The Effect of Bifidobacterium animalis ssp. lactis HN019 on Cellular Immune Function in Healthy Elderly Subjects: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
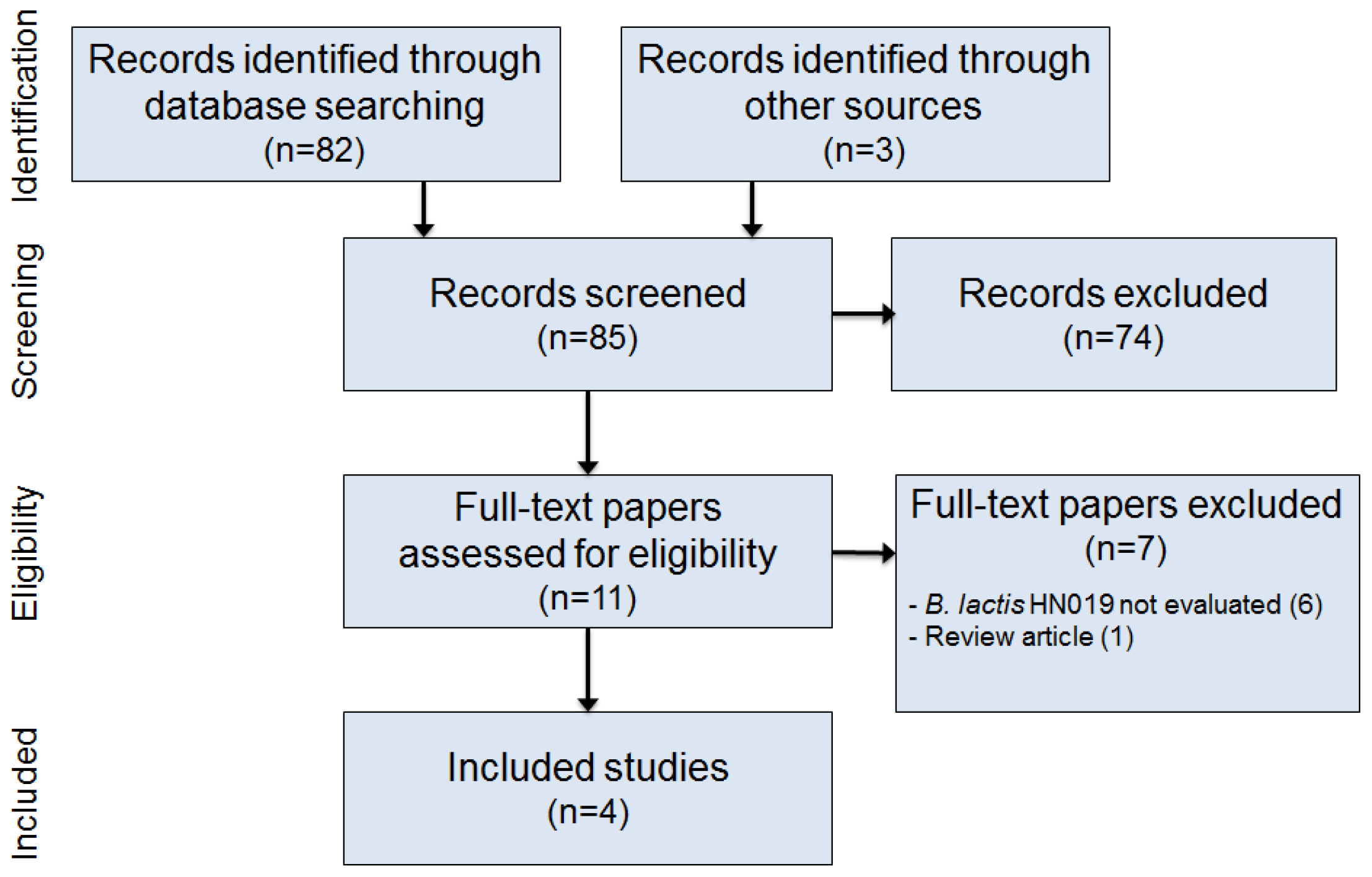
2.1. Literature Search
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis
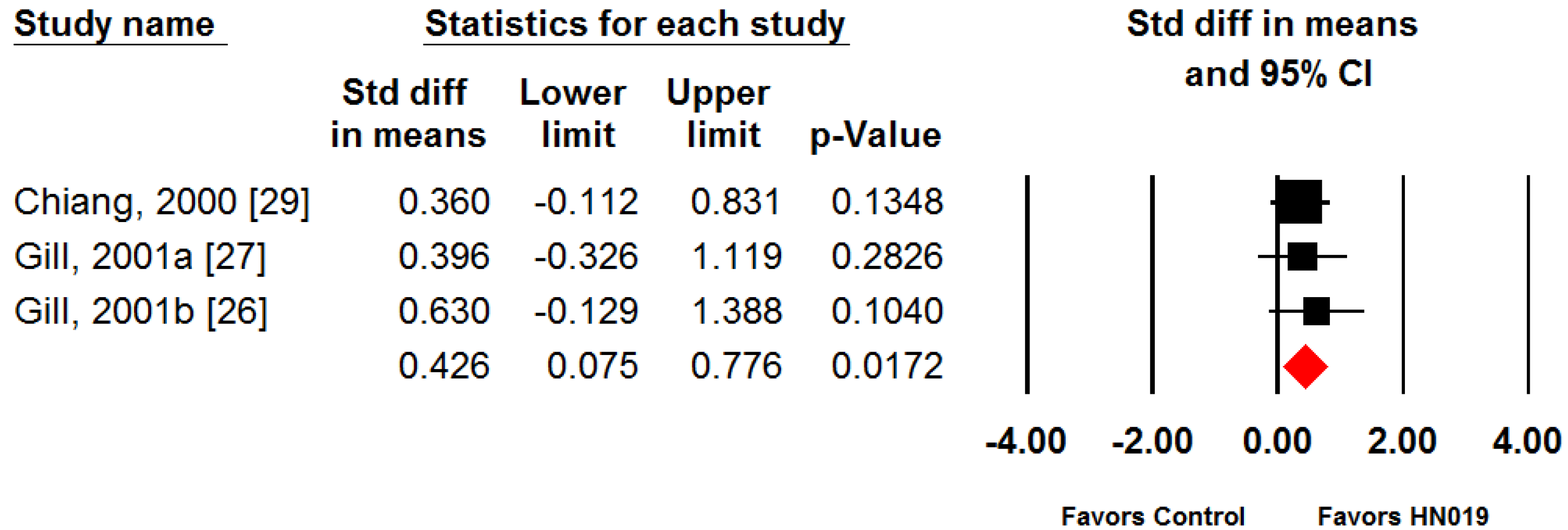
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chou, J.P.; Effros, R.B. T cell replicative senescence in human aging. Curr. Pharm. Des. 2013, 19, 1680–1698. [Google Scholar] [PubMed]
- Hazeldine, J.; Lord, J.M. Innate immunesenescence: Underlying mechanisms and clinical relevance. Biogerontology 2015, 16, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Hallmarks of human “immunosenescence”: Adaptation or dysregulation? Immun. Ageing I A 2012, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Ademokun, A.; Wu, Y.C.; Dunn-Walters, D. The ageing B cell population: Composition and function. Biogerontology 2010, 11, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Joshi, S.; Greenwood, H.; Panda, A.; Lord, J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010, 22, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Butcher, S.K.; Chahal, H.; Nayak, L.; Sinclair, A.; Henriquez, N.V.; Sapey, E.; O’Mahony, D.; Lord, J.M. Senescence in innate immune responses: Reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J. Leukoc. Biol. 2001, 70, 881–886. [Google Scholar] [PubMed]
- Hazeldine, J.; Lord, J.M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res. Rev. 2013, 12, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Le Garff-Tavernier, M.; Beziat, V.; Decocq, J.; Siguret, V.; Gandjbakhch, F.; Pautas, E.; Debre, P.; Merle-Beral, H.; Vieillard, V. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell 2010, 9, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Mocchegiani, E.; Giacconi, R.; Cipriano, C.; Malavolta, M. NK and NKT cells in aging and longevity: Role of zinc and metallothioneins. J. Clin. Immunol. 2009, 29, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Panda, A.; Joshi, S.R.; Qian, F.; Allore, H.G.; Montgomery, R.R. Dysregulation of human Toll-like receptor function in aging. Ageing Res. Rev. 2011, 10, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Mello, A.M.; Paroni, G.; Daragjati, J.; Pilotto, A. Gastrointestinal microbiota and their contribution to healthy aging. Dig. Dis. 2016, 34, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Candela, M.; Turroni, S.; Garagnani, P.; Franceschi, C.; Brigidi, P. Ageing and gut microbes: Perspectives for health maintenance and longevity. Pharmacol. Res. 2013, 69, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Palva, A.M.; de Vos, W.M.; Satokari, R. Contribution of the intestinal microbiota to human health: From birth to 100 years of age. Curr. Top. Microbiol. Immunol. 2013, 358, 323–346. [Google Scholar] [PubMed]
