Maternal Consumption of Hesperidin and Naringin Flavanones Exerts Transient Effects to Tibia Bone Structure in Female CD‐1 Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Study Design
2.3. In Vivo Analysis of Structure of Tibias by μCT
2.4. Ex Vivo Analysis of Structure of Femurs and Lumbar Vertebrae by μCT
2.5. Reconstruction of In Vivo and Ex Vivo Images Obtained Using μCT
2.6. Regions of Interest (ROI) and Segmentation of In Vivo and Ex Vivo Images Obtained Using μCT
2.7. Bone Mineral Density (BMD) and Peak Load of Tibias, Femurs and LV1-3
2.8. Statistical Analyses
3. Results
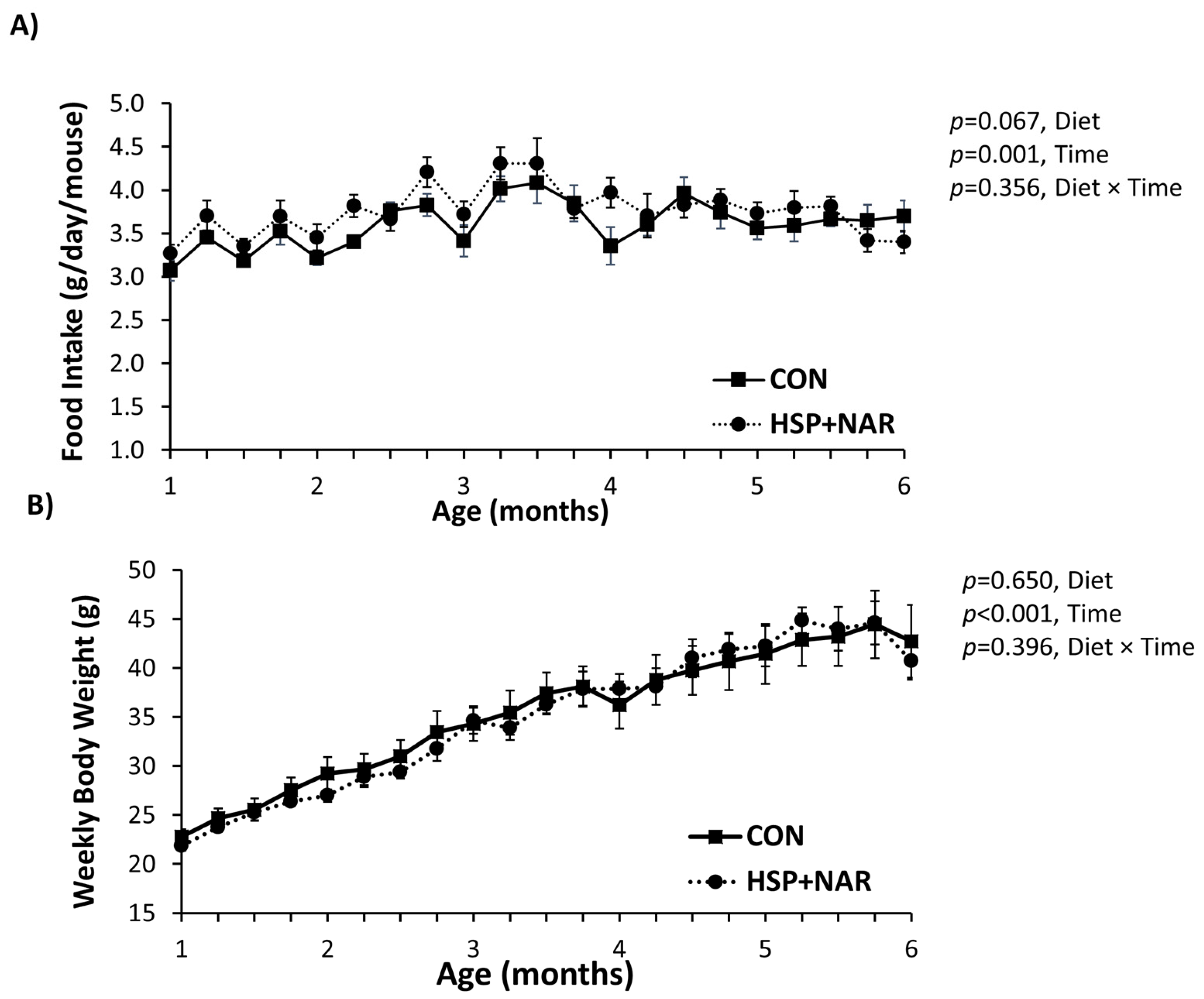
3.1. Food Intake and Body Weight
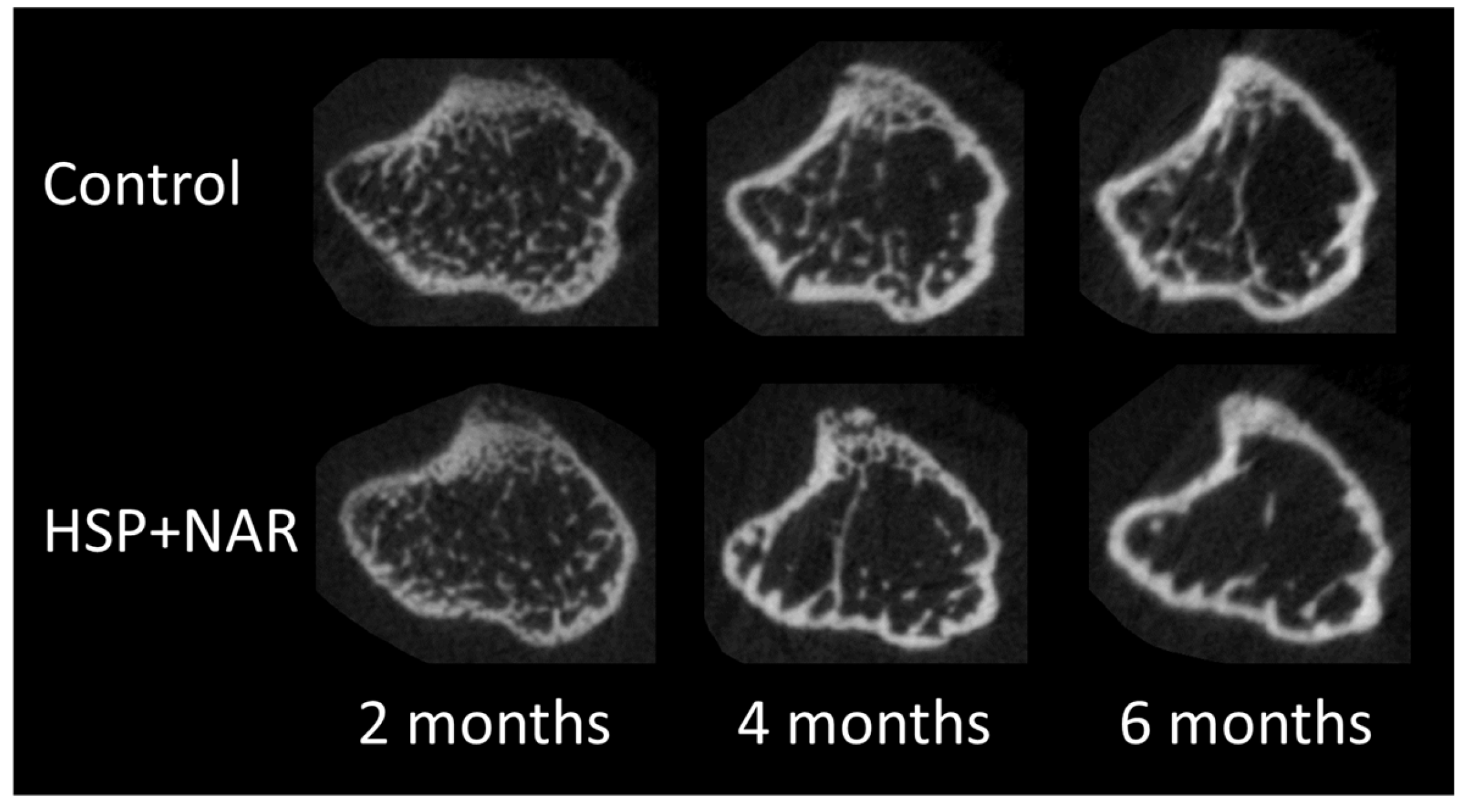
3.2. In Vivo Measurements of Trabecular and Cortical Bone Mineral Density (BMD) and Bone Structure at the Proximal Tibia
3.3. Ex Vivo Measurements of Trabecular and Cortical Bone Structure
3.4. Ex Vivo Measurement of Bone Mineral Density (BMD) and Peak Load
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaludjerovic, J.; Ward, W.E. Bone-specific gene expression patterns and whole bone tissue of female mice are programmed by early life exposure to soy isoflavones and folic acid. J. Nutr. Biochem. 2015, 26, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Kaludjerovic, J.; Ward, W.E. Neonatal administration of isoflavones attenuates deterioration of bone tissue in female but not male mice. J. Nutr. 2010, 140, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, E.C.; Kaludjerovic, J.; Ward, W.E. Isoflavone exposure throughout suckling results in improved adult bone health in mice. J. Dev. Orig. Health Dis. 2012, 3, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Kaludjerovic, J.; Ward, W.E. Neonatal exposure to daidzein, genistein, or the combination modulates bone development in female CD-1 mice. J. Nutr. 2009, 139, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Kim, H.; Matsumoto, A.; Akiyama, S.; Ishimi, Y.; Suzuki, K.; Uehara, M. Hesperidin prevents