1. Introduction
Insomnia is defined by disturbances in sleep quality together with impairment of daytime functioning, for example fatigue and low mood [
1]. Disturbances in sleep quality include difficulty getting to sleep, staying asleep or experiencing non-restorative sleep despite adequate opportunity for sleep [
1]. An estimated 13%–33% of Australians experience some form of insomnia, similar to the estimated rates of insomnia in Western countries including Canada and the United States and in low-income countries across Africa and Asia [
2,
3,
4]. Insomnia can occur as an acute episode, usually triggered by factors such as ill health, change of medication or circumstances, or stress [
5]. Such sleep disturbances generally resolve without treatment once the trigger is eliminated. However, people can also turn to short-term use of medications (typically hypnotics such as a benzodiazepine) or herbal supplements during these episodes of insomnia [
5,
6,
7]. In contrast, long-term or chronic insomnia can involve the development of maladaptive behaviors and a different treatment approach is required [
5].
Commonly used herbal supplements for insomnia often include single or combined formulations of lemon balm (
Melissa officinalis), chamomile (
Matricaria recutita), valerian (
Valeriana spp.), hops (
Humulus lupulus), passionflower (
Passiflora incanata), lactium™ (α
S1-casein hydrolysate) and sour date (
Zizyphus jujube var. spinosa) [
8]. A new combined formulation, LZComplex3, contains lactium, sour date and hops, plus magnesium and vitamin B6 (pyridoxine) to provide nutritional support for metabolic pathways involved in sleep regulation. The rationale for the use of lactium as a sleeping aid originates from the observation that milk calms and soothes newborns [
9]. The milk compound thought to be responsible for the calming or anxiolytic effects is a hydrolysate of α
S1-casein, the bioactive peptide α-casozepine [
10]. Lactium is the manufactured form of α
S1-casein hydrolysate containing the α-casozepine peptide. Clinical studies have demonstrated that lactium reduces some symptoms related to stress [
11,
12]. Lactium has also been shown to have anxiolytic effects and to improve stress-induced sleep disturbance in animal studies [
10,
13,
14].
Sour date (
Zizyphus jujube var. spinosa; alternative spelling
Ziziphus) is a fruit used in traditional Chinese medicine for its mild sedative and calming properties, to relieve irritability and aid sleep [
15,
16]. In combination with other herbs, it has been reported to improve mood and performance in individuals with anxiety and to improve sleep quality and a sense of well-being in individuals with sleep disorders [
17,
18]. Hops (
Humulus lupulus) have been used in traditional western medicine for the treatment of mood disturbances such as restlessness and anxiety and in sleep disturbances due to reported calming and sleep-promoting properties [
19,
20,
21,
22,
23,
24]. Magnesium is involved in more than 300 metabolic reaction pathways including the production of melatonin, which regulates the sleep cycle [
25]. Human and animal studies have implicated magnesium in the modulation of sleep [
26,
27,
28,
29,
30,
31]. Vitamin B6 may indirectly promote sleep quality through its role in the synthesis of a number of neurotransmitters involved in sleep regulation, including dopamine, serotonin, glutamate,
γ-aminobutyric acid (GABA), and histamine [
32,
33].
Although the individual components in LZComplex3 have been studied with respect to their effects on sleep and/or stress (a common cause of sleeping difficulties), the efficacy of the combined formulation as a treatment for sleep disturbance has not been investigated. We report here the results of a clinical trial the primary objective of which was to investigate the short-term effect of LZComplex3 on sleep quality, mood and cognitive function in individuals with sleeping difficulties not caused by a primary sleeping disorder or other diagnosed condition.
