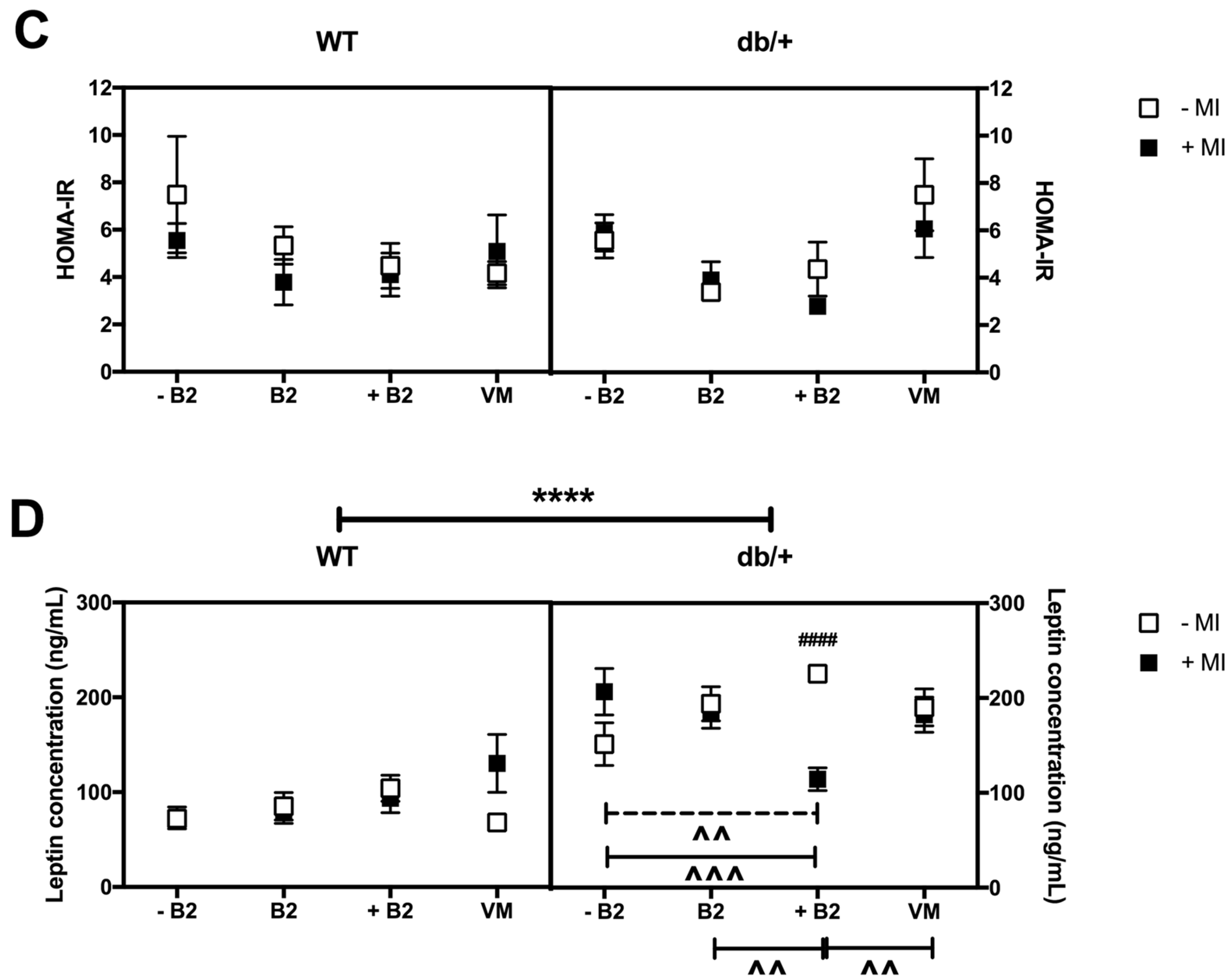
3.4. Fasting Plasma Glucose, Insulin, HOMA-IR, and Leptin at Day 18.5 of Pregnancy
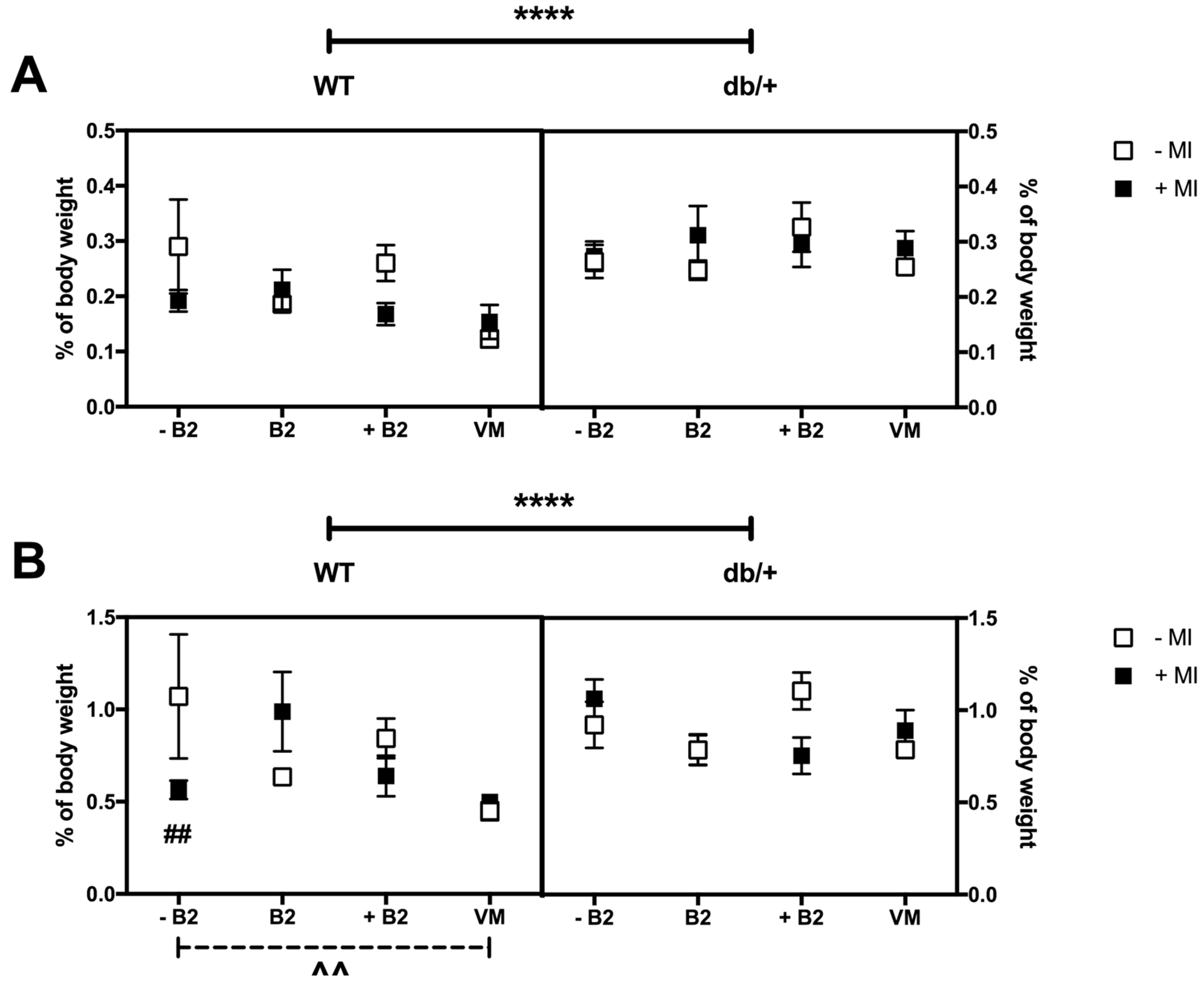
3.5. Weights of Major Organs at Day 18.5 of Pregnancy
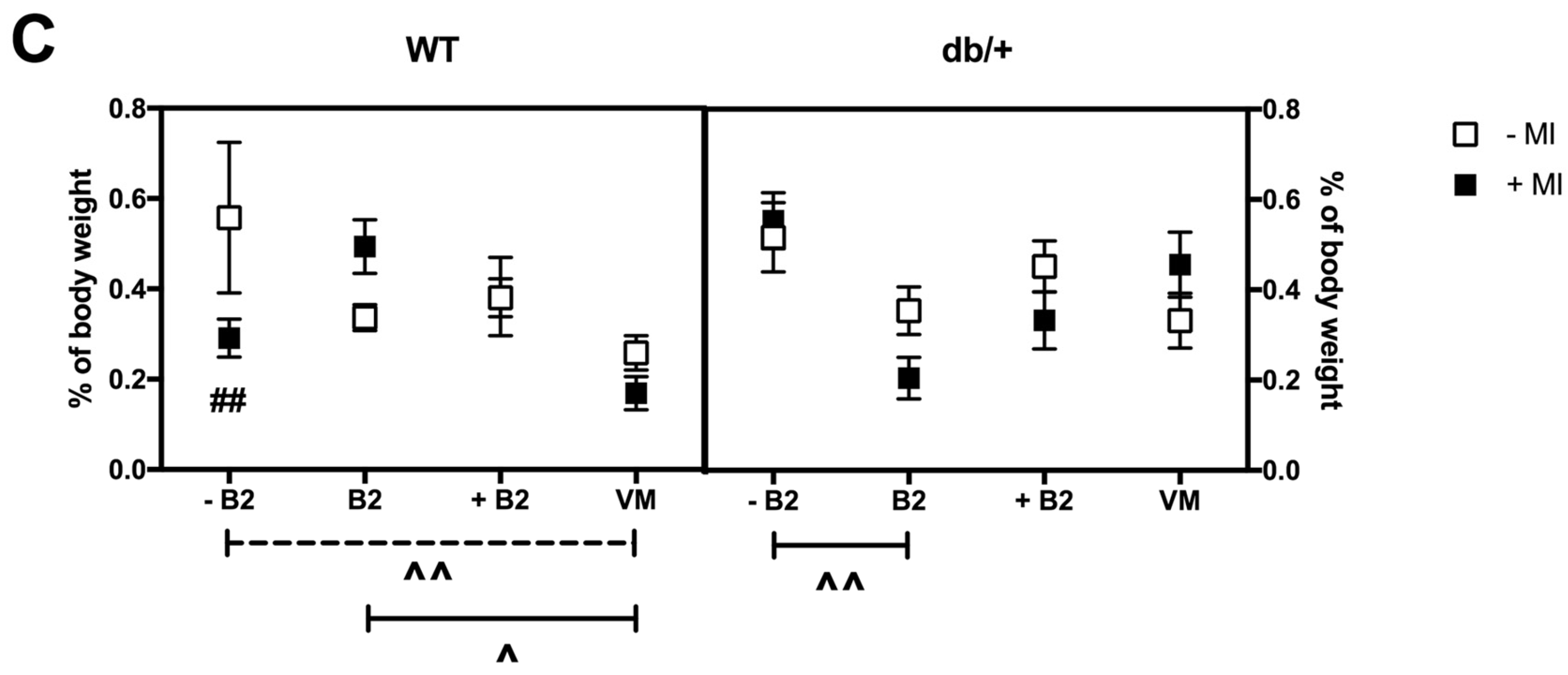
3.6. Fat Distribution at Day 18.5 of Pregnancy
3.6.1. Retroperitoneal Fat
3.6.2. Gonadal Fat
3.6.3. Perirenal Fat
3.7. Foetal Growth at Day 18.5 of Pregnancy
3.8. Gene Expression in Adipose Tissue at Day 18.5 of Pregnancy
3.8.1. Insulin and Leptin Signalling
- (a)
- Akt2: There were no significant three- or two-way interactions between the groups on Akt2 expression. There was a main effect of vitamin status, whereby mice on normal B2 had higher expression of Akt2 than mice on vitamin mix (p = 0.010).