androgen deficiency-induced bone loss in male mice. Phytother. Res. 2014, 28, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Nielsen, I.L.; Gil-Izquierdo, A.; Trzeciakiewicz, A.; Morand, C.; Chee, W.; Barron, D.; Lebecque, P.; Davicco, M.J.; Williamson, G.; et al. Increased bioavailability of hesperetin-7-glucoside compared with hesperidin results in more efficient prevention of bone loss in adult ovariectomised rats. Br. J. Nutr. 2009, 102, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, M.N.; Habauzit, V.; Trzeciakiewicz, A.; Morand, C.; Gil-Izquierdo, A.; Mardon, J.; Lebecque, P.; Davicco, M.J.; Chee, W.S.; Coxam, V.; et al. Hesperidin inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J. Appl. Physiol. (1985) 2008, 104, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Chiba, H.; Uehara, M.; Wu, J.; Wang, X.; Masuyama, R.; Suzuki, K.; Kanazawa, K.; Ishimi, Y. Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J. Nutr. 2003, 133, 1892–1897. [Google Scholar] [PubMed]
- Habauzit, V.; Sacco, S.M.; Gil-Izquierdo, A.; Trzeciakiewicz, A.; Morand, C.; Barron, D.; Pinaud, S.; Offord, E.; Horcajada, M.N. Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone 2011, 49, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, Y.; Wooley, P.H.; Xu, Z.; Yang, S.Y. Naringin promotes osteoblast differentiation and effectively reverses ovariectomy-associated osteoporosis. J. Orthop. Sci. 2013, 18, 478–485. [Google Scholar] [PubMed]
- Pang, W.Y.; Wang, X.L.; Mok, S.K.; Lai, W.P.; Chow, H.K.; Leung, P.C.; Yao, X.S.; Wong, M.S. Naringin improves bone properties in ovariectomized mice and exerts oestrogen-like activities in rat osteoblast-like (UMR-106) cells. Br. J. Pharmacol. 2010, 159, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Song, B.J.; Jouni, Z.E.; Ferruzzi, M.G. Assessment of phytochemical content in human milk during different stages of lactation. Nutrition 2013, 29, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Bai, Y.; Peng, W.; Su, W. Identification of naringin metabolites in human urine and feces. Eur. J. Drug Metab. Pharmacokinet. 