2. Materials and Methods
2.1. Trial Design
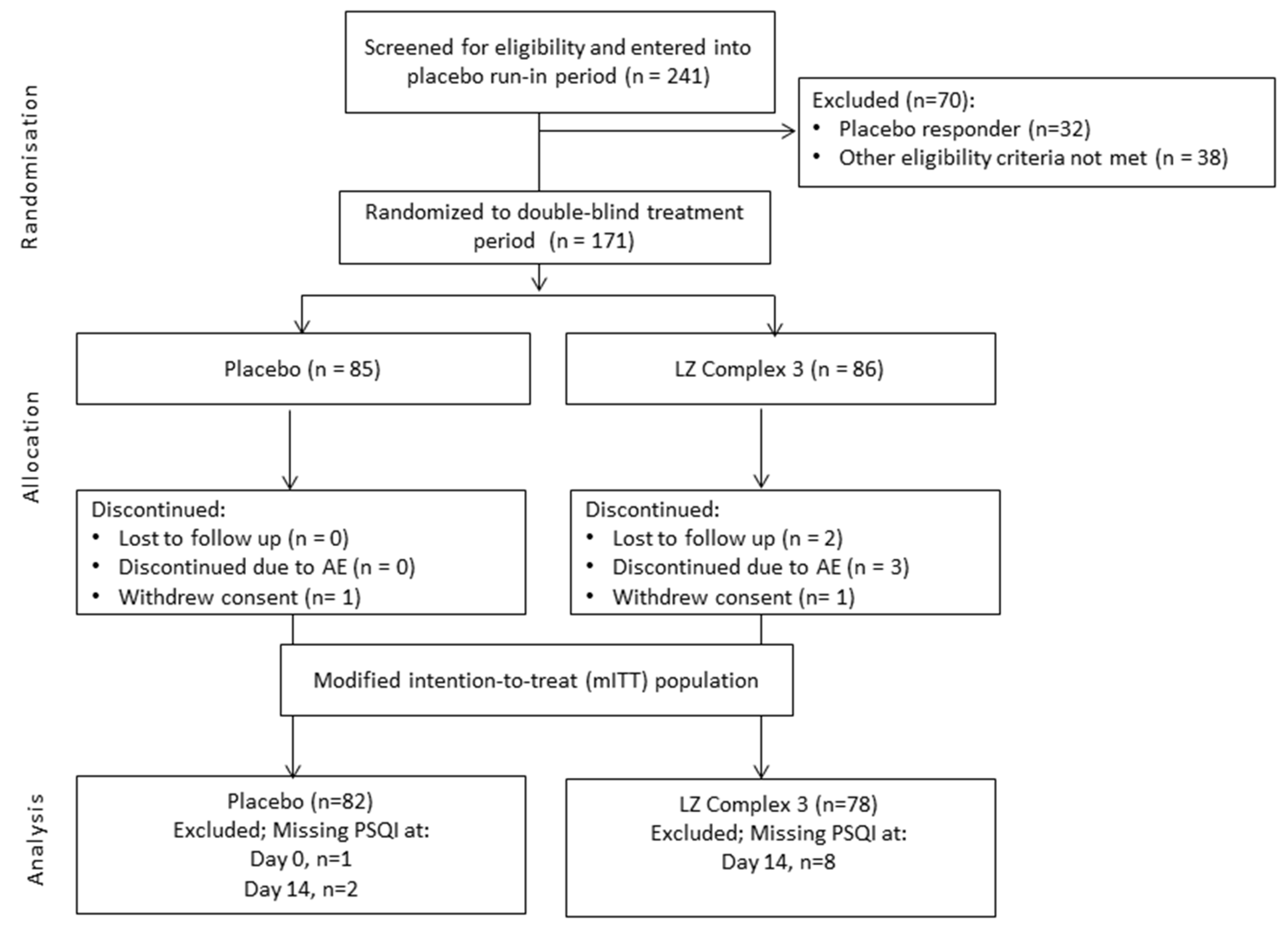
This study was a placebo-controlled, double-blind, randomized, parallel group phase III trial with a single blind placebo run-in period (
Figure 1). The trial was conducted at the Centre for Human Psychopharmacology, Swinburne University of Technology, Hawthorn, Victoria, Australia between 6 January 2014 and 23 December 2014. Ethical approval was granted by Bellberry Ltd., Eastwood, SA, Australia. The trial is registered with the Australian New Zealand Clinical Trials Registry (number ACTRN: 12613001363774) and was performed in accordance with the requirements for the conduct of clinical studies set by the Clinical Trial Notification (CTN) scheme of the Australian Therapeutic Goods Administration (TGA) and the Declaration of Helsinki.
Assessments of sleep quality, daytime functioning and physical fatigue, mood and anxiety, stress-reactivity, and cognitive function were completed by participants during the baseline and end of treatment visits, as well as during a final follow-up visit one week after the end of treatment. All assessment visits followed a procedure identical to that used at the baseline visit. In addition, participants completed all subjective sleep, daytime functioning, physical fatigue, mood and anxiety assessments at home 1, 3 and 7 days after baseline. All participant data were collected either at the study site (at screening, baseline, end of treatment, and final follow-up visits) or at the participants’ homes (interim assessments between baseline and end of treatment).
