A Mediterranean Diet to Improve Cardiovascular and Cognitive Health: Protocol for a Randomised Controlled Intervention Study
Abstract
:1. Introduction
1.1. Background
1.2. Dietary Intervention
The Mediterranean Diet
1.3. Dairy Foods
1.4. Objectives
2. Materials and Methods
2.1. Ethics
2.2. Participants
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.2.3. Recruitment and Screening
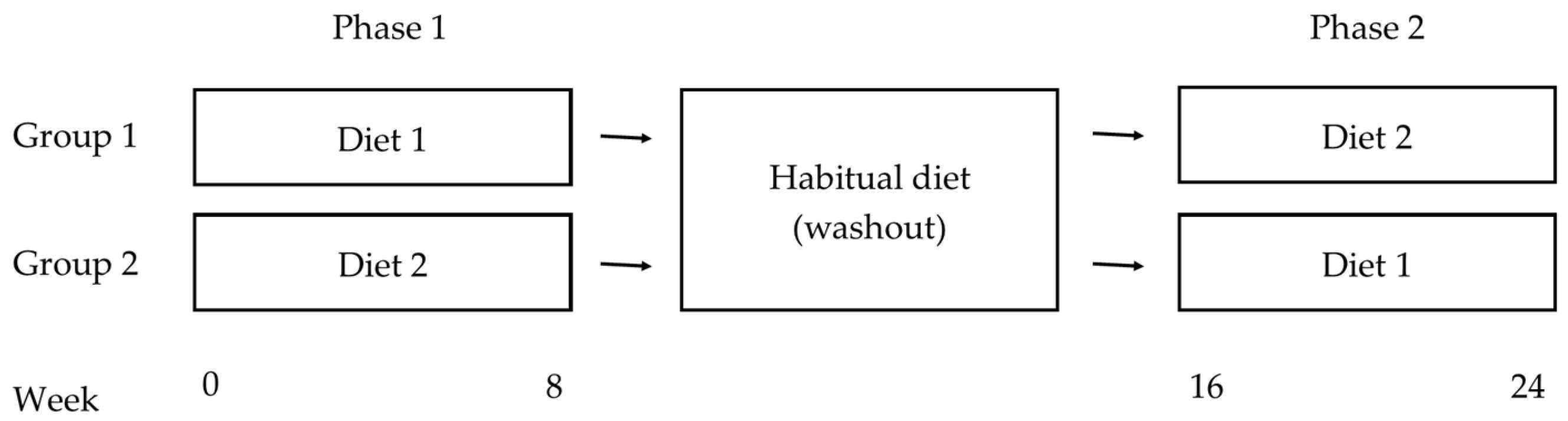
2.3. Design
2.4. Dietary Interventions
2.4.1. The Low-Fat Diet (LFD)
2.4.2. The Mediterranean Diet Supplemented with Dairy (MedD)
- 3–4 daily servings of dairy foods (one serve = 250 mL low fat milk, 40–120 g hard and/or semisoft to soft cheese, 200 g low fat Greek yoghurt, or 200 g tzatziki dip);
- No more than one serving of cheese (any type) per day (one serve = 40 g hard, 50 g semi-soft, or 120 g soft cheese);
- Minimum of one tablespoon (20 mL) of EVOO per day;
- ≥2–3 daily servings of fresh fruit (one serve = 150 g fresh, 40 g dried, or one cup canned in juice);
- ≥3 weekly servings of legumes (one serve = 75 g);
- ≥3 weekly servings of fish and seafood (at least one serving of oily fish) (one serve = 100 g cooked);
- ≥3 weekly serving of nuts or seeds (one serve = 30 g; 7.5 g hazelnuts, 15 g walnuts, 7.5 g almonds supplied for each serve);
- Ad-libitum consumption of wholegrain cereal products (bread, pasta, rice, cereal), nuts, fish, eggs and raw and cooked vegetables;
- Select white meats (poultry without skin) instead of red meats or processed meats;
- Limit consumption of red meat (remove all visible fat), cured ham and chocolate to ≤1 serve/week (one serve of red meat/cured ham = 100 g; one serve of chocolate = 50 g);
- Use EVOO for cooking and dressing vegetables and salad;
- Cook regularly (at least twice a week) with a tomato based sauce (EVOO, tomato, garlic and onion);
- Dress vegetables, pasta, rice and other dishes with EVOO, tomato, garlic and onion sauce;
- Eliminate or limit the consumption of cream, butter, margarine, cold meat, pate, duck, carbonated and or sugared beverages, pastries, commercial bakery products (cakes, donuts, cookies), desserts (puddings), French fries, potato crisps, sweets;
- For usual drinkers, red wine is recommended as the main source of alcohol with a maximum of two standard drinks per day (200 mL = two standard drinks) [125].