2016. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Remesy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Rouseff, R.L.; Martin, S.F.; Youtsey, C.O. Quantitative survey of narirutin, naringin, hesperidin, and neohesperidin in citrus. J. Agric. Food Chem. 1987, 35, 1027–1030. [Google Scholar] [CrossRef]
- Booth, A.N.; Jones, F.T.; Deeds, F. Metabolic and glucosuria studies on naringin and phloridzin. J. Biol. Chem. 1958, 233, 280–282. [Google Scholar] [PubMed]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [PubMed]
- Trzeciakiewicz, A.; Habauzit, V.; Mercier, S.; Lebecque, P.; Davicco, M.J.; Coxam, V.; Demigne, C.; Horcajada, M.N. Hesperetin stimulates differentiation of primary rat osteoblasts involving the bmp signalling pathway. J. Nutr. Biochem. 2010, 21, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Trzeciakiewicz, A.; Habauzit, V.; Mercier, S.; Barron, D.; Urpi-Sarda, M.; Manach, C.; Offord, E.; Horcajada, M.N. Molecular mechanism of hesperetin-7-o-glucuronide, the main circulating metabolite of hesperidin, involved in osteoblast differentiation. J. Agric. Food Chem. 2010, 58, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Fong, Y.C.; Tsai, H.Y.; Chen, Y.F.; Tsuzuki, M.; Tang, C.H. Naringin-induced bone morphogenetic protein-2 expression via PI3K, Akt, c-Fos/c-Jun and AP-1 pathway in osteoblasts. Eur. J. Pharmacol. 2008, 588, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Dai, K.R.; Yan, S.G.; Yan, W.Q.; Zhang, C.; Chen, D.Q.; Xu, B.; Xu, Z.W. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur. J. Pharmacol. 2009, 607, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
