Protein Nutrition and Malnutrition in CKD and ESRD
Abstract
:1. Introduction
2. Protein Nutrition in Healthy Adults and in CKD and ESRD
3. Metabolic and Regulatory Derangements in CKD and ESRD
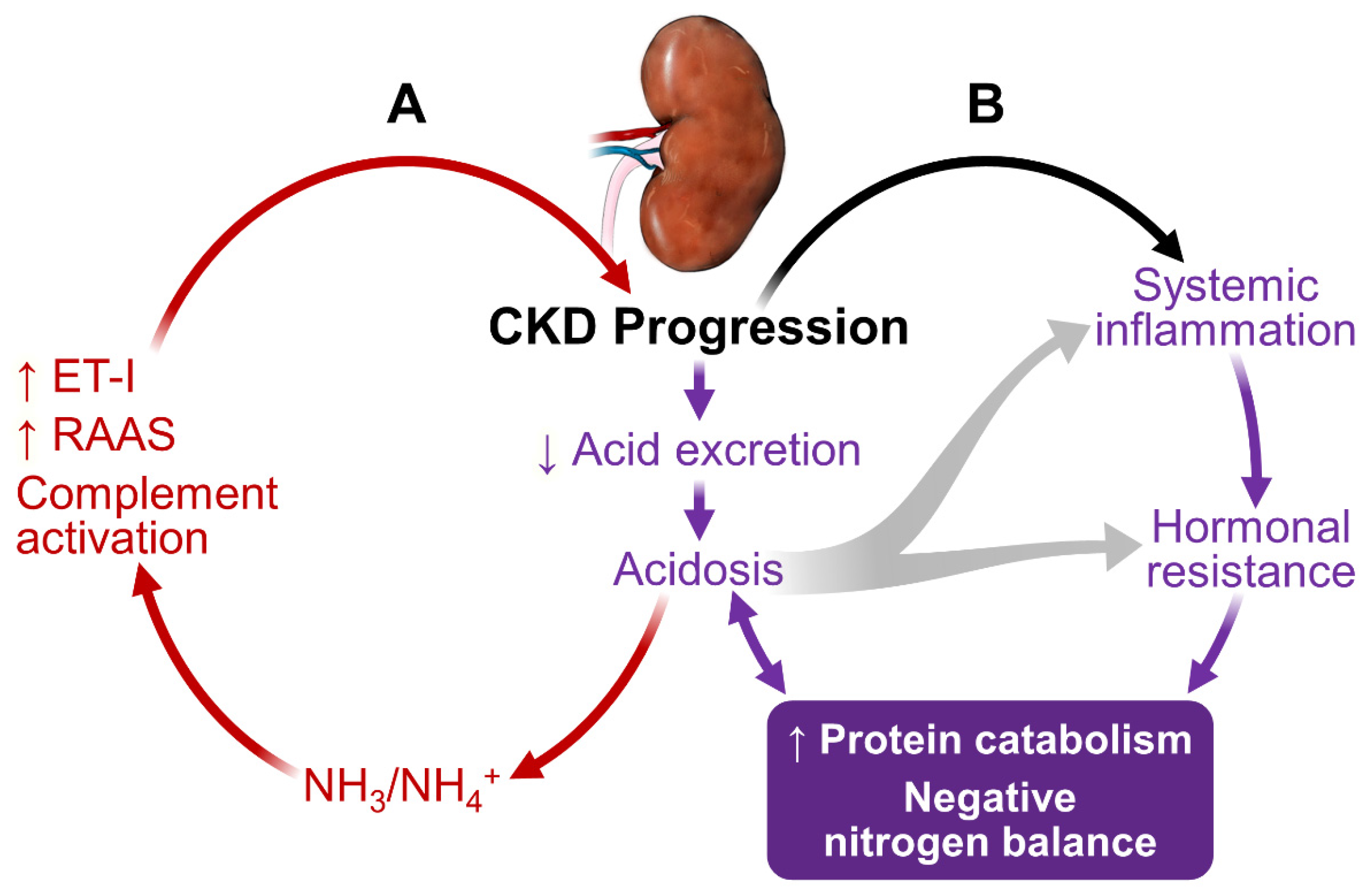
3.1. Metabolic Acidosis
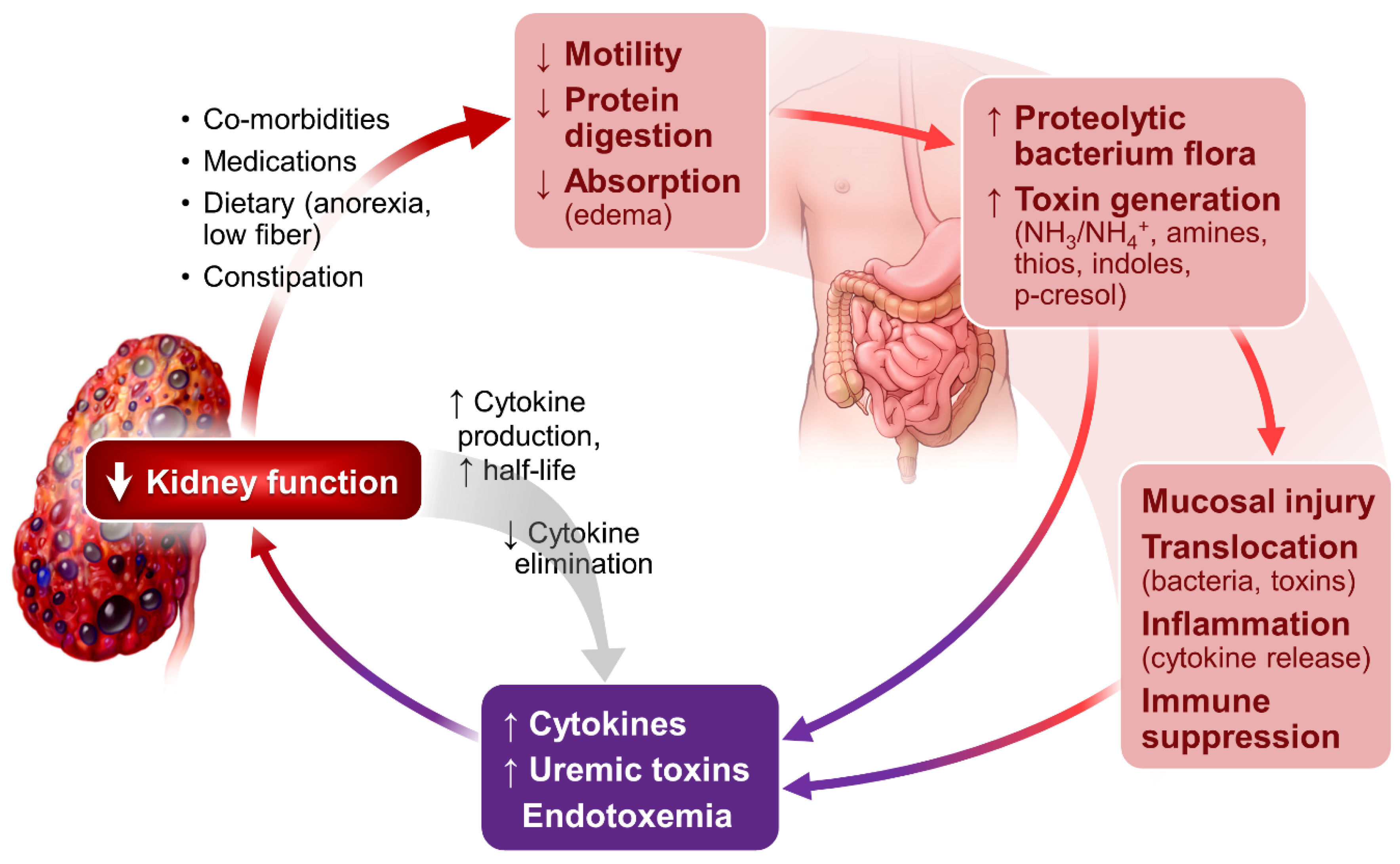
3.2. Sustained Inflammation
3.3. Hormonal Disorders
4. Energy Prescription and Protein-Energy Wasting (PEW) in CKD and ESRD
5. Clinical Recommendations
5.1. Optimizing Nutritional Therapy
5.2. Correcting Metabolic Acidosis
5.3. Eliminating Correctible Inflammatory Factors
5.4. Minimizing Hormonal Alterations
5.5. Increasing Physical Activity
6. Summary
Key Points:
- Protein malnutrition is common in patients with CKD and ESRD, a growing patient population worldwide.
- Protein malnutrition is associated with increased morbidity and mortality.
- Protein malnutrition can be prevented and substantially reversed with ongoing dietary monitoring and nutritional therapy.
- Protein intake of 0.6–0.8 g/kg/day for non-dialysis CKD patients and 1.0–1.2 g/kg/day for patients on peritoneal dialysis or hemodialysis, with >50% HBV proteins, are advised.
- Daily energy intake in CKD and ESRD patients should be 30–35 kcal/kg (ideal body weight).
- Metabolic acidosis is related to multiple metabolic derangements, adversely affects kidney and patient outcome, and should be corrected.
- In patients with stage 3 CKD and without evidence of metabolic acidosis, the dietary modification with increased basis such as vegetables and fruits may be initiated to prevent metabolic acidosis.