2.4.3. Dietetic Counselling
2.5. Outcomes
2.5.1. Blood Pressure
2.5.2. Secondary Outcomes
Cognitive Performance, Dementia Risk and Mood
Diet Adherence
2.6. Procedure
Cognitive Measures
2.7. Power Calculation and Statistical Analyses
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A


Appendix B

Appendix C

Appendix D

Appendix E

Appendix F

Appendix G

References
- World Health Organisation. Global Status Report on Noncommunicable Diseases 2014; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Alzheimer’s Disease International. World Alzheimer Report 2015 the Global Impact of Dementia; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Goss, J. Projection of Australian Health Care Expenditure by Disease, 2003 to 2033; Australian Institute of Health and Welfare: Canberra, Australia, 2008. [Google Scholar]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the future of cardiovascular disease in the united states: A policy statement from the american heart association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. Mech. Dis. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Hofman, A.; Ott, A.; Breteler, M.M.; Bots, M.L.; Slooter, A.J.; van Harskamp, F.; van Duijn, C.N.; Van Broeckhoven, C.; Grobbee, D.E. Atherosclerosis, apolipoprotein E, and prevalence of dementia and alzheimer’s disease in the rotterdam study. Lancet 1997, 349, 151–154. [Google Scholar] [CrossRef]
- Mendis, S.; Puska, P.; Norrving, B. Global Atlas on Cardiovascular Disease Prevention and Control; WHO: Geneva, Switzerland, 2011; pp. 2–14. [Google Scholar]
- Debette, S.; Seshadri, S.; Beiser, A.; Au, R.; Himali, J.J.; Palumbo, C.; Wolf, P.A.; DeCarli, C. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011, 77, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.I.L.; Leritz, E.C.; Williams, V.J.; van Boxtel, M.P.J.; Elst, W.V.D.; Jolles, J.; Verhey, F.R.J.; McGlinchey, R.E.; Milberg, W.P.; Salat, D.H. Association between white matter microstructure, executive functions, and processing speed in older adults: The impact of vascular health. Hum. Brain Mapp. 2013, 34, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Rodrigue, K.M.; Acker, J.D. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behav. Neurosci. 2003, 117, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Rosas, H.D.; Salat, D.H. Age-associated reductions in cerebral blood flow are independent from regional atrophy. NeuroImage 2011, 55, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Justin, B.N.; Turek, M.; Hakim, A.M. Heart disease as a risk factor for dementia. Clin. Epidemiol. 2013, 5, 135–145. [Google Scholar] [PubMed]
- Kelleher, R.J.; Soiza, R.L. Evidence of endothelial dysfunction in the development of Alzheimer’s disease: Is alzheimer’s a vascular disorder? Am. J. Cardiovasc. Dis. 2013, 3, 197–226. [Google Scholar] [PubMed]
- Norton, S.; Matthews, F.E.; Barnes, D.E.; Yaffe, K.; Brayne, C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014, 13, 788–794. [Google Scholar] [CrossRef]
- Hobbs, F.D.R. Cardiovascular disease: Different strategies for primary and secondary prevention? Heart 2004, 90, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.; Flock, M.; Kris-Etherton, P. The role of diet in the prevention and treatment of cardiovascular disease. Nutr. Prev. Treat. Dis. 2013, 2, 541–567. [Google Scholar]
- Andersen, C.J.; Fernandez, M.L. Dietary strategies to reduce metabolic syndrome. Rev. Endocr. Metab. Disord. 2013, 14, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. Clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Chanalia, M.; Garg, A.; Lutjhonatann, D.; von Bergmann, K.; Grundy, S.M.; Brinkley, L.J. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N. Engl. J. Med. 2015, 342, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Cutler, J.A.; Allender, P.S. Randomized trials of sodium reduction: An overview. Am. J. Clin. Nutr. 1997, 65, 643–651. [Google Scholar]
- Flock, M.R.; Fleming, J.A.; Kris-Etherton, P. Macronutrient replacement options for saturated fat: Effects on cardiovascular health author. Curr. Opin. Lipidol. 2014, 25, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Jonge, L.D.; Frisard, M.I.; Delany, J.P.; Enette, D.; Meyer, L.; Rood, J.; Nguyen, T.; Martin, C.K.; Volaufova, J.; et al. Effect of 6 month calorie restriction on biomarkers of longevity, metabolic adaptation and oxidative stress in overweight subjects. J. Am. Med. Assoc. 2006, 295, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Skeaff, C.M.; Chisholm, W.A. The effect of replacing dietary saturated fat with polyunsaturated or monounsaturated fat on plasma lipids in free-living young adults. Eur. J. Clin. Nutr. 2001, 55, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, M.; Yang, H.; Min, C. British food journal dietary fiber role in type 2 diabetes prevention. Br. Food J. Br. 2016, 118, 961–975. [Google Scholar] [CrossRef]