- Sacco, S.M.; Saint, C.; Longo, A.B.; Wakefield, C.B.; Salmon, P.L.; LeBlanc, P.J.; Ward, W.E. Repeated irradiation from micro-computed tomography scanning at 2, 4 and 6 months of age does not induce damage to tibial bone microstructure in male and female CD-1 mice. BoneKEy Rep. 2017. [Google Scholar] [CrossRef]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man. Cyber. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Bruker. Method Note: Bone Mineral Density (BMD) and Tissue Mineral Density (TMD) Calibration and Measurement by Micro-CT Using Bruker-MicroCT CT-Analyser; Bruker microCT: Kontich, Belgium, 2014. [Google Scholar]
- Fonseca, D.; Ward, W.E. Daidzein together with high calcium preserve bone mass and biomechanical strength at multiple sites in ovariectomized mice. Bone 2004, 35, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Breinholt, V.; Larsen, J.C. Detection of weak estrogenic flavonoids using a recombinant yeast strain and a modified MCF7 cell proliferation assay. Chem. Res. Toxicol. 1998, 11, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [PubMed]

| Outcomes | CON | HSP + NAR | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (Months) | |||||||||
| 2 | 4 | 6 | 2 | 4 | 6 | Time | Diet | Time × Diet | |
| Trabecular Structure | |||||||||
| BMD (g/cm3) | 0.212 ± 0.007 a | 0.198 ± 0.003 a | 0.154 ± 0.006 b | 0.190 ± 0.010 a | 0.162 ± 0.008 †,b | 0.142 ± 0.008 b | <0.001 | 0.007 | 0.015 |
| TV (mm3) | 1.185 ± 0.064 a | 0.936 ± 0.045 b | 0.898 ± 0.046 b | 1.022 ± 0.065 a | 0.853 ± 0.052 b | 0.831 ± 0.058 b | <0.001 | 0.184 | 0.151 |
| BV (mm3) | 0.097 ± 0.010 a | 0.050 ± 0.006 b | 0.025 ± 0.004 c | 0.061 ± 0.009 †,a | 0.029 ± 0.005 †,b | 0.017 ± 0.004 b | <0.001 | 0.013 | 0.099 |
| BV/TV (%) | 8.177 ± 0.645 a | 5.282 ± 0.464 b | 2.764 ± 0.298 c | 5.782 ± 0.698 †,a | 3.260 ± 0.434 †,b | 2.018 ± 0.526 b | <0.001 | 0.011 | 0.161 |
| Tb.Th (mm) | 0.059 ± 0.001 b | 0.070 ± 0.001 a | 0.073 ± 0.001 a | 0.059 ± 0.001 b | 0.068 ± 0.002 a | 0.069 ± 0.002 a | <0.001 | 0.243 | 0.406 |
| Tb.N (1/mm) | 1.386 ± 0.105 a | 0.751 ± 0.058 b | 0.379 ± 0.42 c | 0.976 ± 0.104 †,a | 0.479 ± 0.063 †,b | 0.284 ± 0.071 b | <0.001 | 0.009 | 0.094 |