2.2. Study Participants and Randomization
Potentially eligible participants were identified in an initial telephone screen. Participant eligibility was confirmed at the initial screening visit; each eligible participant was allocated a unique participant number as soon as written informed consent was obtained and prior to any screening assessments. Mood questionnaires including the Hospital Anxiety and Depression Scale (HADS) [
34] and State-Trait Anxiety Inventory Trait subscale (STAI-T) [
35] were completed and participants were required to familiarize themselves with all the study assessments and procedures in order to reduce errors at baseline and practice effects.
Eligible participants were healthy adults aged 18–65 years with no significant diagnosed diseases (as judged by the Investigator) who had self-reported sleeping difficulties over one month prior to the screening call. Following accepted practice [
36], sleeping difficulties were defined as a Pittsburgh Sleep Quality Index (PSQI) Score >5 (PSQI scores range from 0 to 21 and higher scores indicate worse sleep quality). Participants with a primary sleep disorder (sleep apnoea-hypopnoea, periodic limb movement disorder, restless legs syndrome, narcolepsy, idiopathic hypersomnia, Kleine-Levin syndrome, as determined by subjective report and compilation of participants' medical history during the initial telephone screening assessment prior to randomization) were excluded. The full lists of inclusion and exclusion criteria are provided in
Appendix A:
Table A1.
Eligible participants entered a one-week single-blind placebo run-in period, during which a daily sleep questionnaire (Consensus Sleep Diary; CSD [
37]) was used to establish baseline sleeping criteria and detect placebo responders. Placebo response was based on CSD scores, and defined as sleep efficiency above 85%, sleep onset latency below 31 min, and wake after sleep onset below 31 min.
At the baseline visit following the placebo run-in, placebo responders were excluded and all other participants were randomly assigned to treatment for two weeks (placebo or LZComplex3 in a 1:1 ratio) based on a randomization list generated centrally by an external independent third party. Participants and investigators were blinded to study treatment in the treatment phase and did not have access to the randomization codes except under exceptional medical circumstances.
Participants were invited at the baseline visit to wear an actiwatch to collect objective sleep data for exploratory cross-validation of the subjective sleep outcome measures. Participants who agreed were given an actiwatch to wear for the duration of the two-week treatment period.
2.3. Study Treatment
LZComplex3 tablets (
Table 1) and placebo tablets were provided in blister packs and were matched for size, appearance, colour, smell and taste. The tablets were supplied to participants at the start of the placebo run-in and treatment phases in kit boxes. Each kit box contained sufficient blister packs of tablets to last for the duration of each phase of the trial plus an additional week to cover for any delays in attending the next scheduled visit. For the duration of each phase, participants were required to take two tablets daily, 30 min before retiring for sleep.
2.4. Primary and Secondary Outcome Measurments
The primary outcome was the change in overall sleep quality after two weeks of daily supplementation with LZComplex3. The primary outcome was measured by the change in PSQI scores from baseline to end of treatment at day 14. Secondary outcomes were the safety of LZComplex3 and the change in sleep quality, daytime functioning and physical fatigue, mood and anxiety, cognitive performance, and stress reactivity at 1, 3, 7 and 14 days after treatment with LZComplex3 and after one week post-treatment. The secondary outcomes were measured using validated assessments as outlined in
Table 2. Objective measurement of sleep efficiency and time asleep using actigraph data from a subset of up to 90 participants was a pre-specified exploratory outcome designed to assess the use of actigraphy as a means of cross-validation of the primary and secondary endpoints. The Mini-Mitter Actiwatch-L (Respironics, Inc., Bend, Oregon) was used to collect actigraph data. Adverse Events (AEs), including Serious Adverse Events (SAEs) and Adverse Events of Special Interest (AESI), were collected at every visit. The AE observation period commenced the day of consent and finished at the final follow-up visit.