- Lindström, J.; Peltonen, M.; Eriksson, J.G.; Louheranta, A.; Fogelholm, M.; Uusitupa, M.; Tuomilehto, J. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: The finnish diabetes prevention study. Diabetologia 2006, 49, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A dietary approach to prevent hypertension: A review of the dietary approaches to stop hypertension (dash) study. Clin. Cardiol. 1999, 22, III6–III10. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadou, K.; Weech, M.; Altowaijiri, H.; Todd, S.; Yaqoob, P.; Jackson, K.G.; Lovegrove, J.A. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: Results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am. J. Clin. Nutr. 2015, 102, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, T.N.; Brunner, E.J.; Ferrie, J.E.; Marmot, M.G.; Kivimaki, M.; Singh-Manoux, A. Dietary pattern and depressive symptoms in middle age. Br. J. Psychiatry 2009, 195, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Daffner, K.R. Promoting successful cognitive aging: A comprehensive review. J. Alzheimer’s Dis. 2010, 19, 1101–1122. [Google Scholar]
- Fusco, S.; Pani, G. Brain response to calorie restriction. Cell. Mol. Life Sci. 2013, 70, 3157–3170. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. Mind diet slows cognitive decline with aging. Alzheimer’s Dement. 2015, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Myint, P.K.; Welch, A.A.; Bingham, S.A.; Surtees, P.G.; Wainwright, N.W.J.; Luben, R.N.; Wareham, N.J.; Smith, R.D.; Harvey, I.M.; Day, N.E.; et al. Fruit and vegetable consumption and self-reported functional health in men and women in the European prospective investigation into cancer-norfolk (epic-norfolk): A population-based cross-sectional study. Public Health Nutr. 2007, 10, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Opie, R.S.; O’Neil, A.; Itsiopoulos, C.; Jacka, F.N. The impact of whole-of-diet interventions on depression and anxiety: A systematic review of randomised controlled trials. Public Health Nutr. 2014, 18, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Blumenthal, J.A.; Babyak, M.A.; Craighead, L.; Welsh-Bohmer, K.A.; Browndyke, J.N.; Strauman, T.A.; Sherwood, A. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension 2010, 55, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Shinde, S.; Moodley, Y.; Lundberg, J.O.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Absence of an effect of high nitrate intake from beetroot juice on blood pressure in treated hypertensive individuals: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The attica study. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [PubMed]
- Hodgson, J.M.; Croft, K.D.; Woodman, R.J.; Puddey, I.B.; Fuchs, D.; Draijer, R.; Lukoshkova, E.; Head, G.A. Black tea lowers the rate of blood pressure variation: A randomized. Am. J. Clin. Nutr. 2013, 97, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Luna, R.; Muñoz-Hernandez, R.; Miranda, M.L.; Costa, A.F.; Jimenez-Jimenez, L.; Vallejo-Vaz, A.J.; Muriana, F.J.G.; Villar, J.; Stiefel, P. Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. Am. J. Hypertens. 2012, 25, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Garcia-Arellano, A.; Estruch, R.; Marquez-Sandoval, F.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Viñoles, E.; Arós, F.; Herrera, C.; et al. Components of the mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur. J. Clin. Nutr. 2008, 62, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of olive oil on markers of inflammation and endothelial function: A systematic review and meta-analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [PubMed]
- Andres-Lacueva, C.; Shukitt-Hale, B.; Galli, R.L.; Jauregui, O.; Lamuela-Raventos, R.M.; Joseph, J.A. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr. Neurosci. 2005, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Field, D.T.; Williams, C.M.; Butler, L.T. Consumption of cocoa flavanols results in an acute improvement in visual and cognitive functions. Physiol. Behav. 2011, 103, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The cocoa, cognition, and aging (cocoa) study—A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.M.; El Mohsen, M.A.; Vauzour, D.; Rendeiro, C.; Butler, L.T.; Ellis, J.A.; Whiteman, M.; Spencer, J.P.E. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal creb phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 2008, 45, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The cocoa, cognition, and aging (cocoa) study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.R.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean diet: A literature review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Féart, C.; Samieri, C.; Rondeau, V.; Amieva, H.; Portet, F.; Dartigues, J.-F.; Scarmeas, N.; Barberger-Gateau, P. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. J. Am. Med. Assoc. 2009, 302, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. Psychiatry 2009, 66, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Stern, Y.; Tang, M.