- Constipation and abnormal bowel habits can compromise gut epithelial cell integrity causing dysbiosis, promoting inflammation and uremic toxin accumulation.
- Exercise, based on patient capacity, should be incorporated in as a part of the CKD and ESRD management.
- Anabolic hormone replacement is controversial and has not been routinely used for adult CKD and ESRD patients.
- Current recommendation is to supplement 25(OH)-vitamin D for CKD and ESRD patients with suboptimal vitamin D status.
Conflicts of Interest
Abbreviations
| UPS | Proteasome-ubiquitin system |
| CKD | Chronic kidney disease |
| ESRD | End-stage renal disease |
| HBV | High biological value |
| IS | Indoxyl sulfate |
| pCS | p-Cresyl sulfate |
| HCl | Hydrogen chloride |
| H2SO4 | Sulfuric acid |
| H3PO4 | Phosphoric acids |
| NaHCO3 | Sodium bicarbonate |
| TWEAK | TNF-related weak inducer of apoptosis |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| NF-kappa B | Nuclear factor kappa light chain enhancer of activated B cells |
| SOCS3 | Suppressor of cytokine signaling |
| IRS-1 | Insulin receptor substrate 1 |
| GH-IGF-IGFBP | Growth factor-insulin-like-growth factor binding protein |
| IGF-1 | Insulin like growth factor-1 |
| KDOQI | Kidney Disease Outcomes Quality Initiative |
| PEW | Protein-energy wasting |
References
- De Nicola, L.; Zoccali, C. Chronic kidney disease prevalence in the general population: Heterogeneity and concerns. Nephrol. Dial. Transplant. 2016, 31, 331–335. [Google Scholar] [CrossRef] [PubMed]
- 2015 USRDS Annual Data Report. Volume 2. Available online: https://www.usrds.org/2015/download/vol2_USRDS_ESRD_15.pdf (accessed on 14 January 2017).