| Tb.Sp (mm) | 0.297 ± 0.010 c | 0.386 ± 0.003 b | 0.429 ± 0.006 a | 0.350 ± 0.015 †,b | 0.430 ± 0.008 †,a | 0.445 ± 0.006 a | <0.001 | 0.002 | 0.004 |
| DA (no unit) | 2.334 ± 0.095 a | 1.970 ± 0.072 b | 2.150 ± 0.116 a,b | 2.562 ± 0.087 | 2.212 ± 0.126 | 2.962 ± 0.401 | <0.001 | 0.030 | 0.305 |
| Conn.D (1/mm3) | 46.6 ± 4.7 a | 20.0 ± 2.1 b | 7.9 ± 1.8 c | 30.1 ± 2.1 †,a | 11.3 ± 3.1 †,b | 8.5 ± 2.3 b | <0.001 | 0.004 | 0.051 |
| Cortical Structure | |||||||||
| TMD (g/cm3) | 0.802 ± 0.007 c | 0.941 ± 0.112 b | 0.996 ± 0.010 a | 0.810 ± 0.007 c | 0.932 ± 0.004 b | 0.995 ± 0.007 a | <0.001 | 0.969 | 0.298 |
| Ct.Ar (mm2) | 1.295 ± 0.041 b | 1.396 ± 0.025 a,b | 1.456 ± 0.021 a | 1.230 ± 0.035 b | 1.299 ± 0.037 †,a | 1.367 ± 0.049 a | <0.001 | 0.061 | 0.798 |
| Tt.Ar (mm2) | 3.951 ± 0.127 a | 3.493 ± 0.104 b | 3.517 ± 0.107 b | 3.544 ± 0.158 a | 3.236 ± 0.137 b | 3.284 ± 0.159 b | <0.001 | 0.119 | 0.085 |
| Ct.Ar/Tt.Ar (%) | 33.1 ± 1.6 b | 40.2 ± 1.1 a | 41.6 ± 1.1 a | 35.0 ± 1.0 b | 40.4 ± 0.9 a | 41.9 ± 1.2 a | <0.001 | 0.634 | 0.384 |
| Ct.Th (mm) | 0.110 ± 0.005 c | 0.164 ± 0.005 b | 0.187 ± 0.004 a | 0.120 ± 0.004 c | 0.167 ± 0.005 b | 0.185 ± 0.003 a | <0.001 | 0.492 | 0.139 |
| Ps.Pm (mm) | 8.231 ± 0.123 a | 7.851 ± 0.104 b | 7.939 ± 0.097 b | 7.780 ± 0.169 † | 7.609 ± 0.159 | 7.731 ± 0.179 | <0.001 | 0.135 | 0.046 |
| Ec.Pm (mm) | 6.535 ± 0.178 a | 5.804 ± 0.153 b | 5.724 ± 0.154 b | 6.005 ± 0.193 a | 5.457 ± 0.184 b | 5.440 ± 0.214 b | <0.001 | 0.134 | 0.231 |
| Ma.Ar (mm2) | 2.656 ± 0.139 a | 2.097 ± 0.097 b | 2.061 ± 0.099 b | 2.314 ± 0.131 a | 1.937 ± 0.106 b | 1.917 ± 0.125 b | <0.001 | 0.196 | 0.116 |
| Ecc (no unit) | 0.459 ± 0.032 a,b | 0.370 ± 0.026 b | 0.426 ± 0.021 a | 0.459 ± 0.028 | 0.458 ± 0.034 | 0.502 ± 0.034 | 0.041 | 0.054 | 0.452 |
| CON | HSP + NAR | p Value | |
|---|---|---|---|
| Femur BMD (g/cm2) | 0.092 ± 0.002 | 0.090 ± 0.003 | 0.427 |
| Femur Neck Trabecular Structure | |||
| BV/TV (%) | 15.6 ± 0.7 | 15.0 ± 0.9 | 0.621 |
| Tb.Th (mm) | 0.098 ± 0.002 | 0.099 ± 0.002 | 0.835 |