-X.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2011, 59, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Dimitrios, T. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E. Benefits of the Mediterranean diet: Insights from the predimed study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Romero-Mamani, E.S.; Gilabert, R.; Núñez, I.; de La Torre, R.; Corella, D.; Ruiz-Gutiérrez, V.; López-Sabater, M.C.; Pint, X.; Rekondo, J.; et al. Changes in ultrasound-assessed carotid intima-media thickness and plaque with a Mediterranean diet: A substudy of the predimed trial. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Pase, M.; Pipingas, A.; Raubenheimer, J.; Thurgood, M.; Villalon, L.; Macpherson, H.; Gibbs, A.; Scholey, A. Switching to a 10-day Mediterranean-style diet improves mood and cardiovascular function in a controlled crossover study. Nutrition 2015, 31, 647–652. [Google Scholar] [CrossRef] [PubMed]
- McMillan, L.; Owen, L.; Kras, M.; Scholey, A. Behavioural effects of a 10-day Mediterranean diet. Results from a pilot study evaluating mood and cognitive performance. Appetite 2011, 56, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Ngandu, T.; Laatikainen, T.; Winblad, B.; Soininen, H.; Tuomilehto, J. Risk score for the prediction of dementia risk in 20 years among middle aged people: A longitudinal, population-based study. Lancet Neurol. 2006, 5, 735–741. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (finger): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lapiscina, E.H.; Clavero, P.; Toledo, E.; Estruch, R.; Salas-Salvado, J.; San Julian, B.; Sanchez-Tainta, A.; Ros, E.; Valls-Pedret, C.; Martinez-Gonzalez, M.A. Mediterranean diet improves cognition: The predimed-navarra randomised trial. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Valls-Pedret, C.; Lamuela-Raventós, R.M.; Medina-Remón, A.; Quintana, M.; Corella, D.; Pintó, X.; Martínez-González, A.; Estruch, R.; Ros, E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J. Alzheimer’s Dis. 2012, 29, 773–782. [Google Scholar]
- Creegan, R.; Hunt, W.; McManus, A.; Rainey-Smith, S.R. Diet, nutrients and metabolism: Cogs in the wheel driving Alzheimer’s disease pathology? Br. J. Nutr. 2015, 113, 1499–1517. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.; Calvaresi, E.; Hughes, D. Short-term folate, vitamin B-12 or vitamin B-6 supplementation slightly affects memory performance but not mood in women of various ages. J. Nutr. 2002, 132, 1345–1356. [Google Scholar] [PubMed]
- Mazloom, Z.; Ekramzadeh, M.; Hejazi, N. Efficacy of supplementary vitamins C and E on anxiety, depression and stress in type 2 diabetic patients: A randomized, single-blind, placebo-controlled trial. Pak. J. Biol. Sci. 2013, 16, 1597–1600. [Google Scholar] [PubMed]
- Naqvi, A.Z.; Harty, B.; Mukamal, K.J. Monounsaturated, trans & saturated fatty acids and cognitive decline in women. J. Am. Geriatr. Soc. 2011, 59, 837–843. [Google Scholar] [PubMed]
- Pase, M.P.; Grima, N.; Cockerell, R.; Stough, C.; Scholey, A.; Sali, A.; Pipingas, A. The effects of long-chain omega-3 fish oils and multivitamins on cognitive and cardiovascular function: A randomized, controlled clinical trial. J. Am. Coll. Nutr. 2015, 34, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Hermannstädter, H.M.; Fiebach, J.B.; Schreiber, S.J.; Schuchardt, J.P.; Hahn, A.; Flöel, A. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb. Cortex 2014, 24, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Sinn, N.; Milte, C.; Howe, P.R.C. Oiling the brain: A review of randomized controlled trials of omega-3 fatty acids in psychopathology across the lifespan. Nutrients 2010, 2, 128–170. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Bloom, M.; Broadhurst, C.L.; Schmidt, W.F.; Cunnane, S.C.; Galli, C.; Ghebremeskel, K.; Linseisen, F.; Lloyd-Smith, J.; Parkington, J. Evidence for the unique function of docosahexaenoic acid during the evolution of the modern hominid brain. Lipids 1999, 34, 39–47. [Google Scholar] [CrossRef]
- Schroeter, H.; Spencer, J.P.; Rice-Evans, C.; Williams, R.J. Flavonoids protect neurons from oxidized low-density-lipoprotein-induced apoptosis involving c-Jun N-terminal kinase (JNK), c-Jun and caspase-3. Biochem. J. 2001, 358, 547–557. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health Human and Human Services, U.S. Department of Agriculture. Dietary Guidelines for Americans, 2015–2010, 8th ed.U.S. Department of Health Human and Human Services, U.S. Department of Agriculture: Washington, DC, USA, 2011.
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 63, 2960–2984. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Australian Dietary Guidelines; Australian Government: Canberra, Australia, 2013.
- The Australian National Health and Medical Research Council. Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; The Australian National Health and Medical Research Council: Canberra, Australia, 2006; pp. 155–163.