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; TerWee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Gracia-Iguacel, C.; González-Parra, E.; Pérez-Gómez, M.V.; Mahíllo, I.; Egido, J.; Ortiz, A.; Carrero, J.J. Prevalence of protein-energy wasting syndrome and its association with mortality in haemodialysis patients in a centre in Spain. Nefrologia 2013, 33, 495–505. [Google Scholar] [PubMed]
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.W.; Byham-Gray, L.D.; Scott Parrott, J.; Rigassio-Radler, D.; Mandayam, S.; Jones, S.L.; Mitch, W.E.; Gaber, A.O. The mean dietary protein intake at different stages of chronic kidney disease is higher than current guidelines. Kidney Int. 2013, 83, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Koeppen, B.M. The kidney and acid-base regulation. Adv. Physiol. Educ. 2009, 33, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Wrong, O.; Davies, H.E. The excretion of acid in renal disease. Q. J. Med. 1959, 28, 259–313. [Google Scholar] [PubMed]
- Kraut, J.A.; Kurtz, I. Metabolic acidosis of CKD: Diagnosis, clinical characteristics, and treatment. Am. J. Kidney Dis. 2005, 45, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D.; Kalantar-Zadeh, K.; Mehrotra, R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int. Suppl. 2005. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L.; Zhang, Y.; Ying, J.; Greene, T. Prevalence of and risk factors for reduced serum bicarbonate in chronic kidney disease. Nephrology 2014, 19, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Driver, T.H.; Shlipak, M.G.; Katz, R.; Goldenstein, L.; Sarnak, M.J.; Hoofnagle, A.N.; Siscovick, D.S.; Kestenbaum, B.; de Boer, I.H.; Ix, J.H. Low serum bicarbonate and kidney function decline: The Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Kidney Dis. 2014, 64, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Baylis, C.; Verlander, J.W.; Han, K.H.; Reungjui, S.; Handlogten, M.E.; Weiner, I.D. Effect of reduced renal mass on renal ammonia transporter family, Rh C glycoprotein and Rh B glycoprotein, expression. Am. J. Physiol. Renal. Physiol. 2007, 293, F1238–F1247. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.; Attmane-Elakeb, A.; Bichara, M. Renal handling of NH4+ in relation to the control of acid-base balance by the kidney. J. Nephrol. 2002, 15 (Suppl. 5), S128–S134. [Google Scholar] [PubMed]
- Nath, K.A.; Hostetter, M.K.; Hostetter, T.H. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J. Clin. Investig. 1985, 76, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Simoni, J. Acid retention during kidney failure induces endothelin and aldosterone production which lead to progressive GFR decline, a situation ameliorated by alkali diet. Kidney Int. 2010, 78, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Simoni, J.; Broglio, K.; Sheather, S. Acid retention accompanies reduced GFR in humans and increases plasma levels of endothelin and aldosterone. Am. J. Physiol. Renal. Physiol. 2011, 300, F830–F837. [Google Scholar] [CrossRef] [PubMed]
- Reaich, D.; Channon, S.M.; Scrimgeour, C.M.; Goodship, T.H. Ammonium chloride-induced acidosis increases protein breakdown and amino acid oxidation in humans. Am. J. Physiol. 1992, 263, E735–E739. [Google Scholar] [PubMed]
- Ballmer, P.E.; McNurlan, M.A.; Hulter, H.N.; Anderson, S.E.; Garlick, P.J.; Krapf, R. Chronic metabolic acidosis decreases albumin synthesis and induces negative nitrogen balance in humans. J. Clin. Investig. 1995, 95, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Pickering, W.P.; Price, S.R.; Bircher, G.; Marinovic, A.C.; Mitch, W.E.; Walls, J. Nutrition in CAPD: Serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int. 2002, 61, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Vallet, M.; Metzger, M.; Haymann, J.P.; Flamant, M.; Gauci, C.; Thervet, E.; Boffa, J.J.; Vrtovsnik, F.; Froissart, M.; Stengel, B.; et al. Urinary ammonia and long-term outcomes in chronic kidney disease. Kidney Int. 2015, 88, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.; Abramowitz, M.; Hostetter, T.H.; Melamed, M.L. Serum bicarbonate levels and the progression of kidney disease: A cohort study. Am. J. Kidney Dis. 2009, 54, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Raphael, K.L.; Wei, G.; Baird, B.C.; Greene, T.; Beddhu, S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 2011, 79, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Kanda, E.; Ai, M.; Yoshida, M.; Kuriyama, R.; Shiigai, T. High serum bicarbonate level within the normal range prevents the progression of chronic kidney disease in elderly chronic kidney disease patients. BMC Nephrol. 2013, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- De Brito-Ashurst, I.; Varagunam, M.; Raftery, M.J.; Yaqoob, M.M. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J. Am. Soc. Nephrol. 2009, 20, 2075–2084. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Simoni, J.; Sheather, S.J.; Broglio, K.R.; Rajab, M.H.; Wesson, D.E. Daily oral sodium bicarbonate preserves glomerular filtration rate by slowing its decline in early hypertensive nephropathy. Kidney Int. 2010, 78, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Phisitkul, S.; Khanna, A.; Simoni, J.; Broglio, K.; Sheather, S.; Rajab, M.H.; Wesson, D.E. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 2010, 77, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Abramowitz, M.K.; Melamed, M.L.; Bauer, C.; Raff, A.C.; Hostetter, T.H. Effects of oral sodium bicarbonate in patients with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and Progression of CKD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Snaedal, S.; Qureshi, A.R.; Lund, S.H.; Germanis, G.; Hylander, B.; Heimbürger, O.; Carrero, J.J.; Stenvinkel, P.; Bárány, P. Dialysis modality and nutritional status are associated with variability of inflammatory markers. Nephrol. Dial. Transplant. 2016, 31, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Raj, D.S. The gut microbiome, kidney disease, and targeted interventions. J. Am. Soc. Nephrol. 