- Biagi, E.; Candela, M.; Franceschi, C.; Brigidi, P. The aging gut microbiota: New perspectives. Ageing Res. Rev. 2011, 10, 428–429. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.J.; Macfarlane, G.T. Changes in predominant bacterial populations in human faeces with age and with clostridium difficile infection. J. Med. Microbiol. 2002, 51, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, S.; Fite, A.; Macfarlane, G.T.; McMurdo, M.E. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 2004, 70, 3575–3581. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, O.; Coakley, M.; Lakshminarayanan, B.; Conde, S.; Claesson, M.J.; Cusack, S.; Fitzgerald, A.P.; O’Toole, P.W.; Stanton, C.; Ross, R.P. Alterations in intestinal microbiota of elderly Irish subjects post-antibiotic therapy. J. Antimicrob. Chemother. 2012. [Google Scholar] [CrossRef] [PubMed]
- Van Tongeren, S.P.; Slaets, J.P.; Harmsen, H.J.; Welling, G.W. Fecal microbiota composition and frailty. Appl. Environ. Microbiol. 2005, 71, 6438–6442. [Google Scholar] [CrossRef] [PubMed]
- Perez Martinez, G.; Bauerl, C.; Collado, M.C. Understanding gut microbiota in elderly’s health will enable intervention through probiotics. Benef. Microbes 2014, 5, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Bergsma, N.; Parhiala, R.; Lahtinen, S.; Gueimonde, M.; Finne-Soveri, H.; Strandberg, T.; Pitkala, K.; Salminen, S. Bifidobacterium microbiota and parameters of immune function in elderly subjects. FEMS Immunol. Med. Microbiol. 2008, 53, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Mendez, M.; Perez, M.; Farran, A.; Fuentes, M.C.; Cune, J. Lactobacillus plantarum CECT7315 and CECT7316 stimulate immunoglobulin production after influenza vaccination in elderly. Nutr. Hosp. 2012, 27, 504–509. [Google Scholar] [PubMed]
- Boge, T.; Remigy, M.; Vaudaine, S.; Tanguy, J.; Bourdet-Sicard, R.; van der Werf, S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine 2009, 27, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W65–W94. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Lawrence Erlbaum Associates: Hillside, NJ, USA, 1987. [Google Scholar]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: An investigation of age-related immunological changes. J. Clin. Immunol. 2001, 21, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Rutherfurd, K.J.; Cross, M.L.; Gopal, P.K. Enhancement of immunity in the elderly by dietary supplementation with the probiotic bifidobacterium lactis HN019. Am. J. Clin. Nutr. 2001, 74, 833–839. [Google Scholar] [PubMed]
- Arunachalam, K.; Gill, H.S.; Chandra, R.K. Enhancement of natural immune function by dietary consumption of Bifidobacterium lactis (HN019). Eur. J. Clin. Nutr. 2000, 54, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Chiang, B.L.; Sheih, Y.H.; Wang, L.H.; Liao, C.K.; Gill, H.S. Enhancing immunity by dietary consumption of a probiotic lactic acid bacterium (Bifidobacterium lactis HN019): Optimization and definition of cellular immune responses. Eur. J. Clin. Nutr. 2000, 54, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Clements, S.J.; Carding, S.R. Diet, the intestinal microbiota and immune health in ageing. Crit. Rev. Food Sci. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.T. Epidemiology and unique aspects of aging and infectious diseases. Clin. Infect. Dis. 2000, 30, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Wick, G.; Grubeck-Loebenstein, B. The aging immune system: Primary and secondary alterations of immune reactivity in the elderly. Exp. Gerontol. 1997, 32, 401–413. [Google Scholar] [CrossRef]
- Maneerat, S.; Lehtinen, M.J.; Childs, C.E.; Forssten, S.D.; Alhoniemi, E.; Tiphaine, M.; Yaqoob, P.; Ouwehand, A.C.; Rastall, R.A. Consumption of Bifidobacterium lactis Bi-07 by healthy elderly adults enhances phagocytic activity of monocytes and granulocytes. J. Nutr. Sci. 2013, 2, e44. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.J.; Parlesak, A.; Bode, C.; Bode, J.C.; van’t Hof, M.A.; Grathwohl, D.; Guigoz, Y. Probiotic yogurt in the elderly with intestinal bacterial overgrowth: Endotoxaemia and innate immune functions. Br. J. Nutr. 2009, 101, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; An, E.; Shioi, Y.; Nakamura, K.; Luo, S.; Yokose, N.; Minami, S.; Dan, K. Association between natural killer cell activity and infection in immunologically normal elderly people. Clini. Exp. Immunol. 2001, 124, 392–397. [Google Scholar] [CrossRef]
- Morales, A.; Ottenhof, P.C. Clinical application of a whole blood assay for human natural killer (NK) cell activity. Cancer 1983, 52, 667–670. [Google Scholar] [CrossRef]
- Imai, K.; Matsuyama, S.; Miyake, S.; Suga, K.; Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: An 11-year follow-up study of a general population. Lancet 2000, 356, 1795–1799. [Google Scholar] [CrossRef]
- Ibrahim, F.; Ruvio, S.; Granlund, L.; Salminen, S.; Viitanen, M.; Ouwehand, A.C. Probiotics and immunosenescence: Cheese as a carrier. FEMS Immunol. Med. Microbiol. 2010, 59, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Akatsu, H.; Iwabuchi, N.; Xiao, J.Z.; Matsuyama, Z.; Kurihara, R.; Okuda, K.; Yamamoto, T.; Maruyama, M. Clinical effects of probiotic Bifidobacterium longum BB536 on immune function and intestinal microbiota in elderly patients receiving enteral tube feeding. JPEN J. Parenter. Enter. Nutr. 2013, 37, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Kawase, M.; Kubota, A.; Yoda, K.; Harata, G.; Hosoda, M.; He, F. Heat-killed Lactobacillus gasseri can enhance immunity in the elderly in a double-blind, placebo-controlled clinical study. Benef. Microbes 2015, 6, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Suzuki, T.; Shimada, S.I.; Shida, K.; Nanno, M.; Okumura, K. Interleukin-12 is involved in the enhancement of human natural killer cell activity by Lactobacillus casei Shirota. Clin. Exp. Immunol. 2006, 146, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Rowland, I.; Thomas, L.V.; Yaqoob, P. Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur. J. Nutr. 2013, 52, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Okumura, K. Effects of a fermented milk drink containing Lactobacillus casei strain Shirota on the human NK-cell activity. J. Nutr. 2007, 137, S791–S793. [Google Scholar]