- Burger, H.; de Laet, C.E.; van Daele, P.L.; Weel, A.E.; Witteman, J.C.; Hofman, A.; Pols, H.A. Risk factors for increased bone loss in an elderly population: The rotterdam study. Am. J. Epidemiol. 1998, 147, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.T.; Felson, D.T.; Anderson, J.J. Bone mineral density in elderly men and women: Results from the framingham osteoporosis study. J. Bone Miner. Res. 1992, 7, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef] [PubMed]
- Kafatos, A.; Verhagen, H.; Moschandreas, J.; Apostolaki, I.; Van Westerop, J.J.M. Mediterranean diet of crete: Foods and nutrient content. J. Am. Diet. Assoc. 2000, 100, 1487–1493. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Vasilopoulou, E.; Georga, K.; Soukara, S.; Dilis, V. Traditional foods: Why and how to sustain them. Trends Food Sci. Technol. 2006, 17, 498–504. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on dietary reference values for calcium. EFSA J. 2015, 13, 1–82. [Google Scholar]
- Australian Institute of Health and Welfare. Estimating the Prevalence of Osteoporosis in Australia; Australian Institute of Health and Welfare: Canberra, Australia, 2014. [Google Scholar]
- Svedbom, A.; Hernlund, E.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the european union: A compendium of country-specific reports. Arch. Osteoporos. 2013, 8, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warensjo, E.; Byberg, L.; Melhus, H.; Gedeborg, R.; Mallmin, H.; Wolk, A.; Michaelsson, K. Dietary calcium intake and risk of fracture and osteoporosis: Prospective longitudinal cohort study. BMJ 2011, 342. [Google Scholar] [CrossRef] [PubMed]
- Teegarden, D. The influence of dairy product consumption on body composition. J. Nutr. 2005, 135, 2749–2752. [Google Scholar] [PubMed]
- Van Meijl, L.E.C.; Vrolix, R.; Mensink, R.P. Dairy product consumption and the metabolic syndrome. Nutr. Res. Rev. 2008, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Lawson, A.B.; Liese, A.D.; Bell, R.A.; Mayer-Davis, E.J. Dairy, magnesium, and calcium intake in relation to insulin sensitivity: Approaches to modeling a dose-dependent association. Am. J. Epidemiol. 2006, 164, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Sontia, B.; Touyz, R.M. Role of magnesium in hypertension. Arch. Biochem. Biophys. 2007, 458, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hadjistavri, L.S.; Sarafidis, P.A.; Panagiotis, G.I.; Tziolas, I.M.; Aroditis, C.P.; Hitoglou-Makedou, A.; Zebekakis, P.E.; Pikilidou, M.I.; Lasaridis, A.N. Beneficial effects of oral magnesium supplementation on insulin sensitivity and serum lipid profile. Med. Sci. Monit. 2010, 16, 307–312. [Google Scholar]
- Alonso, A.; Nettleton, J.A.; Ix, J.H.; de Boer, I.H.; Folsom, A.R.; Bidulescu, A.; Kestenbaum, B.R.; Chambless, L.E.; Jacobs, D.R. Dietary phosphorus, blood pressure, and incidence of hypertension in the atherosclerosis risk in communities study and the multi-ethnic study of atherosclerosis. Hypertension 2010, 55, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Beunza, J.J.; Delgado-Rodriguez, M.; Martínez, J.A.; Martínez-González, M.A. Low-fat dairy consumption and reduced risk of hypertension: Seguiemiento universidad de navarra (sun) cohort. Am. J. Clin. Nutr. 2005, 82, 972–979. [Google Scholar] [PubMed]
- Soedamah-Muthu, S.S.; Ding, E.L.; Al-Delaimy, W.K.; Hu, F.B.; Engberink, M.F.; Willett, W.C.; Geleijnse, J.M. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: Dose-response meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2010, 93, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Engberink, M.F.; Geleijnse, J.M.; de Jong, N.; Smit, H.A.; Kok, F.J.; Verschuren, W.M.M. Dairy intake, blood pressure, and incident hypertension in a general dutch population. J. Nutr. 2009, 139, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Jacobs, D.R., Jr.; van Horn, L.; Slattery, M.L.; Kartashov, A.I.; Ludwig, D.S. Dairy consumption, obesity, and the insulin resistance syndrome in young adults: The cardia study. JAMA 2002, 287, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Delgado-Rodríguez, M.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Gomez-Gracia, E.; Fiol, M.; Lamuela-Raventós, R.M.; Schröder, H.; Arós, F.; et al. Low-fat dairy products and blood pressure: Follow-up of 2290 older persons at high cardiovascular risk participating in the predimed study. Br. J. Nutr. 2008, 101, 59. [Google Scholar] [CrossRef] [PubMed]
- Camfield, D.A.; Owen, L.; Scholey, A.B.; Pipingas, A.; Stough, C. Dairy constituents and neurocognitive health in ageing. Br. J. Nutr. 2011, 106, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Bryan, J.; Murphy, K.J.; Buckley, J. Review of dairy consumption and cognitive performance in adults: Findings and methodological issues. Dement. Geriatr. Cogn. Disord. 2010, 30, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Calvo, D.; Spitznagel, M.B.; Sweet, L.; Josephson, R.; Hughes, J.; Gunstad, J. Dairy intake is associated with memory and pulsatility index in heart failure. Int. J. Neurosci. 2015, 125, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Ogata, S.; Tanaka, H.; Omura, K.; Honda, C.; Hayakawa, K. Association between intake of dairy products and short-term memory with and without adjustment for genetic and family environmental factors: A twin study. Clin. Nutr. 2015, 35, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.; Davies, M.; Yates, T.; Khunti, K. Primary prevention of cardiovascular disease using validated risk scores: A systematic review. J. R. Soc. Med. 2012, 105, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Bopp, K.L.; Verhaeghen, P. Aging and verbal memory span: A meta-analysis. J. Gerontol. Psychol. Sci. 2005, 60, 223–233. [Google Scholar] [CrossRef]