2014, 25, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Hida, M.; Aiba, Y.; Sawamura, S.; Suzuki, N.; Satoh, T.; Koga, Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 1996, 74, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Bammens, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int. 2003, 64, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Yuan, J.; Rahimi, A.; Ni, Z.; Said, H.; Subramanian, V.S. Disintegration of colonic epithelial tight junction in uremia: A likely cause of CKD-associated inflammation. Nephrol. Dial. Transplant. 2012, 27, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Goshtasbi, N.; Yuan, J.; Jellbauer, S.; Moradi, H.; Raffatellu, M.; Kalantar-Zadeh, K. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am. J. Nephrol. 2012, 36, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Yuan, J.; Norris, K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am. J. Nephrol. 2013, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pugin, J.; Heumann, I.D.; Tomasz, A.; Kravchenko, V.V.; Akamatsu, Y.; Nishijima, M.; Glauser, M.P.; Tobias, P.S.; Ulevitch, R.J. CD14 is a pattern recognition receptor. Immunity 1994, 1, 509–516. [Google Scholar] [CrossRef]
- Freudenberg, M.A.; Tchaptchet, S.; Keck, S.; Fejer, G.; Huber, M.; Schütze, N.; Beutler, B.; Galanos, C. Lipopolysaccharide sensing an important factor in the innate immune response to Gram-negative bacterial infections: Benefits and hazards of LPS hypersensitivity. Immunobiology 2008, 213, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Hatch, M.; Vaziri, N.D. Enhanced enteric excretion of urate in rats with chronic renal failure. Clin. Sci. (Lond.) 1994, 86, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Piceno, Y.M.; Desantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Anfora, A.T.; Liu, J.; Schultz, P.G.; Lesley, S.A.; Peters, E.C.; Siuzdak, G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 2009, 106, 3698–3703. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Ise, M.; Seo, H.; Niwa, T. Indoxyl sulfate increases the gene expressions of TGF-beta 1, TIMP-1 and pro-alpha 1(I) collagen in uremic rat kidneys. Kidney Int. Suppl. 1997, 62, S15–S22. [Google Scholar] [PubMed]
- Watanabe, H.; Miyamoto, Y.; Honda, D.; Tanaka, H.; Wu, Q.; Endo, M.; Noguchi, T.; Kadowaki, D.; Ishima, Y.; Kotani, S.; et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013, 83, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.L.; Bonan, N.B.; Dias, G.; Brehm, F.; Steiner, T.M.; Souza, W.M.; Stinghen, A.E.; Barreto, F.C.; Elifio-Esposito, S.; Pecoits-Filho, R.; et al. p-Cresyl sulfate affects the oxidative burst, phagocytosis process, and antigen presentation of monocyte-derived macrophages. Toxicol. Lett. 2016, 263, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Chang, S.C.; Wu, M.S. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS ONE 2012, 7, e34026. [Google Scholar] [CrossRef] [PubMed]
- Adijiang, A.; Higuchi, Y.; Nishijima, F.; Shimizu, H.; Niwa, T. Indoxyl sulfate, a uremic toxin, promotes cell senescence in aorta of hypertensive rats. Biochem. Biophys. Res. Commun. 2010, 399, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; European Uremic Toxin Work Group (EUTox). Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.; Claes, K.; Bammens, B.; de Loor, H.; Viaene, L.; Verbeke, K.; Kuypers, D.; Vanrenterghem, Y.; Evenepoel, P. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, H.A.; Engelke, U.F.; Wilmer, M.J.; Wetzels, J.F.; Wevers, R.A.; van den Heuvel, L.P.; Hoenderop, J.G.; Masereeuw, R. Optimized metabolomic approach to identify uremic solutes in plasma of stage 3–4 chronic kidney disease patients. PLoS ONE 2013, 8, e71199. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Witasp, A.; Carrero, J.J.; Heimbürger, O.; Lindholm, B.; Hammarqvist, F.; Stenvinkel, P.; Nordfors, L. Increased expression of pro-inflammatory genes in abdominal subcutaneous fat in advanced chronic kidney disease patients. J. Intern. Med. 2011, 269, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Rashid Qureshi, A.; Suliman, M.E.; Honda, H.; Pecoits-Filho, R.; Heimbürger, O.; Lindholm, B.; Cederholm, T.; Stenvinke, P. Truncal fat mass as a contributor to inflammation in end-stage renal disease. Am. J. Clin. Nutr. 2004, 80, 1222–1229. [Google Scholar] [PubMed]
- Gohda, T.; Gotoh, H.; Tanimoto, M.; Sato, M.; Io, H.; Kaneko, K.; Hamada, C.; Tomino, Y. Relationship between abdominal fat accumulation and insulin resistance in hemodialysis patients. Hypertens. Res. 2008, 31, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Odamaki, M.; Furuya, R.; Ohkawa, S.; Yoneyama, T.; Nishikino, M.; Hishida, A.; Kumagai, H. Altered abdominal fat distribution and its association with the serum lipid profile in non-diabetic haemodialysis patients. Nephrol. Dial. Transplant. 1999, 14, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Odamaki, M.; Furuya, R.; Kinumura, Y.; Ikegaya, N.; Kumagai, H. Association between plasma adiponectin concentration and visceral fat accumulation in hemodialysis patients. Nephron. Clin. Pract. 2006, 102, c8–c13. [Google Scholar] [CrossRef] [PubMed]
- Witasp, A.; Carrero, J.J.; Hammarqvist, F.; Qureshi, A.R.; Heimbürger, O.; Schalling, M.; Lindholm, B.; Nordfors, L.; Stenvinkel, P. Expression of osteoprotegerin in human fat tissue; implications for chronic kidney disease. Eur. J. Clin. Investig. 2011, 41, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, M.A.; Carrero, J.J.; Canziani, M.E.; Watanabe, R.; Lemos, M.M.; Cuppari, L. Visceral obesity assessed by computed tomography predicts cardiovascular events in chronic kidney disease patients. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Luger, A.; Kovarik, J.; Stummvoll, H.K.; Urbanska, A.; Luger, T.A. Blood-membrane interaction in hemodialysis leads to increased cytokine production. Kidney Int. 1987, 32, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Borazan, A.; Ustün, H.; Ustundag, Y.; Aydemir, S.; Bayraktaroglu, T.; Sert, M.; Yilmaz, A. The effects of peritoneal dialysis and hemodialysis on serum tumor necrosis factor-alpha, interleukin-6, interleukin-10 and C-reactive-protein levels. Mediat. Inflamm. 2004, 13, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P. Inflammation in end-stage renal disease—What have we learned in 10 years? Semin. Dial. 2010, 23, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.J.; Moldawer, L.L. The origins of cachexia in acute and chronic inflammatory diseases. Nutr. Clin. Pract. 