| Tb.N (1/mm) | 1.591 ± 0.083 | 1.523 ± 0.102 | 0.610 |
| Tb.Sp (mm) | 0.426 ± 0.014 | 0.440 ± 0.013 | 0.462 |
| Femur Midpoint Cortical Structure | |||
| Ct.Ar (mm2) | 1.312 ± 0.034 | 1.181 ± 0.076 | 0.101 |
| Ct.Th (mm) | 0.264 ± 0.009 | 0.252 ± 0.010 | 0.422 |
| Distal Femur Trabecular Structure | |||
| BV/TV (%) | 11.0 ± 0.9 | 10.8 ± 2.8 | 0.932 |
| Tb.Th (mm) | 0.081 ± 0.002 | 0.078 ± 0.005 | 0.520 |
| Tb.N (1/mm) | 1.361 ± 0.105 | 1.294 ± 0.292 | 0.813 |
| Tb.Sp (mm) | 0.463 ± 0.031 | 0.544 ± 0.069 | 0.324 |
| Femur Neck Peak Load (N) | 23.5 ± 1.2 | 24.3 ± 1.8 | 0.697 |
| Midpoint Peak Load (N) | 25.5 ± 1.1 | 25.4 ± 2.4 | 0.602 |
| CON | HSP + NAR | p Value | |
|---|---|---|---|
| LV1-3 BMD (g/cm2) | 0.075 ± 0.002 | 0.078 ± 0.004 | 0.490 |
| LV2 Trabecular Structure | |||
| BV/TV (%) | 25.1 ± 1.6 | 22.5 ± 2.3 | 0.360 |
| Tb.Th (mm) | 0.081 ± 0.001 | 0.087 ± 0.005 | 0.304 |
| Tb.N (1/mm) | 3.106 ± 0.179 | 2.619 ± 0.228 | 0.110 |
| Tb.Sp (mm) | 0.296 ± 0.012 | 0.315 ± 0.017 | 0.359 |
| LV2 Cortical Structure | |||
| Ct.Ar (mm2) | 0.525 ± 0.013 | 0.523 ± 0.032 | 0.957 |
| Ct.Th (mm) | 0.083 ± 0.002 | 0.085 ± 0.005 | 0.706 |
| LV2 Peak Load (N) | 32.2 ± 2.7 | 35.8 ± 6.3 | 0.544 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, S.M.; Saint, C.; LeBlanc, P.J.; Ward, W.E. Maternal Consumption of Hesperidin and Naringin Flavanones Exerts Transient Effects to Tibia Bone Structure in Female CD‐1 Offspring. Nutrients 2017, 9, 250. https://doi.org/10.3390/nu9030250
Sacco SM, Saint C, LeBlanc PJ, Ward WE. Maternal Consumption of Hesperidin and Naringin Flavanones Exerts Transient Effects to Tibia Bone Structure in Female CD‐1 Offspring. Nutrients. 2017; 9(3):250. https://doi.org/10.3390/nu9030250
Chicago/Turabian StyleSacco, Sandra M., Caitlin Saint, Paul J. LeBlanc, and Wendy E. Ward. 2017. "Maternal Consumption of Hesperidin and Naringin Flavanones Exerts Transient Effects to Tibia Bone Structure in Female CD‐1 Offspring" Nutrients 9, no. 3: 250. https://doi.org/10.3390/nu9030250