- Johnson, J.K.; Lui, L.-Y.; Yaffe, K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J. Geronotol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 1134–1141. [Google Scholar] [CrossRef]
- Kerchner, G.A.; Racine, C.A.; Hale, S.; Wilheim, R.; Laluz, V.; Miller, B.L.; Kramer, J.H. Cognitive processing speed in older adults: Relationship with white matter integrity. PLoS ONE 2012, 7, e50425. [Google Scholar] [CrossRef] [PubMed]
- Park, D.C.; Lautenschlager, G.; Hedden, T.; Davidson, N.S.; Smith, A.D.; Smith, P.K. Models of visuospatial and verbal memory across the adult life span. Psychol. Aging 2002, 17, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120391. [Google Scholar] [CrossRef] [PubMed]
- Zonderman, A.B.; Giambra, L.M.; Arenberg, D.; Resnick, S.M.; Costa, P.T., Jr.; Kawas, C.H. Changes in immediate visual memory predict cognitive impairment. Arch. Clin. Neuropsychol. 1995, 10, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Fahle, M.; Daum, I. Visual learning and memory as functions of age. Neuropsychologia 1997, 35, 1583–1589. [Google Scholar] [CrossRef]
- Alipour, H.; Goldust, M. The association between blood pressure components and cognitive functions and cognitive reserve. Clin. Exp. Hypertens. 2016, 38, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Borghesani, P.R.; Madhyastha, T.M.; Aylward, E.H.; Reiter, M.A.; Swarny, B.R.; Warner Schaie, K.; Willis, S.L. The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia 2013, 51, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.V.; Robinson, D.J.; O’Connell, H.; Hamilton, F.; Bruce, I.; Coen, R.; Walsh, B.; Coakley, D.; Molloy, A.; Scott, J.; et al. Vascular biomarkers of cognitive performance in a community-based elderly population: The dublin healthy ageing study. Age Ageing 2008, 37, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Grant, H.; Bhambhani, Y.; Singhal, A. Hemodynamic changes in the prefrontal cortex during working memory in essential hypertension. J. Am. Soc. Hypertens. 2015, 9, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Harrington, F.; Saxby, B.K.; McKeith, I.G.; Wesnes, K.; Ford, G. Cognitive performance in hypertensive and normotensive older subjects. Hypertension 2000, 36, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Kalra, L.; Jackson, S.H.; Swift, C.G. Psychomotor performance in elderly hypertensive patients. J. Hum. Hypertens. 1993, 7, 279–284. [Google Scholar] [PubMed]
- Kesse-Guyot, E.; Lassale, C.; Assmann, K.E.; Andreeva, V.A.; Julia, C.; Blacher, J.; Fezeu, L.; Hercberg, S.; Galan, P. Are different vascular risk scores calculated at midlife uniformly associated with subsequent poor cognitive performance? Atherosclerosis 2015, 243, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.K.; Sorond, F.; Iloputaife, I.; Gagnon, M.; Milberg, W.; Lipsitz, L. Effect of blood pressure on cognitive functions in elderly persons. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Love, S.; Miners, J.S. Cerebrovascular disease in ageing and Alzheimer’s disease. Acta Neuropathol. 2016, 131, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Lowry, J.; Austin, A.; Al-Sayegh, H.; Yan, F.; Liu, F.; Zhang, J. Impaired verbal memory is a significant predictor of early cerebral-cardiovascular death, an 18-year follow-up of a national cohort. Int. J. Geriatr. Psychiatry 2014, 29, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, B.P.; Zakzanis, K.K. The neuropsychological profile of vascular cognitive impairment not demented: A meta-analysis. J. Neuropsychol. 2015, 9, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Mioshi, E.; Dawson, K.; Mitchell, J.; Arnold, R.; Hodges, J.R. The addenbrooke’s cognitive examination revised (ace-r): A brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry 2006, 21, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Radeborg, K.; Salo, I.; Bjorck, I. Effects of supplementation with n-3 polyunsaturated fatty acids on cognitive performance and cardiometabolic risk markers in healthy 51 to 72 years old subjects: A randomized controlled cross-over study. Nutr. J. 2012, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Case, C.C.; Jones, P.H.; Nelson, K.; O’Brian Smith, E.; Ballantyne, C.M. Impact of weight loss on the metabolic syndrome. Diabetes Obes. Metab. 2002, 4, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Siervo, M.; Arnold, R.; Wells, J.C.K.; Tagliabue, A.; Colantuoni, A.; Albanese, E.; Brayne, C.; Stephan, B.C.M. Intentional weight loss in overweight and obese individuals and cognitive function: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 968–983. [Google Scholar] [CrossRef] [PubMed]
- The Australian National Health and Medical Research Council. Australian Guidelines to Reduce Health Risks from Drinking Alcohol; The Australian National Health and Medical Research Council: Canberra, Australia, 2009.