- (b)
- Igf1R: There were no significant three- or two-way interactions between the groups on Igf1R expression. There was a main effect of MI status, whereby mice on MI supplementation had higher expression of Igf1R (p = 0.020). There was also a main effect of vitamin status, whereby mice in the normal B2 group had decreased Igf1R expression compared to those in both the suboptimal B2 group (p = 0.025) and the supplemented B2 group (p = 0.019).
- (c)
- LepR: There was a three-way interaction between genotype, MI, and vitamin status (p = 0.023), as a result of a simple two-way interaction between MI and vitamin status in db/+ mice (p = 0.028). Within the db/+ mice that were supplemented with B2, those not receiving MI had increased LepR expression compared with those receiving MI (p = 0.014). Within the db/+ mice that were not treated with MI, those on supplemented B2 had higher LepR expression than mice on both suboptimal B2 (p = 0.005) and mice on normal B2 (p = 0.010).
- (d)
- IRS1: There were no main effects or interactions between any of the groups on IRS1 expression.
- (e)
- GLUT4: There were no significant three- or two-way interactions between the groups on GLUT4 expression. There was a main effect of vitamin status, whereby mice on normal B2 had higher GLUT4 expression than mice on both suboptimal B2 (p = 0.049) and mice on vitamin mix (p = 0.015).
3.8.2. Lipid Metabolism
- (f)
- Acsl1: There were no main effects or interactions between any of the groups on Acsl1 expression.
3.8.3. Inflammatory Markers
- (g)
- IL-1β: There were no significant three- or two-way interactions between the groups on IL-1β expression. There was a main effect of vitamin status, whereby mice on normal B2 had higher IL-1β expression than those on both supplemented B2 (p = 0.035) and vitamin mix (p = 0.026).
- (h)
- Tlr4: There was no significant three-way interaction between genotype, MI, and vitamin status. There was, however, a significant two-way interaction between MI and vitamin status. Within the mice supplemented with MI, those on supplemented B2 (p = 0.013) and vitamin mix (p = 0.015) had reduced Tlr4 expression compared with those on normal B2.
- (i)
- Tnfrsf1b: There were no main effects or interactions between any of the groups on Tnfrsf1b expression.
- (j)
- Ccr5: There was a three-way interaction between genotype, MI, and vitamin status (p = 0.049), due to a simple two-way interaction between MI and vitamin status in WT mice (p = 0.001). Within the WT mice on normal B2, those receiving MI had greater Ccr5 expression than without MI (p = 0.001). In contrast, Ccr5 expression was reduced when WT mice on suboptimal B2 were supplemented with MI (p = 0.014). Within WT mice not receiving MI, those on suboptimal B2 had higher Ccr5 expression than those on normal B2 (p = 0.002), supplemented B2 (p = 0.016), and vitamin mix (p = 0.001). However, in the presence of MI, there was only a difference between normal B2 and supplemented B2—whereby those on supplemented B2 had reduced Ccr5 expression compared to those mice on normal B2 (p = 0.014).
- (k)
- Nlrp3: db/+ mice had increased Nlrp3 expression compared to WT mice (p = 0.012). There were no significant three- or two-way interactions between the groups on Nlrp3 expression.
3.8.4. Glucose Metabolism
- (l)
- G6pc: There were no significant three- or two-way interactions between the groups on G6pc expression. There was a main effect of MI, whereby those mice supplemented with MI had lower expression of G6pc than those not on MI (p = 0.023).
- (m)
- Gck: There was no significant three-way interaction between genotype, MI, and vitamin status. There was, however, a significant two-way interaction between MI and vitamin status (p = 0.018). Within the mice on normal B2, those receiving MI had increased glucokinase expression (p < 0.0001). This expression was greater than that seen in mice on both suboptimal (p = 0.016) and supplemented B2 (p = 0.017).
- (n)
- Gys1: There were no main effects or interactions between any of the groups on Gys1 expression.
- (o)
- Pck1: There were no significant three- or two-way interactions between the groups on Pck1 expression. There was a main effect of MI, whereby those mice on MI had higher expression of Pck1 (p = 0.043). There was also a main effect of vitamin status, whereby mice on normal B2 had higher expression of Pck1 than those on supplemented B2 (p = 0.003).
3.8.5. Apoptotic Marker
- (p)
- Fas: There was no significant three-way interaction between genotype, MI, and vitamin status on Fas expression. There was, however, a significant two-way interaction between MI and vitamin status (p = 0.047). In the suboptimal B2 group, mice receiving MI had lower expression of Fas compared to those not supplemented with MI (p = 0.045). Within those mice not receiving MI, those on vitamin mix had lower expression of Fas than those in the normal B2 (mean difference of 0.403 (p = 0.031) and suboptimal B2 groups (p = 0.001).