2006, 21, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Meuwese, C.L.; Stenvinkel, P.; Dekker, F.W.; Carrero, J.J. Monitoring of inflammation in patients on dialysis: Forewarned is forearmed. Nat. Rev. Nephrol. 2011, 7, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Dogra, C.; Changotra, H.; Wedhas, N.; Qin, X.; Wergedal, J.E.; Kumar, A. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J. 2007, 21, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Ortiz, A.; Qureshi, A.R.; Martín-Ventura, J.L.; Bárány, P.; Heimbürger, O.; Marrón, B.; Metry, G.; Snaedal, S.; Lindholm, B.; et al. Additive effects of soluble TWEAK and inflammation on mortality in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Szabo, G.; Dallmann, G.; Müller, G.; Patthy, L.; Soller, M.; Varga, L. A deletion in the myostatin gene causes the compact (Cmpt) hypermuscular mutation in mice. Mamm. Genome 1998, 9, 671–672. [Google Scholar] [CrossRef] [PubMed]
- Han, H.Q.; Mitch, W.E. Targeting the myostatin signaling pathway to treat muscle wasting diseases. Curr. Opin. Support Palliat. Care 2011, 5, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Hu, Z.; Klein, J.D.; Zhang, L.; Fang, F.; Mitch, W.E. Decreased miR-29 suppresses myogenesis in CKD. J. Am. Soc. Nephrol. 2011, 22, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Rajan, V.; Lin, E.; Hu, Z.; Han, H.Q.; Zhou, X.; Song, Y.; Min, H.; Wang, X.; Du, J.; Mitch, W.E. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 2011, 25, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Brandt, C.; Nielsen, A.R.; Hojman, P.; Whitham, M.; Febbraio, M.A.; Pedersen, B.K.; Plomgaard, P. Exercise induces a marked increase in plasma follistatin: Evidence that follistatin is a contraction-induced hepatokine. Endocrinology 2011, 152, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Leehey, D.J.; Moinuddin, I.; Bast, J.P.; Qureshi, S.; Jelinek, C.S.; Cooper, C.; Edwards, L.C.; Smith, B.M.; Collins, E.G. Aerobic exercise in obese diabetic patients with chronic kidney disease: A randomized and controlled pilot study. Cardiovasc Diabetol. 2009, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wang, H.; Lee, I.H.; Du, J.; Mitch, W.E. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J. Clin. Investig. 2009, 119, 3059–3069. [Google Scholar] [CrossRef] [PubMed]
- Spindler, S.R. Caloric restriction: From soup to nuts. Ageing Res. Rev. 2010, 9, 324–353. [Google Scholar] [CrossRef] [PubMed]
- Finn, P.F.; Dice, J.F. Proteolytic and lipolytic responses to starvation. Nutrition 2006, 22, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Keusch, G.T. The history of nutrition: Malnutrition, infection and immunity. J. Nutr. 2003, 133, 336S–340S. [Google Scholar] [PubMed]
- Zhang, L.; Du, J.; Hu, Z.; Han, G.; Delafontaine, P.; Garcia, G.; Mitch, W.E. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 2009, 20, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.S.; Moseley, P.; Dominic, E.A.; Onime, A.; Tzamaloukas, A.H.; Boyd, A.; Shah, V.O.; Glew, R.; Wolfe, R.; Ferrando, A. Interleukin-6 modulates hepatic and muscle protein synthesis during hemodialysis. Kidney Int. 2008, 73, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Boivin, M.A.; Battah, S.I.; Dominic, E.A.; Kalantar-Zadeh, K.; Ferrando, A.; Tzamaloukas, A.H.; Dwivedi, R.; Ma, T.A.; Moseley, P.; Raj, D.S. Activation of caspase-3 in the skeletal muscle during haemodialysis. Eur. J. Clin. Investig. 2010, 40, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Feneberg, R.; Schaefer, F.; Veldhuis, J.D. Neuroendocrine adaptations in renal disease. Pediatr. Nephrol. 2003, 18, 492–497. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Taylor, S.I.; Olefsky, J.M. Physiological action of insulin. In Diabetes Mellitus: A Fundamental and Clinical Text; Leroith, D.T.S., Olefsky, J., Eds.; Williams & Wilkins: Philadelphia, PA, USA, 2000; pp. 148–161. [Google Scholar]
- DeFronzo, R.A.; Alvestrand, A.; Smith, D.; Hendler, R.; Hendler, E.; Wahren, J. Insulin resistance in uremia. J. Clin. Investig. 1981, 67, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Pupim, L.B.; Flakoll, P.J.; Majchrzak, K.M.; Aftab Guy, D.L.; Stenvinkel, P.; Ikizler, T.A. Increased muscle protein breakdown in chronic hemodialysis patients with type 2 diabetes mellitus. Kidney Int. 2005, 68, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Siew, E.D.; Pupim, L.B.; Majchrzak, K.M.; Shintani, A.; Flakoll, P.J.; Ikizler, T.A. Insulin resistance is associated with skeletal muscle protein breakdown in non-diabetic chronic hemodialysis patients. Kidney Int. 2007, 71, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Pillon, N.J.; Vella, R.E.; Croze, M.L.; Pelletier, C.C.; Chambert, S.; Massy, Z.; Glorieux, G.; Vanholder, R.; Dugenet, Y. p-Cresyl sulfate promotes insulin resistance associated with CKD. J. Am. Soc. Nephrol. 2013, 24, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Moller, J. Effects of growth hormone on fluid homeostasis. Clinical and experimental aspects. Growth Horm. IGF Res. 2003, 13, 55–74. [Google Scholar] [CrossRef]
- Powell, D.R. Effects of renal failure on the growth hormone-insulin-like growth factor axis. J. Pediatr. 1997, 131, S13–S16. [Google Scholar] [CrossRef]
- Tonshoff, B.; Blum, W.F.; Mehls, O. Derangements of the somatotropic hormone axis in chronic renal failure. Kidney Int. Suppl. 1997, 58, S106–S113. [Google Scholar] [PubMed]
- Haffner, D.; Schaefer, F.; Girard, J.; Ritz, E.; Mehls, O. Metabolic clearance of recombinant human growth hormone in health and chronic renal failure. J. Clin. Investig. 1994, 93, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, F.; Chen, Y.; Tsao, T.; Nouri, P.; Rabkin, R. Impaired JAK-STAT signal transduction contributes to growth hormone resistance in chronic uremia. J. Clin. Investig. 2001, 108, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.F.; Zheng, Z.; Tummala, P.; Oh, J.; Schaefer, F.; Rabkin, R. Chronic uremia attenuates growth hormone-induced signal transduction in skeletal muscle. J. Am. Soc. Nephrol. 2004, 15, 2630–2636. [Google Scholar] [CrossRef] [PubMed]