- Davis, C.R.; Bryan, J.; Hodgson, J.M.; Wilson, C.; Dhillon, V.; Murphy, K. A randomised controlled intervention trial evaluating the efficacy of a mediterranean dietary pattern on cognitive function and psychological wellbeing in healthy older adults: The medley study. BMC Geriatr. 2015, 15. [Google Scholar] [CrossRef]
- Mule, G.; Caimi, G.; Cottone, S.; Nardi, E.; Andronico, G.; Piazza, G.; Volpe, V.; Federico, M.R.; Cerasola, G. Value of home blood pressures as predictor oftarget organ damage in mild arterial hypertension. J. Cardiovasc. Risk 2002, 9, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, T.; Imai, Y.; Tsuji, I.; Nagai, K.; Kato, J.; Kikuchi, N.; Nishiyama, A.; Aihara, A.; Sekino, M.; Kikuya, M.; et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: A population-based observation in Ohasama, Japan. J. Hypertens. 1998, 16, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Jula, A.; Puukka, P.; Karanko, H. Multiple clinic and home blood pressure measurements versus ambulatory blood pressure monitoring. Hypertension 1999, 34, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Thompson, P.L.; Stojceski, B.; Prince, R.L. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Watson, N.A.; Dyer, K.A.; Buckley, J.D.; Brinkworth, G.D.; Coates, A.M.; Parfitt, G.; Howe, P.R.; Noakes, M.; Dye, L.; Chadwick, H.; et al. A randomised trial comparing low-fat diets differing in carbohydrate and protein ratio, combined with regular moderate intensity exercise, on glycaemic control, cardiometabolic risk factors, food cravings, cognitive function and psychological wellbeing in adults with type 2 diabetes: Study protocol. Contemp. Clin. Trials 2015, 45, 217–225. [Google Scholar] [PubMed]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.; Saggiani, F.; Zenere, M.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.C.; Muhlhausler, B.S.; Yelland, L.N.; Gibson, R.A. Correlations between blood and tissue omega-3 LCPUFA status following dietary ALA intervention in rats. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Ehrenhaft, A.; Griesser, K.; Pfeufer, A.; Muller, J.; Schomig, A.; Kastrati, A. Taqman systems for genotyping of disease-related polymorphisms present in the gene encoding apolipoprotein E. Clin. Chem. Lab. Med. 2002, 40, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Amen, D.G.; Taylor, D.V.; Ojala, K.; Kaur, J.; Willeumier, K. Effects of brain-directed nutrients on cerebral blood flow and neuropsychological testing: A randomized, double-blind, placebo-controlled, crossover trial. Adv. Mind-Body Med. 2013, 27, 24–33. [Google Scholar] [PubMed]
- Bauer, I.; Hughes, M.; Rowsell, R.; Cockerell, R.; Pipingas, A.; Crewther, S.; Crewther, D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum. Psychopharmacol. Clin. Exp. 2014, 29, 133–144. [Google Scholar] [CrossRef] [PubMed]
- File, S.E.; Jarrett, N.; Fluck, E.; Duffy, R.; Casey, K.; Wiseman, H. Eating soya improves human memory. Psychopharmacology 2001, 157, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Strike, S.C.; Carlisle, A.; Gibson, E.L.; Dyall, S.C. A high omega-3 fatty acid multinutrient supplement benefits cognition and mobility in older women: A randomized, double-blind, placebo-controlled pilot study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.W.; James, M.; Owen, A.M.; Sahakian, B.J.; McInnes, L.; Rabbitt, P. Cambridge neuropsychological test automated battery (cantab): A factor analytic study of a large sample of normal elderly volunteers. Dementia 1994, 5, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Cognition. Test-Retest Reliabilities and Detecting Reliable Change; Cambridge Cognition: Cambridge, UK, 2008; pp. 1–4. [Google Scholar]
- Lowe, C.; Rabbitt, P. Test/re-test reliability of the cantab and ispocd neuropsychological batteries: Theoretical and practical issues. Neuropsychologia 1998, 36, 915–923. [Google Scholar] [CrossRef]
- D’Agostino, R.B.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The framingham heart study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; Sajjad, A.; Bramer, W.M.; Ikram, M.A.; Tiemeier, H.; Stephan, B.C.M. Exploring strategies to operationalize cognitive reserve: A systematic review of reviews. J. Clin. Exp. Neuropsychol. 2015, 37, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Kaffashian, S.; Dugravot, A.; Elbaz, A. Predicting cognitive decline: A dementia risk score vs. the framingham vascular risk scores predicting cognitive decline. Neurology 2013, 80, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E. Sf-36® Health Survey (Version 1.0); Quality Metric Incorporate: Lincoln, RI, USA, 2005; pp. 1–15. [Google Scholar]
- Carillon, J.; Notin, C.; Schmitt, K.; Simoneau, G.; Lacan, D. Dietary supplementation with a superoxide dismutase-melon concentrate reduces stress, physical and mental fatigue in healthy people: A randomised, double-blind, placebo-controlled trial. Nutrients 2014, 6, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Bryan, J.; Murphy, K.J. Dietary antioxidants, cognitive function and dementia—A systematic review. Plant Foods Hum. Nutr. 2013, 68, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R.C. Effects of n-3 fatty acids, epa v. Dha, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Curran, S.L.; Andrykowski, M.A.; Studts, J.L. Short form of the profile of mood states (POMS-SF): Psychometric information. Psychol. Assess. 1995, 7, 80–83. [Google Scholar] [CrossRef]