2.4.1. Screening Assessments
Screening assessments included the HADS, STAI-T, Leeds Sleep Evaluation Questionnaire (LSEQ), Bond-Lader Visual Analogue Scale (VAS), and the Stress and Fatigue Visual Analogue Mood Scales (VAMS). The HADS is a 14-item questionnaire designed to measure levels of anxiety and depression and was administered at screening to exclude participants with depression and/or anxiety [
34]. The STAI-T comprises 20 different statements (e.g., “Some unimportant thought runs through my mind and bothers me”) [
35]. Participants indicate how they generally feel on a scale ranging from “almost never” to “almost always”. Scores on the STAI-T range from 20 to 80, with higher scores indicating more anxiety. The Trait subscale of the STAI was to be used at screening to detect those participants who may have excessive levels of trait anxiety prior to commencing the study.
2.4.2. Treatment Assessments
2.5. Statistical Analyses
With an anticipated drop-out/non-compliance rate of 33%, it was estimated that a total of 170 participants would be required for 80% power to detect a medium effect size of approximately 0.5 at the 5% level of significance, with respect to the primary outcome. All analyses were conducted on a modified intention-to-treat (mITT) population, representing a per protocol/completer analysis and defined as all participants who were randomized and who had valid PSQI measures at both baseline and end of treatment. The primary outcome was also measured in the per protocol (PP) population, which included all participants in the mITT population who were at least 80% and less than 120% compliant with randomized treatment medication and had no major protocol deviations. Safety analyses were conducted on the safety population, which included all participants who were randomized and received at least one dose of study drug.
All measures were analyzed using SAS software (V9.4, SAS Statistical Institute, Cart, NC, USA). A general repeated measures mixed model was fitted to explore the difference between placebo and LZComplex3 in unadjusted change of total PSQI across all PSQI assessments for the mITT population. Day numbers and treatment group were included as fixed effects, participant as a random effect, the change in PSQI from baseline as the dependent variable and the baseline value of PSQI as a covariate. Secondary endpoints except ISI scores were analyzed in the same form as the primary endpoint. For the STAI-S, Bond-Lader VAS and VAMS scores, the mixed model also included time point (before and after administration of the MTF) as a fixed effect. A multinomial distribution and cumulative logit link function using PROC GLIMMIX was used to explore the difference between placebo and LZComplex3 in unadjusted change in ISI. The intended analysis of actigraphy data was not performed due to insufficient participant numbers (n = 16).
4. Discussion
This study evaluated the efficacy of LZComplex3 in improving sleep quality in otherwise-well individuals with sleeping difficulties. There were no group differences in the primary outcome. Improvements in sleep quality were seen over a two-week treatment period with LZComplex3, however a persistent placebo response was observed and there was no significant treatment effect compared with placebo. Although the study included a one-week placebo run-in period designed to identify and exclude placebo-responders, it appears that that the run-in period may not have been of sufficient length. It is also possible that the persistent placebo response occurring after randomization may have been due to participants’ increased focus on overall sleep hygiene as a result of study visits and assessments, filling in a daily sleep diary and observing the protocol-mandated study parameters regarding stimulant use and sleep times. This degree of attention was not required in the placebo run-in phase. It has previously been suggested that having a patient keep a sleep diary for 2 weeks will aid with identification of behaviors that may worsen insomnia, thus providing a useful behavioral intervention [
45]. Participants were required to fill in the CSD daily for the duration of the run-in and treatment periods, which may have contributed to the improvements in sleep quality observed in both treatment groups.
An improvement in sleep quality with LZComplex3 was expected, as previous studies have supported the use of individual components of the formulation as an aid for sleeping difficulties or insomnia (see Introduction). However, rigorous clinical studies are lacking. Effects of lactium on sleep quality have been investigated in a double-blind, controlled, parallel study of 32 Japanese patients experiencing poor sleep as determined by a global PSQI score greater than 4 [
46]. As in the current study, improvements in sleep quality were observed over 4 weeks within the lactium group, however there were no significant differences between the placebo and lactium groups for any of the sleep components evaluated. It is possible that an effect of lactium was not detected due to a placebo response and the small sample size. Clinical evidence for the anxiolytic effects of lactium is more supportive. In a double-blind, randomized, controlled study of 42 healthy men treated with 3 doses each 12 h apart, experimental stress-induced elevations in blood pressure were significantly lower in the lactium group compared with the placebo group, supporting an anti-stress activity of lactium. As stress is a common cause of sleeping difficulties, it is thought that lactium may promote good quality sleep through its anti-stress activity.