4. Discussion
4.1. Suitability of db/+ Mice as a Model of GDM
4.2. Effects of Myo-Insoitol
4.3. Effects of Suboptimal B2
4.4. Effects of Supplemented B2
4.5. Effects of Vitamin Mix (B2, B6, B12, and D)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kordella, T. American Diabetes Association. Resource guide 2005. New products. Diabetes For. 2005, 58, RG2–RG4. [Google Scholar]
- Ferrara, A. Increasing Prevalence of Gestational Diabetes Mellitus A public health perspective. Diabetes Care 2007, 30, S141–S146. [Google Scholar] [CrossRef] [PubMed]
- Balaji, V.; Madhuri, B.S.; Ashalatha, S.; Sheela, S.; Suresh, S.; Seshiah, V. A1C in Gestational Diabetes Mellitus in Asian Indian Women. Diabetes Care 2007, 30, 1865–1867. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-W.; Yang, H.-X.; Wei, Y.-M.; Yan, J.; Wang, Z.-L.; Li, X.-L.; Wu, H.-R.; Li, N.; Zhang, M.-H.; Liu, X.-H.; et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes Care 2013, 36, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Dong, L.; Zhang, C.P.; Li, B.; Wen, J.; Gao, W.; Sun, S.; Lv, F.; Tian, H.; Tuomilehto, J.; et al. Increasing prevalence of gestational diabetes mellitus in Chinese women from 1999 to 2008. Diabetes Med. J. Br. Diabet. Assoc. 2011, 28, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, I.; Haglund, B.; Hanson, U. Gestational diabetes and preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 113, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, J.; Grant, A.M. The genetics of gestational diabetes mellitus: Evidence for relationship with type 2 diabetes mellitus. Genet. Med. 2008, 10, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Löbner, K.; Knopff, A.; Baumgarten, A.; Mollenhauer, U.; Marienfeld, S.; Garrido-Franco, M.; Bonifacio, E.; Ziegler, A.-G. Predictors of Postpartum Diabetes in Women with Gestational Diabetes Mellitus. Diabetes 2006, 55, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, H. Gestational diabetes mellitus, programing and epigenetics. J. Matern.-Fetal Neonatal Med. 2014, 27, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.A.; Templeton, L.J.; Gertz, S.J. Intrauterine Growth Retardation Leads to the Development of Type 2 Diabetes in the Rat. Diabetes 2001, 50, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Boloker, J.; Gertz, S.J.; Simmons, R.A. Gestational Diabetes Leads to the Development of Diabetes in Adulthood in the Rat. Diabetes 2002, 51, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Brustman, L.E.; Langer, O.; Bimson, B.; Scarpelli, S.; El Daouk, M. Weight gain in gestational diabetes: The effect of treatment modality. J. Matern.-Fetal Neonatal Med. 2016, 29, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Ainuddin, J.; Karim, N.; Hasan, A.A.; Naqvi, S.A. Metformin versus insulin treatment in gestational diabetes in pregnancy in a developing country: A randomized control trial. Diabetes Res. Clin. Pract. 2015, 107, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.S.; Moses, R.G. Metformin Therapy during Pregnancy Good for the goose and good for the gosling too? Diabetes Care 2011, 34, 2329–2330. [Google Scholar] [CrossRef] [PubMed]
- Burton, J.H.; Johnson, M.; Johnson, J.; Hsia, D.S.; Greenway, F.L.; Heiman, M.L. Addition of a Gastrointestinal Microbiome Modulator to Metformin Improves Metformin Tolerance and Fasting Glucose Levels. J. Diabetes Sci. Technol. 2015, 9, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Refuerzo, J.S.; Viteri, O.A.; Hutchinson, M.; Pedroza, C.; Blackwell, S.C.; Tyson, J.E.; Ramin, S.M. The effects of metformin on weight loss in women with gestational diabetes: A pilot randomized, placebo-controlled trial. Am. J. Obstet. Gynecol. 2015, 212, 389.e1–389.e9. [Google Scholar] [CrossRef] [PubMed]
- Gatford, K.L.; Houda, C.M.; Lu, Z.X.; Coat, S.; Baghurst, P.A.; Owens, J.A.; Sikaris, K.; Rowan, J.A.; Hague, W.M. Vitamin B12 and homocysteine status during pregnancy in the metformin in gestational diabetes trial: Responses to maternal metformin compared with insulin treatment. Diabetes Obes. Metab. 2013, 15, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-F.; Chen, X.-Y.; Ding, T.; Wang, X.-F.; Zhu, Z.-N.; Su, S.-W. Comparative efficacy and safety of OADs in management of GDM: Network meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2015, 100, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- Camelo Castillo, W.; Boggess, K.; Stürmer, T.; Brookhart, M.A.; Benjamin, D.K.; Jonsson Funk, M. Association of Adverse Pregnancy Outcomes with Glyburide vs. Insulin in Women with Gestational Diabetes. JAMA Pediatr. 2015, 169, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Chiu, T.T.Y.; Unfer, V.; Carlomagno, G.; Bizzarri, M. The rationale of the myo-inositol and D-chiro-inositol combined treatment for polycystic ovary syndrome. J. Clin. Pharmacol. 2014, 54, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Artini, P.G.; Di Berardino, O.M.; Papini, F.; Genazzani, A.D.; Simi, G.; Ruggiero, M.; Cela, V. Endocrine and clinical effects of myo-inositol administration in polycystic ovary syndrome. A randomized study. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2013, 29, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-F.; Zhang, M.-N.; Wang, T.-X.; Wu, T.-C.; Ai, R.-D.; Zhang, Z.-S. Hypoglycemic effect of d-chiro-inositol in type 2 diabetes mellitus rats through the PI3K/Akt signaling pathway. Mol. Cell. Endocrinol. 2016, 433, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Matarrelli, B.; Vitacolonna, E.; D’Angelo, M.; Pavone, G.; Mattei, P.A.; Liberati, M.; Celentano, C. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: A randomized controlled trial. J. Matern.-Fetal Neonatal Med. 2013, 26, 967–972. [Google Scholar] [CrossRef] [PubMed]