- Didari, T.; Solki, S.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. A systematic review of the safety of probiotics. Expert Opin. Drug Saf. 2014, 13, 227–239. [Google Scholar] [CrossRef] [PubMed]

| Intervention Search Terms |
|---|
| 1. Probiotic |
| 2. Synbiotic |
| 3. Bifidobacteri * |
| 4. Lactis |
| 5. B. lactis |
| 6. HN019 |
| 7. Yogurt (yoghurt) |
| 8. Fermented milk |
| Outcomes Search Terms |
| 9. Phagocyt * |
| 10. Polymorphonuclear |
| 11. PMN |
| 12. Natural killer cell |
| 13. NK cell |
| 14. Tumoricidal |
| 15. Immun * |
| Combination Terms |
| 16. or/1–8 |
| 17. or/9–15 |
| 18. and/16–17 |
| Study | No. Subjects (HN019:Control) | Female (%) | Age (Median, Range) | Delivery Vehicle | HN019 Daily Dose (cfu) | Intervention Duration c |
|---|---|---|---|---|---|---|
| Arunachalam, 2000 [28] | 13:12 | 72 | 69 (60–83) | Low-fat milk | 3 × 1011 | 6 weeks |
| Chiang, 2000 [29] | 27 a | 70 | 60 (41–81) | Low-fat milk | 5 × 1010 | 3 weeks |
| Gill, 2001a [27] | 15 a,b | 60 | 69 (63–84) | Low-fat milk | 5 × 1010 b | 3 weeks |
| Gill, 2001b [26] | 14 a | 57 | 70 (60–84) | Low-fat milk | 5 × 109 | 3 weeks |
| Study | Randomization | Blinding | Control | Definitions | |
|---|---|---|---|---|---|
| Phagocytic Capacity | NK Cell Tumoricidal Activity | ||||
| Arunachalam, 2000 [28] | Yes | Yes | Parallel group | Relative increase in % PMN cells showing phagocytic activity | -- |
| Chiang, 2000 [29] | No | No | 3-week run-in period | % PMN cells showing phagocytic activity | % NK cell tumor killing activity |
| Gill, 2001a [27] | No | No | 3-week run-in period | % PMN cells showing phagocytic activity | % NK cell tumor killing activity |
| Gill, 2001b [26] | No | Yes | 3-week run-in period | -- | % NK cell tumor killing activity |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, L.E.; Lehtoranta, L.; Lehtinen, M.J. The Effect of Bifidobacterium animalis ssp. lactis HN019 on Cellular Immune Function in Healthy Elderly Subjects: Systematic Review and Meta-Analysis. Nutrients 2017, 9, 191. https://doi.org/10.3390/nu9030191
Miller LE, Lehtoranta L, Lehtinen MJ. The Effect of Bifidobacterium animalis ssp. lactis HN019 on Cellular Immune Function in Healthy Elderly Subjects: Systematic Review and Meta-Analysis. Nutrients. 2017; 9(3):191. https://doi.org/10.3390/nu9030191
Chicago/Turabian StyleMiller, Larry E., Liisa Lehtoranta, and Markus J. Lehtinen. 2017. "The Effect of Bifidobacterium animalis ssp. lactis HN019 on Cellular Immune Function in Healthy Elderly Subjects: Systematic Review and Meta-Analysis" Nutrients 9, no. 3: 191. https://doi.org/10.3390/nu9030191