The efficacy of sour date (
Zizyphus jujube var. spinosa) in treating insomnia has been investigated in one clinical trial in the form of suanzaorentang [
17,
47], a popular Chinese herbal formula consisting of sour date (
Zizyphus jujube var. spinosa), Fu Ling (mushroom) (
Poria cocos), szechuan lovage (
Ligusticum wallichii), Zhi Mu (
Anemarrhenae rhizoma), and liquorice root (
Glycyrrhizae radix) in a ratio of 7:5:2:1:1 [
48]. The study compared self-rated measures of sleep quality in 60 participants with insomnia who received placebo for one week, followed by suanzaorentang for two weeks, followed by another week of placebo. All ratings of sleep quality significantly improved during the suanzaorentang treatment phase compared with the placebo periods. A number of trials evaluating the efficacy of suanzaorentang using benzodiazepines as the comparator showed favorable results for suanzaorentang in improving sleep, although the studies lacked methodological rigor [
47].
Hops (
Humulus lupulus) is considered to be a sedative agent, a view that originated from the observation of sleepiness in European hops-pickers [
49]. It is commonly used in combination preparations with other herbs such as valerian (
Valeriana spp.) and passionflower (
Passiflora incanata) [
50,
51]. Although hops (
Humulus lupulus) is listed as an approved herb for mood disturbances including sleep disturbances in The Complete German Commission E Monographs [
52], there are no randomized controlled trials investigating the efficacy of hops (
Humulus lupulus) alone in the treatment of insomnia. Thus it remains unclear whether hops (
Humulus lupulus) has independent sedative effects, works as a synergist, or lacks sedative activity.
The rationale for inclusion of magnesium and vitamin B6 in LZComplex3 is based on in vitro and animal studies suggesting they may promote sleep quality by supporting metabolic pathways involved in sleep regulation, rather than due to any direct sedative activity [
26,
27,
28,
29,
30,
31,
32,
33]. Given the current clinical evidence base for the individual components in LZComplex3, it is difficult to determine whether the primary endpoint of this study was not met because of an absence of sedative activity, unknown complex pharmacokinetic interaction between the individual active ingredients, or because of methodological factors.
It is possible that the two-week treatment duration in our study was not long enough to observe a treatment effect, and/or the one-week placebo run-in period was not long enough to eliminate all placebo responders. Another possibility is that the study population may have included individuals with chronic insomnia, which unlike brief or acute insomnia generally requires a cognitive behavioral approach to treatment [
5]. The study population was selected based on a global PSQI score greater than 5, which indicates poor sleep [
38]. However, individual’s responses on the PSQI questionnaire could not be used to differentiate between acute and chronic insomnia. Conversely, our ability to detect a treatment effect may be due to eligibility here being set at a minor level of sleeping difficulties, while individuals with more severe insomnia would be expected to benefit most from treatment.
Overall, the secondary outcome measures did not support a benefit of LZComplex3 over placebo during the two week treatment period. Participants completed a battery of questionnaires assessing various aspects of sleep quality, daytime functioning and physical fatigue, mood and anxiety, cognitive performance, and stress. Poor sleep can contribute to impairment of daytime functioning and physical fatigue thus these outcomes were anticipated to improve with better quality sleep. However, given that no significant difference between LZComplex3 and placebo was detected with respect to improvement in sleep quality as measured by the PSQI, it is not surprising that daytime functioning and physical fatigue were also not significantly different between the treatment groups. An improvement in ISI at day 3 favoring LZComplex3 and a between-group difference in stress reactivity as measured by change in STAI-S total score from pre- to post-administration of the MTF which appeared to be due to a slight increase in stress reactivity in the placebo group at baseline and at day 14 were the only significant treatment effects detected. Potential improvements in mood, anxiety and stress with LZComplex3 were anticipated as there is evidence that they may be positively impacted by some of the individual components of the formulation [
10,
11,
12,
13,
14,
17,
18,
19,
20,
21,
22,
23,
24]. In our study, a clinically significant treatment effect may not have been detected for the same reasons as described for the primary outcome measure.
The safety data collected in this study indicate that LZComplex3 is well-tolerated at the dose of two tablets prior to sleep. Overall the safety profile of LZComplex3 was similar to placebo, with similar proportions of patients reporting AEs in both groups, the majority of which were mild or moderate and none of which were serious. There were more severe AEs reported in the LZComplex3 group and only two were considered possibly, probably or definitely related to treatment (both gastrointestinal disorders).