- DʼAnna, R.; Di Benedetto, A.; Scilipoti, A.; Santamaria, A.; Interdonato, M.L.; Petrella, E.; Neri, I.; Pintaudi, B.; Corrado, F.; Facchinetti, F. Myo-inositol Supplementation for Prevention of Gestational Diabetes in Obese Pregnant Women: A Randomized Controlled Trial. Obstet. Gynecol. 2015, 126, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Zheng, Y.; Qi, Q.; Xu, M.; Ley, S.H.; Li, Y.; Kang, J.H.; Wiggs, J.; Pasquale, L.R.; Chan, A.T.; et al. DNA Methylation Variants at HIF3A Locus, B-Vitamin Intake, and Long-term Weight Change: Gene-Diet Interactions in Two U.S. Cohorts. Diabetes 2015, 64, 3146–3154. [Google Scholar] [CrossRef] [PubMed]
- Zykova, S.N.; Storhaug, H.M.; Toft, I.; Chadban, S.J.; Jenssen, T.G.; White, S.L. Cross-sectional analysis of nutrition and serum uric acid in two Caucasian cohorts: The AusDiab Study and the Tromsø study. Nutr. J. 2015, 14, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, D.; Bird, J.K.; McBurney, M.I.; Chapman-Novakofski, K.M. Nutritional status as assessed by nutrient intakes and biomarkers among women of childbearing age—Is the burden of nutrient inadequacies growing in America? Public Health Nutr. 2015, 18, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Nix, W.A.; Zirwes, R.; Bangert, V.; Kaiser, R.P.; Schilling, M.; Hostalek, U.; Obeid, R. Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes Res. Clin. Pract. 2015, 107, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.; Ratner, R.; Summergrad, P. Kynurenines and vitamin B6: Link between diabetes and depression. J. Bioinform. Diabetes 2013, 1. [Google Scholar] [CrossRef]
- Oxenkrug, G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 2013, 48, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, G.V.; Hill, J.C.; Veena, S.R.; Bhat, D.S.; Wills, A.K.; Karat, C.L.S.; Yajnik, C.S.; Fall, C.H.D. Low plasma vitamin B12 in pregnancy is associated with gestational “diabesity” and later diabetes. Diabetologia 2009, 52, 2350–2358. [Google Scholar] [CrossRef] [PubMed]
- Knight, B.A.; Shields, B.M.; Brook, A.; Hill, A.; Bhat, D.S.; Hattersley, A.T.; Yajnik, C.S. Lower Circulating B12 Is Associated with Higher Obesity and Insulin Resistance during Pregnancy in a Non-Diabetic White British Population. PLoS ONE 2015, 10, e0135268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukumar, N.; Venkataraman, H.; Wilson, S.; Goljan, I.; Selvamoni, S.; Patel, V.; Saravanan, P. Vitamin B12 Status among Pregnant Women in the UK and Its Association with Obesity and Gestational Diabetes. Nutrients 2016, 8, 768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-X.; Pan, G.-T.; Guo, J.-F.; Li, B.-Y.; Qin, L.-Q.; Zhang, Z.-L. Vitamin D Deficiency Increases the Risk of Gestational Diabetes Mellitus: A Meta-Analysis of Observational Studies. Nutrients 2015, 7, 8366–8375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qiu, C.; Hu, F.B.; David, R.M.; van Dam, R.M.; Bralley, A.; Williams, M.A. Maternal Plasma 25-Hydroxyvitamin D Concentrations and the Risk for Gestational Diabetes Mellitus. PLoS ONE 2008, 3, e3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, O.; Li, W.; Ma, L.; Ping, F.; Chen, L.; Nie, M. Variants in Vitamin D Binding Protein Gene Are Associated with Gestational Diabetes Mellitus. Medicine (Baltimore) 2015, 94, e1693. [Google Scholar] [CrossRef] [PubMed]
- Meinilä, J.; Koivusalo, S.B.; Valkama, A.; Rönö, K.; Erkkola, M.; Kautiainen, H.; Stach-Lempinen, B.; Eriksson, J.G. Nutrient intake of pregnant women at high risk of gestational diabetes. Food Nutr. Res. 2015, 59, 26676. [Google Scholar] [CrossRef] [PubMed]
- Powers, H.J.; Corfe, B.M.; Nakano, E. Riboflavin in development and cell fate. Subcell. Biochem. 2012, 56, 229–245. [Google Scholar] [PubMed]
- Christensen, B.C.; Marsit, C.J. Epigenomics in Environmental Health. Front. Genet. 2011, 2, 84. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Wang, J.; Yang, M.; Shao, Y.; Liu, J.; Wu, Q.; Xu, Q.; Wang, H.; He, X.; Chen, Y.; et al. Serum homocysteine level and gestational diabetes mellitus: A meta-analysis. J. Diabetes Investig. 2016, 7, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Guven, M.A.; Kilinc, M.; Batukan, C.; Ekerbicer, H.C.; Aksu, T. Elevated second trimester serum homocysteine levels in women with gestational diabetes mellitus. Arch. Gynecol. Obstet. 2006, 274, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Seghieri, G.; Breschi, M.C.; Anichini, R.; De Bellis, A.; Alviggi, L.; Maida, I.; Franconi, F. Serum homocysteine levels are increased in women with gestational diabetes mellitus. Metabolism 2003, 52, 720–723. [Google Scholar] [CrossRef]