- Ferry, R.J., Jr.; Cerri, R.W.; Cohen, P. Insulin-like growth factor binding proteins: New proteins, new functions. Horm. Res. 1999, 51, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Roelfsema, V.; Lane, M.H.; Clark, R.G. Insulin-like growth factor binding protein (IGFBP) displacers: Relevance to the treatment of renal disease. Pediatr. Nephrol. 2000, 14, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Pak, Y.K. End-organ resistance to growth hormone and IGF-I in epiphyseal chondrocytes of rats with chronic renal failure. Kidney Int. 1996, 50, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, S.K.; Kim, J.S. Insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children with nephrotic syndrome. J. Clin. Endocrinol. Metab. 1996, 81, 1856–1860. [Google Scholar] [PubMed]
- Du, J.; Wang, X.; Miereles, C.; Bailey, J.L.; Debigare, R.; Zheng, B.; Price, S.R.; Mitch, W.E. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J. Clin. Investig. 2004, 113, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zhang, Y.; Ren, J. IGF-1 deficiency resists cardiac hypertrophy and myocardial contractile dysfunction: Role of microRNA-1 and microRNA-133a. J. Cell Mol. Med. 2012, 16, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Elia, L.; Contu, R.; Quintavalle, M.; Varrone, F.; Chimenti, C.; Russo, M.A.; Cimino, V.; De Marinis, L.; Frustaci, A.; Catalucci, D.; et al. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation 2009, 120, 2377–2385. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.B.; Xu, H.; Xie, S.J.; Zhou, H.; Qu, L.H. Insulin-like growth factor-1 receptor is regulated by microRNA-133 during skeletal myogenesis. PLoS ONE 2011, 6, e29173. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P. The vulnerable man: Impact of testosterone deficiency on the uraemic phenotype. Nephrol. Dial. Transplant. 2012, 27, 4030–4041. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J.; Dong, Q. Hypothalamo-pituitary gonadal axis in chronic renal failure. Endocrinol. Metab. Clin. North Am. 1993, 22, 145–161. [Google Scholar] [PubMed]
- Holley, J.L. The hypothalamic-pituitary axis in men and women with chronic kidney disease. Adv. Chronic Kidney Dis. 2004, 11, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Phadke, A.G.; MacKinnon, K.J.; Dossetor, J.B. Male fertility in uremia: Restoration by renal allografts. Can. Med. Assoc. J. 1970, 102, 607–608. [Google Scholar] [PubMed]
- Lim, V.S.; Fang, V.S. Gonadal dysfunction in uremic men. A study of the hypothalamo-pituitary-testicular axis before and after renal transplantation. Am. J. Med. 1975, 58, 655–662. [Google Scholar] [CrossRef]
- Cigarran, S.; Pousa, M.; Castro, M.J.; González, B.; Martínez, A.; Barril, G.; Aguilera, A.; Coronel, F.; Stenvinkel, P.; Carrero, J.J. Endogenous testosterone, muscle strength, and fat-free mass in men with chronic kidney disease. J. Ren. Nutr. 2013, 23, e89–e95. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.E.; Bailey, J.L.; Wang, X.; Jurkovitz, C.; Newby, D.; Price, S.R. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am. J. Physiol. 1999, 276, C1132–C1138. [Google Scholar] [PubMed]
- May, R.C.; Kelly, R.A.; Mitch, W.E. Metabolic acidosis stimulates protein degradation in rat muscle by a glucocorticoid-dependent mechanism. J. Clin. Investig. 1986, 77, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Ohkawa, S.; Li, H.; Roberts-Wilson, T.K.; Price, S.R. FOXO3a mediates signaling crosstalk that coordinates ubiquitin and atrogin-1/MAFbx expression during glucocorticoid-induced skeletal muscle atrophy. FASEB J. 2010, 24, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Nigwekar, S.U.; Bhan, I.; Thadhani, R. Ergocalciferol and cholecalciferol in CKD. Am. J. Kidney Dis. 2012, 60, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; David, V.; Quarles, L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol. Rev. 2012, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Girgis, C.M.; Clifton-Bligh, R.J.; Mokbel, N.; Cheng, K.; Gunton, J.E. Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology 2014, 155, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Harter, H.R.; Birge, S.J.; Martin, K.J.; Klahr, S.; Karl, I.E. Effects of vitamin D metabolites on protein catabolism of muscle from uremic rats. Kidney Int. 1983, 23, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Tajar, A.; Pye, S.R.; Boonen, S.; Vanderschueren, D.; Bouillon, R.; O’Neill, T.W.; Bartfai, G.; Casanueva, F.F.; Finn, J.D.; et al. Association of hypogonadism with vitamin D status: The European Male Ageing Study. Eur. J. Endocrinol. 2012, 166, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gordon, P.L.; Sakkas, G.K.; Doyle, J.W.; Shubert, T.; Johansen, K.L. Relationship between vitamin D and muscle size and strength in patients on hemodialysis. J. Ren. Nutr. 2007, 17, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lin, J.; Qian, Q. Role of Vitamin D in Cognitive Function in Chronic Kidney Disease. Nutrients 2016. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D.; Monteon, F.J.; Shaib, J.K. Effect of energy intake on nitrogen metabolism in nondialyzed patients with chronic renal failure. Kidney Int. 1986, 29, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Sciatti, E.; Lombardi, C.; Ravera, A.; Vizzardi, E.; Bonadei, I.; Carubelli, V.; Gorga, E.; Metra, M. Nutritional Deficiency in Patients with Heart Failure. Nutrients 2016, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Avesani, C.M.; Cuppari, L.; Silva, A.C.; Sigulem, D.M.; Cendoroglo, M.; Sesso, R.; Draibe, S.A. Resting energy expenditure in pre-dialysis diabetic patients. Nephrol. Dial. Transplant. 2001, 16, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Neyra, R.; Chen, K.Y.; Sun, M.; Shyr, Y.; Hakim, R.M.; Ikizler, T.A. Increased resting energy expenditure in patients with end-stage renal disease. JPEN J. Parenter. Enter. Nutr. 2003, 27, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Sea, M.M.; Tang, N.; Sanderson, J.E.; Lui, S.F.; Li, P.K.; Woo, J. Resting energy expenditure and subsequent mortality risk in peritoneal dialysis patients. J. Am. Soc. Nephrol. 2004, 15, 3134–3143. [Google Scholar] [CrossRef] [PubMed]
- Cuppari, L.; de Carvalho, A.B.; Avesani, C.M.; Kamimura, M.A.; Dos Santos Lobão, R.R.; Draibe, S.A. Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. J. Am. Soc. Nephrol. 2004, 15, 2933–2939. [Google Scholar] [CrossRef] [PubMed]