- Louis, W.J.; Mander, A.G. Use of computerized neuropsychological tests (CANTAB) to assess cognitive effects of antihypertensive drugs in the elderly. J. Hypertens. 1999, 17, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Conlon, C.; Podd, J.; Hill, S.R.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 94, 1134–1143. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.Y.; Lavori, P.W.; Cohen, H.J.; Feussner, J.R. An overview of variance inflation factors for sample-size calculation. Eval. Health Prof. 2003, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Changing the Trajectory of Alzheimer’s Disease: How a Treatment by 2025 Saves Lives and Dollars; Alzheimer’s Association: New York, NY, USA, 2015. [Google Scholar]
- Jorm, A.F.; Dear, K.B.G.; Burgess, N.M. Projections of future numbers of dementia cases in Australia with and without prevention. Aust. N. Z. J. Psychiatry 2005, 39, 959–963. [Google Scholar] [CrossRef] [PubMed]
| Test | Description | Cognitive Function |
|---|---|---|
| Motor Orientation Task (MOT) | Familiarisation task. The participant is instructed to touch the centre of a flashing cross that appears across the screen in different locations. | N/A |
| Paired Associates Learning (PAL) | The participant is required to learn the spatial location of patterns across a matrix of boxes. The task progresses in stages, from two to eight patterns. If the participant identifies all patterns of a stage correctly they progress to the next stage. If patterns are not correctly identified after 10 trials the task is terminated. | Visual episodic memory; learning |
| Delayed Matching to Sample (DMS) | A “sample” pattern is presented, followed by four similar “choice” patterns. The participant is instructed to identify the choice pattern that matches the sample. Choice patterns are presented simultaneous to the sample, or after a delay of 0, 4 or 12 s. | Simultaneous visual pattern recognition; short-term visual memory |
| Verbal Recognition Memory (VRM) | A list of 12 words is presented in succession and the participant is instructed to read each word aloud. The participant is then asked to recall as many words as possible, and distinguish between words from the original list and distractor words. | Verbal memory |
| Reaction Time (RTI) | A yellow spot appears on the screen and the participant is instructed to touch the spot as fast as possible. In the “Simple” stage, the spot will appear in only one location for 10 trials. In the “Choice” stage, the spot will appear in one of five locations for 15 trials. | Simple and choice reaction time; processing speed |
| Rapid Visual Information Processing (RVIP) | The digits 2 through 9 are presented at a rate of 100 digits per minute. Participants are instructed to detect three target sequences of digits (3-5-7, 2-4-6 and 4-6-8) and to register their response using a press pad. | Visual sustained attention; processing speed |
| Spatial Working Memory (SWM) | Coloured boxes are presented on the screen. The participant is instructed to find a blue token in each of the boxes using a process of elimination. The task progresses from 3 to 8 boxes. | Spatial memory; spatial working memory; heuristic strategy; executive function |
| One Touch Stockings of Cambridge (OTS) | The screen is divided into two halves, each of which contains a display of three coloured balls arranged across three “stockings”. The participants is asked to determine the minimum number of moves required to match the position of balls in lower display to the upper display, moving only one ball at a time. | Spatial planning, spatial working memory, executive function |
| Attention Switching Task (AST) | Arrows appear on each side of the screen and the participant is cued to indicate the direction the arrow is pointing, or the side of the screen on which the arrow appears using a press pad. | Attentional set-shifting; processing speed; executive function |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wade, A.T.; Davis, C.R.; Dyer, K.A.; Hodgson, J.M.; Woodman, R.J.; Keage, H.A.D.; Murphy, K.J. A Mediterranean Diet to Improve Cardiovascular and Cognitive Health: Protocol for a Randomised Controlled Intervention Study. Nutrients 2017, 9, 145. https://doi.org/10.3390/nu9020145
Wade AT, Davis CR, Dyer KA, Hodgson JM, Woodman RJ, Keage HAD, Murphy KJ. A Mediterranean Diet to Improve Cardiovascular and Cognitive Health: Protocol for a Randomised Controlled Intervention Study. Nutrients. 2017; 9(2):145. https://doi.org/10.3390/nu9020145
Chicago/Turabian StyleWade, Alexandra T., Courtney R. Davis, Kathryn A. Dyer, Jonathan M. Hodgson, Richard J. Woodman, Hannah A. D. Keage, and Karen J. Murphy. 2017. "A Mediterranean Diet to Improve Cardiovascular and Cognitive Health: Protocol for a Randomised Controlled Intervention Study" Nutrients 9, no. 2: 145. https://doi.org/10.3390/nu9020145