- Tarim, E.; Bagis, T.; Kilicdag, E.; Erkanli, S.; Aslan, E.; Sezgin, N.; Kuscu, E. Elevated plasma homocysteine levels in gestational diabetes mellitus. Acta Obstet. Gynecol. Scand. 2004, 83, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Vanhees, K.; Vonhögen, I.G.C.; van Schooten, F.J.; Godschalk, R.W.L. You are what you eat, and so are your children: The impact of micronutrients on the epigenetic programming of offspring. Cell. Mol. Life Sci. CMLS 2014, 71, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Karamali, M.; Taghizadeh, M.; Sharifi, N.; Jafari, Z.; Memarzadeh, M.R.; Mahlouji, M.; Asemi, Z. Vitamin D and Evening Primrose Oil Administration Improve Glycemia and Lipid Profiles in Women with Gestational Diabetes. Lipids 2016, 51, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Lapik, I.A.; Sokol’nikov, A.A.; Sharafetdinov, K.K.; Sentsova, T.B.; Plotnikova, O.A. Assessment of efficiency of dietotherapy with addition of a vitamin-mineral complex in patients with diabetes mellitus type 2. Vopr. Pitan. 2014, 83, 74–81. [Google Scholar] [PubMed]
- Bennink, H.J.; Schreurs, W.H. Improvement of oral glucose tolerance in gestational diabetes by pyridoxine. Br. Med. J. 1975, 3, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Spellacy, W.N.; Buhi, W.C.; Birk, S.A. Vitamin B6 treatment of gestational diabetes mellitus: Studies of blood glucose and plasma insulin. Am. J. Obstet. Gynecol. 1977, 127, 599–602. [Google Scholar] [CrossRef]
- Shahgheibi, S.; Farhadifar, F.; Pouya, B. The effect of vitamin D supplementation on gestational diabetes in high-risk women: Results from a randomized placebo-controlled trial. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2016, 21, 2. [Google Scholar]
- Asemi, Z.; Hashemi, T.; Karamali, M.; Samimi, M.; Esmaillzadeh, A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: A double-blind randomized controlled clinical trial. Am. J. Clin. Nutr. 2013, 98, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Katre, P.; Bhat, D.; Lubree, H.; Otiv, S.; Joshi, S.; Joglekar, C.; Rush, E.; Yajnik, C. Vitamin B12 and folic acid supplementation and plasma total homocysteine concentrations in pregnant Indian women with low B12 and high folate status. Asia Pac. J. Clin. Nutr. 2010, 19, 335–343. [Google Scholar] [PubMed]
- Alam, M.M.; Iqbal, S.; Naseem, I. Ameliorative effect of riboflavin on hyperglycemia, oxidative stress and DNA damage in type-2 diabetic mice: Mechanistic and therapeutic strategies. Arch. Biochem. Biophys. 2015, 584, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Fan, Y.; Xiang, J.; Zhan, M. Effect of the folic acid and vitamin B2 on the diabetes mellitus rats with diabetic nephropathy. Wei Sheng Yan Jiu 2012, 41, 911–915. [Google Scholar] [PubMed]
- Mazur-Bialy, A.I.; Pocheć, E. Riboflavin Reduces Pro-Inflammatory Activation of Adipocyte-Macrophage Co-culture. Potential Application of Vitamin B2 Enrichment for Attenuation of Insulin Resistance and Metabolic Syndrome Development. Molecules 2016, 21, 1724. [Google Scholar] [CrossRef] [PubMed]
- Nutritional Intervention Preconception and during Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health. Available online: https://clinicaltrials.gov/ct2/show/NCT02509988 (accessed on 26 January 2017).
- Yamashita, H.; Shao, J.; Qiao, L.; Pagliassotti, M.; Friedman, J.E. Effect of spontaneous gestational diabetes on fetal and postnatal hepatic insulin resistance in Lepr(db/+) mice. Pediatr. Res. 2003, 53, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Shao, J.; Ishizuka, T.; Klepcyk, P.J.; Muhlenkamp, P.; Qiao, L.; Hoggard, N.; Friedman, J.E. Leptin administration prevents spontaneous gestational diabetes in heterozygous Lepr(db/+) mice: Effects on placental leptin and fetal growth. Endocrinology 2001, 142, 2888–2897. [Google Scholar] [PubMed]
- Nadif, R.; Dilworth, M.R.; Sibley, C.P.; Baker, P.N.; Davidge, S.T.; Gibson, J.M.; Aplin, J.D.; Westwood, M. The maternal environment programs postnatal weight gain and glucose tolerance of male offspring, but placental and fetal growth are determined by fetal genotype in the Leprdb/+ model of gestational diabetes. Endocrinology 2015, 156, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, R.C.; Amankwah, K.S.; Dunaway, G.; Maroun, L.; Arbuthnot, J.; Roddick, J.W., Jr. An animal model of gestational diabetes. Am. J. Obstet. Gynecol. 1981, 141, 479–482. [Google Scholar] [CrossRef]
- Li, D.; Rozen, R. Maternal folate deficiency affects proliferation, but not apoptosis, in embryonic mouse heart. J. Nutr. 2006, 136, 1774–1778. [Google Scholar] [PubMed]
- Li, D.; Pickell, L.; Liu, Y.; Wu, Q.; Cohn, J.S.; Rozen, R. Maternal methylenetetrahydrofolate reductase deficiency and low dietary folate lead to adverse reproductive outcomes and congenital heart defects in mice. Am. J. Clin. Nutr. 2005, 82, 188–195. [Google Scholar] [PubMed]