- Utaka, S.; Avesani, C.M.; Draibe, S.A.; Kamimura, M.A.; Andreoni, S.; Cuppari, L. Inflammation is associated with increased energy expenditure in patients with chronic kidney disease. Am. J. Clin. Nutr. 2005, 82, 801–805. [Google Scholar] [PubMed]
- Kamimura, M.A.; Draibe, S.A.; Dalboni, M.A.; Cendoroglo, M.; Avesani, C.M.; Manfredi, S.R.; Canziani, M.E.; Cuppari, L. Serum and cellular interleukin-6 in haemodialysis patients: Relationship with energy expenditure. Nephrol. Dial. Transplant. 2007, 22, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Deleaval, P.; Teta, D.; Cleaud, C.; Arkouche, W.; Jolivot, A.; Fouque, D. Influence of inflammation on total energy expenditure in hemodialysis patients. J. Ren. Nutr. 2011, 21, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Kaysen, G.A.; Greene, T.; Daugirdas, J.T.; Kimmel, P.L.; Schulman, G.W.; Toto, R.D.; Levin, N.W.; Yan, G.; HEMO Study Group. Longitudinal and cross-sectional effects of C-reactive protein, equilibrated normalized protein catabolic rate, and serum bicarbonate on creatinine and albumin levels in dialysis patients. Am. J. Kidney Dis. 2003, 42, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Tom, K.; Young, V.R.; Chapman, T.; Masud, T.; Akpele, L.; Maroni, B.J. Long-term adaptive responses to dietary protein restriction in chronic renal failure. Am. J. Physiol. 1995, 268, E668–E677. [Google Scholar] [PubMed]
- Masud, T.; Young, V.R.; Chapman, T.; Maroni, B.J. Adaptive responses to very low protein diets: The first comparison of ketoacids to essential amino acids. Kidney Int. 1994, 45, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, X.; Yang, L.; Li, Z.; Qin, W. Effect of restricted protein diet supplemented with keto analogues in chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 2016, 48, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Block, G.; McAllister, C.J.; Humphreys, M.H.; Kopple, J.D. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am. J. Clin. Nutr. 2004, 80, 299–307. [Google Scholar] [PubMed]
- Shinaberger, C.S.; Kilpatrick, R.D.; Regidor, D.L.; McAllister, C.J.; Greenland, S.; Kopple, J.D.; Kalantar-Zadeh, K. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am. J. Kidney Dis. 2006, 48, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Ikizler, T.A.; Block, G.; Avram, M.M.; Kopple, J.D. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am. J. Kidney Dis. 2003, 42, 864–881. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Pupim, L.B.; Brouillette, J.R.; Levenhagen, D.K.; Farmer, K.; Hakim, R.M.; Flakoll, P.J. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E107–E116. [Google Scholar] [PubMed]
- Avesani, C.M.; Trolonge, S.; Deléaval, P.; Baria, F.; Mafra, D.; Faxén-Irving, G.; Chauveau, P.; Teta, D.; Kamimura, M.A.; Cuppari, L.; et al. Physical activity and energy expenditure in haemodialysis patients: An international survey. Nephrol. Dial. Transplant. 2012, 27, 2430–2434. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Regolisti, G.; Karupaiah, T.; Sahathevan, S.; Sadu Singh, B.K.; Khor, B.H.; Salhab, N.; Karavetian, M.; Cupisti, A.; Fiaccadori, E. Protein-energy wasting and nutritional supplementation in patients with end-stage renal disease on hemodialysis. Clin. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Pupim, L.B.; Caglar, K.; Hakim, R.M.; Shyr, Y.; Ikizler, T.A. Uremic malnutrition is a predictor of death independent of inflammatory status. Kidney Int. 2004, 66, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Rambod, M.; Bross, R.; Zitterkoph, J.; Benner, D.; Pithia, J.; Colman, S.; Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Association of Malnutrition-Inflammation Score with quality of life and mortality in hemodialysis patients: A 5-year prospective cohort study. Am. J. Kidney Dis. 2009, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2001, 37 (Suppl. 2), S66–S70. [Google Scholar] [CrossRef] [PubMed]
- Dhondup, T.; Qian, Q. Electrolyte and Acid-base Disorders in Chronic Kidney Disease and End-stage Kidney Failure. Blood Purif. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.M.; You, A.S.; Koontz Parsons, T.; Tortorici, A.R.; Bross, R.; St-Jules, D.E.; Jing, J.; Lee, M.L.; Benner, D.; Kovesdy, C.P.; et al. Effect of high-protein meals during hemodialysis combined with lanthanum carbonate in hypoalbuminemic dialysis patients: Findings from the FrEDI randomized controlled trial. Nephrol. Dial. Transplant. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Cano, N.J.; Budde, K.; Chazot, C.; Kovesdy, C.P.; Mak, R.H.; Mehrotra, R.; Raj, D.S.; Sehgal, A.R.; Stenvinkel, P.; et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat. Rev. Nephrol. 2011, 7, 369–384. [Google Scholar] [CrossRef] [PubMed]
- McSherry, E.; Morris, R.C., Jr. Attainment and maintenance of normal stature with alkali therapy in infants and children with classic renal tubular acidosis. J. Clin. Investig. 1978, 61, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.; Morris, R.C., Jr.; Sebastian, A. Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J. Clin. Endocrinol. Metab. 1997, 82, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Witham, M.D.; Band, M.M.; Littleford, R.C.; Avenell, A.; Soiza, R.L.; McMurdo, M.E.; Sumukadas, D.; Ogston, S.A.; Lamb, E.J.; Hampson, G.; et al. Does oral sodium bicarbonate therapy improve function and quality of life in older patients with chronic kidney disease and low-grade acidosis (the BiCARB trial)? Study protocol for a randomized controlled trial. Trials 2015, 16, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaggl, M.; Cejka, D.; Plischke, M.; Heinze, G.; Fraunschiel, M.; Schmidt, A.; Hörl, W.H.; Sunder-Plassmann, G. Effect of oral sodium bicarbonate supplementation on progression of chronic kidney disease in patients with chronic metabolic acidosis: Study protocol for a randomized controlled trial (SoBic-Study). Trials 2013, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.; Aucella, F.; Conte, G.; Cupisti, A.; Santoro, D. A prospective, multicenter, randomized, controlled study: The correction of metabolic acidosis with use of bicarbonate in Chronic Renal Insufficiency (UBI) Study. J. Nephrol. 2012, 25, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Andrassy, K.M. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int. 2013, 84, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Anderson, J.E.; Kalantar-Zadeh, K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol. Dial. Transplant. 2009, 24, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Dobre, M.; Yang, W.; Pan, Q.; Appel, L.; Bellovich, K.; Chen, J.; Feldman, H.; Fischer, M.J.; Ham, L.L.; Hostetter, T.; et al. Persistent high serum bicarbonate and the risk of heart failure in patients with chronic kidney disease (CKD): A report from the Chronic Renal Insufficiency Cohort (CRIC) study. J. Am. Heart Assoc. 2015, 4, e001599. [Google Scholar] [CrossRef] [PubMed]