- Ciappio, E.D.; Liu, Z.; Brooks, R.S.; Mason, J.B.; Bronson, R.T.; Crott, J.W. Maternal B vitamin supplementation from preconception through weaning suppresses intestinal tumorigenesis in Apc1638N mouse offspring. Gut 2011, 60, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.K.; Belfi, K.; Chua, M.; Wiley, J.; Mackintosh, R.; Nicolson, M.; Boozer, C.N.; Leibel, R.L. Heterozygosity for Lepob or Leprdb affects body composition and leptin homeostasis in adult mice. Am. J. Physiol. 1998, 274, R985–R990. [Google Scholar] [PubMed]
- Lambin, S.; van Bree, R.; Caluwaerts, S.; Vercruysse, L.; Vergote, I.; Verhaeghe, J. Adipose tissue in offspring of Lepr(db/+) mice: Early-life environment vs. genotype. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E262–E271. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.L.; Cheung, C.C.; Rueda-Clausen, C.F.; Sankaralingam, S.; Baker, P.N.; Davidge, S.T. Effect of gestational diabetes on maternal artery function. Reprod. Sci. 2011, 18, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Harrod, J.S.; Rada, C.C.; Pierce, S.L.; England, S.K.; Lamping, K.G. Altered contribution of RhoA/Rho kinase signaling in contractile activity of myometrium in leptin receptor-deficient mice. Am. J. Physiol.—Endocrinol. Metab. 2011, 301, E362–E369. [Google Scholar] [CrossRef] [PubMed]
- Pollock, K.E.; Stevens, D.; Pennington, K.A.; Thaisrivongs, R.; Kaiser, J.; Ellersieck, M.R.; Miller, D.K.; Schulz, L.C. Hyperleptinemia during pregnancy decreases adult weight of offspring and is associated with increased offspring locomotor activity in mice. Endocrinology 2015, 56, 3777–3790. [Google Scholar] [CrossRef] [PubMed]
- Keubler, L.M.; Buettner, M.; Häger, C.; Bleich, A. A Multihit Model: Colitis Lessons from the Interleukin-10–deficient Mouse. Inflamm. Bowel Dis. 2015, 21, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Sviderskaya, E.V.; Novak, E.K.; Swank, R.T.; Bennett, D.C. The murine misty mutation: Phenotypic effects on melanocytes, platelets and brown fat. Genetics 1998, 148, 381–390. [Google Scholar] [PubMed]
- Vickers, M.H.; Sloboda, D.M. Leptin as mediator of the effects of developmental programming. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Maple-Brown, L.; Ye, C.; Hanley, A.J.; Connelly, P.W.; Sermer, M.; Zinman, B.; Retnakaran, R. Maternal Pregravid Weight Is the Primary Determinant of Serum Leptin and Its Metabolic Associations in Pregnancy, Irrespective of Gestational Glucose Tolerance Status. J. Clin. Endocrinol. Metab. 2012, 97, 4148–4155. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Kucuk, M.; Ilgin, A.; Dagdelen, M. Assessment of insulin sensitivity/resistance and their relations with leptin concentrations and anthropometric measures in a pregnant population with and without gestational diabetes mellitus. J. Diabetes Complicat. 2010, 24, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Pétrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate Immune Activation Through Nalp3 Inflammasome Sensing of Asbestos and Silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Halle, A.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Tack, C.J.; Stienstra, R.; Joosten, L.A.B.; Netea, M.G. Inflammation links excess fat to insulin resistance: The role of the interleukin-1 family. Immunol. Rev. 2012, 249, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Saberi, M.; Woods, N.-B.; de Luca, C.; Schenk, S.; Lu, J.C.; Bandyopadhyay, G.; Verma, I.M.; Olefsky, J.M. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009, 10, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Joosten, L.A.B.; Koenen, T.; van Tits, B.; van Diepen, J.A.; van den Berg, S.A.A.; Rensen, P.C.N.; Voshol, P.J.; Fantuzzi, G.; Hijmans, A.; et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010, 12, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, D.F.; Coope, A.; Caricilli, A.M.; Prada, P.O.; Saad, M.J.; Velloso, L.A.; Araujo, E.P. Inhibition of 72 kDa inositol polyphosphate 5-phosphatase E improves insulin signal transduction in diet-induced obesity. J. Endocrinol. 2013, 217, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Rui, L. Recent advances in understanding leptin signaling and leptin resistance. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1247–E1259. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, M.; Claret, M. Recent Insights into the Role of Hypothalamic AMPK Signaling Cascade upon Metabolic Control. Front. Neurosci. 2012, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Softic, S.; Ouaamari, A.E.; Krumpoch, M.T.; Kleinridders, A.; Kulkarni, R.N.; O’Neill, B.T.; Kahn, C.R. Differential Roles of Insulin and IGF-1 Receptors in Adipose Tissue Development and Function. Diabetes 2016, 65, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Jeoung, N.H.; Harris, R.A. Role of pyruvate dehydrogenase kinase 4 in regulation of blood glucose levels. Korean Diabetes J. 2010, 34, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, S.; Franckhauser, S.; Elias, I.; Ferré, T.; Hidalgo, A.; Monteys, A.M.; Molas, M.; Cerdán, S.; Pujol, A.; Ruberte, J.; et al. Chronically increased glucose uptake by adipose tissue leads to lactate production and improved insulin sensitivity rather than obesity in the mouse. Diabetologia 2010, 53, 2417–2430. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.C.; Oeser, J.K.; Svitek, C.A.; Hunter, S.I.; Hutton, J.C.; O’Brien, R.M. Identification and characterization of a human cDNA and gene encoding a ubiquitously expressed glucose-6-phosphatase catalytic subunit-related protein. J. Mol. Endocrinol. 2002, 29, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Seril, D.N.; Yang, A.L.; Lu, G.G.; Yang, G.-Y. Inhibition of chronic ulcerative colitis associated adenocarcinoma development in mice by inositol compounds. Carcinogenesis 2007, 28, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Donà, G.; Sabbadin, C.; Fiore, C.; Bragadin, M.; Giorgino, F.L.; Ragazzi, E.; Clari, G.; Bordin, L.; Armanini, D. Inositol administration reduces oxidative stress in erythrocytes of patients with polycystic ovary syndrome. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 2012, 166, 703–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitade, H.; Sawamoto, K.; Nagashimada, M.; Inoue, H.; Yamamoto, Y.; Sai, Y.; Takamura, T.; Yamamoto, H.; Miyamoto, K.; Ginsberg, H.N.; et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes 2012, 61, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Gunanti, I.R.; Marks, G.C.; Al-Mamun, A.; Long, K.Z. Low serum vitamin B-12 and folate concentrations and low thiamin and riboflavin intakes are inversely associated with greater adiposity in Mexican American children. J. Nutr. 2014, 144, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Salas, P.; Cox, S.E.; Prentice, A.M.; Hennig, B.J.; Moore, S.E. Maternal nutritional status, C1 metabolism and offspring DNA methylation: A review of current evidence in human subjects. Proc. Nutr. Soc. 2012, 71, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Dick, K.J.; Nelson, C.P.; Tsaprouni, L.; Sandling, J.K.; Aïssi, D.; Wahl, S.; Meduri, E.; Morange, P.-E.; Gagnon, F.; Grallert, H.; et al. DNA methylation and body-mass index: A genome-wide analysis. Lancet 2014, 383, 1990–1998. [Google Scholar] [CrossRef]
- Mahalle, N.; Kulkarni, M.V.; Naik, S.S.; Garg, M.K. Association of dietary factors with insulin resistance and inflammatory markers in subjects with diabetes mellitus and coronary artery disease in Indian population. J. Diabetes Complicat. 2014, 28, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Morrison, E.Y.; Smith-Richardson, S.; West, M.; Brooks, S.E.; Pascoe, K.; Fletcher, C. Toxicity of the hyperglycaemic-inducing extract of the annatto (Bixa orellana) in the dog. West Indian Med. J. 1987, 36, 99–103. [Google Scholar] [PubMed]
- Prado, E.L.; Alcock, K.J.; Muadz, H.; Ullman, M.T.; Shankar, A.H. Maternal Multiple Micronutrient Supplements and Child Cognition: A Randomized Trial in Indonesia. Pediatrics 2012, 130, e536–e546. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Sun, C.; Zhou, X.; Wang, H.; Ren, L. Effect of dietary mineral nutrients and vitamins on metabolism of rat fed with high fat. Wei Sheng Yan Jiu 2004, 33, 447–449. [Google Scholar] [PubMed]
- Dror, D.K.; Allen, L.H. Interventions with vitamins B6, B12 and C in pregnancy. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. 1), 55–74. [Google Scholar] [CrossRef] [PubMed]
- Hovdenak, N.; Haram, K. Influence of mineral and vitamin supplements on pregnancy outcome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 164, 127–132. [Google Scholar] [CrossRef] [PubMed]

| Diet | MI (myo-inositol) | B2 | B6, B12, D |
|---|---|---|---|
| Suboptimal B2 (−B2) | - | 1 mg | 7 mg, 25 μg, 1000 IU |
| Suboptimal B2 + MI (−B2 + MI) | 10 g | 1 mg | 7 mg, 25 μg, 1000 IU |
| AIN-93G (B2) | - | 6 mg | 7 mg, 25 μg, 1000 IU |
| MI only (B2 + MI) | 10 g | 6 mg | 7 mg, 25 μg, 1000 IU |
| B2 only (+B2) | - | 24 mg | 7 mg, 25 μg, 1000 IU |
| B2 + MI (+B2 + MI) | 10 g | 24 mg | 7 mg, 25 μg, 1000 IU |
| Vitamin Mix (VM) | - | 24 mg | 28 mg, 215 μg, 4000 IU |
| MI + vitamin mix (VM + MI) | 10 g | 24 mg | 28 mg, 215 μg, 4000 IU |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plows, J.F.; Budin, F.; Andersson, R.A.M.; Mills, V.J.; Mace, K.; Davidge, S.T.; Vickers, M.H.; Baker, P.N.; Silva-Zolezzi, I.; Stanley, J.L. The Effects of Myo-Inositol and B and D Vitamin Supplementation in the db/+ Mouse Model of Gestational Diabetes Mellitus. Nutrients 2017, 9, 141. https://doi.org/10.3390/nu9020141
Plows JF, Budin F, Andersson RAM, Mills VJ, Mace K, Davidge ST, Vickers MH, Baker PN, Silva-Zolezzi I, Stanley JL. The Effects of Myo-Inositol and B and D Vitamin Supplementation in the db/+ Mouse Model of Gestational Diabetes Mellitus. Nutrients. 2017; 9(2):141. https://doi.org/10.3390/nu9020141
Chicago/Turabian StylePlows, Jasmine F., Florence Budin, Rebecka A. M. Andersson, Valerie J. Mills, Katherine Mace, Sandra T. Davidge, Mark H. Vickers, Philip N. Baker, Irma Silva-Zolezzi, and Joanna L. Stanley. 2017. "The Effects of Myo-Inositol and B and D Vitamin Supplementation in the db/+ Mouse Model of Gestational Diabetes Mellitus" Nutrients 9, no. 2: 141. https://doi.org/10.3390/nu9020141