- Reaich, D.; Channon, S.M.; Scrimgeour, C.M.; Daley, S.E.; Wilkinson, R.; Goodship, T.H. Correction of acidosis in humans with CRF decreases protein degradation and amino acid oxidation. Am. J. Physiol. 1993, 265, E230–E235. [Google Scholar] [PubMed]
- Graham, K.A.; Reaich, D.; Channon, S.M.; Downie, S.; Goodship, T.H. Correction of acidosis in hemodialysis decreases whole-body protein degradation. J. Am. Soc. Nephrol. 1997, 8, 632–637. [Google Scholar] [PubMed]
- Graham, K.A.; Reaich, D.; Channon, S.M.; Downie, S.; Gilmour, E.; Passlick-Deetjen, J.; Goodship, T.H. Correction of acidosis in CAPD decreases whole body protein degradation. Kidney Int. 1996, 49, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Moorhouse, J.; Iles-Smith, H.; Baker, F.; Johnstone, J.; James, G.; Troughton, J.; Bircher, G.; Walls, J. Role of an improvement in acid-base status and nutrition in CAPD patients. Kidney Int. 1997, 52, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Wiederkehr, M.R.; Kalogiros, J.; Krapf, R. Correction of metabolic acidosis improves thyroid and growth hormone axes in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Tentori, F.; Karaboyas, A.; Robinson, B.M.; Morgenstern, H.; Zhang, J.; Sen, A.; Ikizler, T.A.; Rayner, H.; Fissell, R.B.; Vanholder, R.; et al. Association of dialysate bicarbonate concentration with mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2013, 62, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Giordano, M.; Lucidi, P.; Ciarambino, T.; Gesuè, L.; Castellino, P.; Cioffi, M.; Gresele, P.; Paolisso, G.; De Feo, P. Effects of dietary protein restriction on albumin and fibrinogen synthesis in macroalbuminuric type 2 diabetic patients. Diabetologia 2008, 51, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cheng, Z.; Qian, Q. Intravenous Fluids and Acute Kidney Injury. Blood Purif. 2017. [Google Scholar] [CrossRef] [PubMed]
- Niebauer, J.; Volk, H.D.; Kemp, M.; Dominguez, M.; Schumann, R.R.; Rauchhaus, M.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 1999, 353, 1838–1842. [Google Scholar] [CrossRef]
- Agarwal, R.; Georgianos, P.I. Con: Nutritional vitamin D replacement in chronic kidney disease and end-stage renal disease. Nephrol. Dial. Transplant. 2016, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Kopple, J.D.; Cheung, A.K.; Christiansen, J.S.; Djurhuus, C.B.; El Nahas, M.; Feldt-Rasmussen, B.; Mitch, W.E.; Wanner, C.; Göthberg, M.; Ikizler, T.A. Opportunity & trade: A large-scale randomized clinical trial of growth hormone in hemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 4095–4103. [Google Scholar] [PubMed]
- Guebre-Egziabher, F.; Juillard, L.; Boirie, Y.; Laville, M.; Beaufrère, B.; Fouque, D. Short-term administration of a combination of recombinant growth hormone and insulin-like growth factor-I induces anabolism in maintenance hemodialysis. J. Clin. Endocrinol. Metab. 2009, 94, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Feldt-Rasmussen, B.; Lange, M.; Sulowicz, W.; Gafter, U.; Lai, K.N.; Wiedemann, J.; Christiansen, J.S.; El Nahas, M.; APCD Study Group. Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J. Am. Soc. Nephrol. 2007, 18, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Gascon, A.; Belvis, J.J.; Berisa, F.; Iglesias, E.; Estopiñán, V.; Teruel, J.L. Nandrolone decanoate is a good alternative for the treatment of anemia in elderly male patients on hemodialysis. Geriatr. Nephrol. Urol. 1999, 9, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Barton Pai, A.; Chretien, C.; Lau, A.H. The effects of nandrolone decanoate on nutritional parameters in hemodialysis patients. Clin. Nephrol. 2002, 58, 38–46. [Google Scholar] [PubMed]
- Navarro, J.F.; Mora, C.; Macía, M.; García, J. Randomized prospective comparison between erythropoietin and androgens in CAPD patients. Kidney Int. 2002, 61, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Orr, R.; Singh, M.F. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: Review of efficacy and safety. Drugs 2004, 64, 725–750. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Du, J.; Klein, J.D.; Bailey, J.L.; Mitch, W.E. Exercise ameliorates chronic kidney disease-induced defects in muscle protein metabolism and progenitor cell function. Kidney Int. 2009, 76, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Storer, T.W.; Casaburi, R.; Sawelson, S.; Kopple, J.D. Endurance exercise training during haemodialysis improves strength, power, fatigability and physical performance in maintenance haemodialysis patients. Nephrol. Dial. Transplant. 2005, 20, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, C.; Gordon, P.L.; Parker, R.C.; Uhlin, K.L.; Roubenoff, R.; Levey, A.S. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am. J. Kidney Dis. 2004, 43, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.; Verdijk, L.B.; van Loon, L.J. The impact of sarcopenia and exercise training on skeletal muscle satellite cells. Ageing Res. Rev. 2009, 8, 328–338. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, Y.; Qian, Q. Protein Nutrition and Malnutrition in CKD and ESRD. Nutrients 2017, 9, 208. https://doi.org/10.3390/nu9030208
Zha Y, Qian Q. Protein Nutrition and Malnutrition in CKD and ESRD. Nutrients. 2017; 9(3):208. https://doi.org/10.3390/nu9030208
Chicago/Turabian StyleZha, Yan, and Qi Qian. 2017. "Protein Nutrition and Malnutrition in CKD and ESRD" Nutrients 9, no. 3: 208. https://doi.org/10.3390/nu9030208



