Dietary Buglossoides Arvensis Oil Increases Circulating n-3 Polyunsaturated Fatty Acids in a Dose-Dependent Manner and Enhances Lipopolysaccharide-Stimulated Whole Blood Interleukin-10—A Randomized Placebo-Controlled Trial
Abstract
:1. Introduction
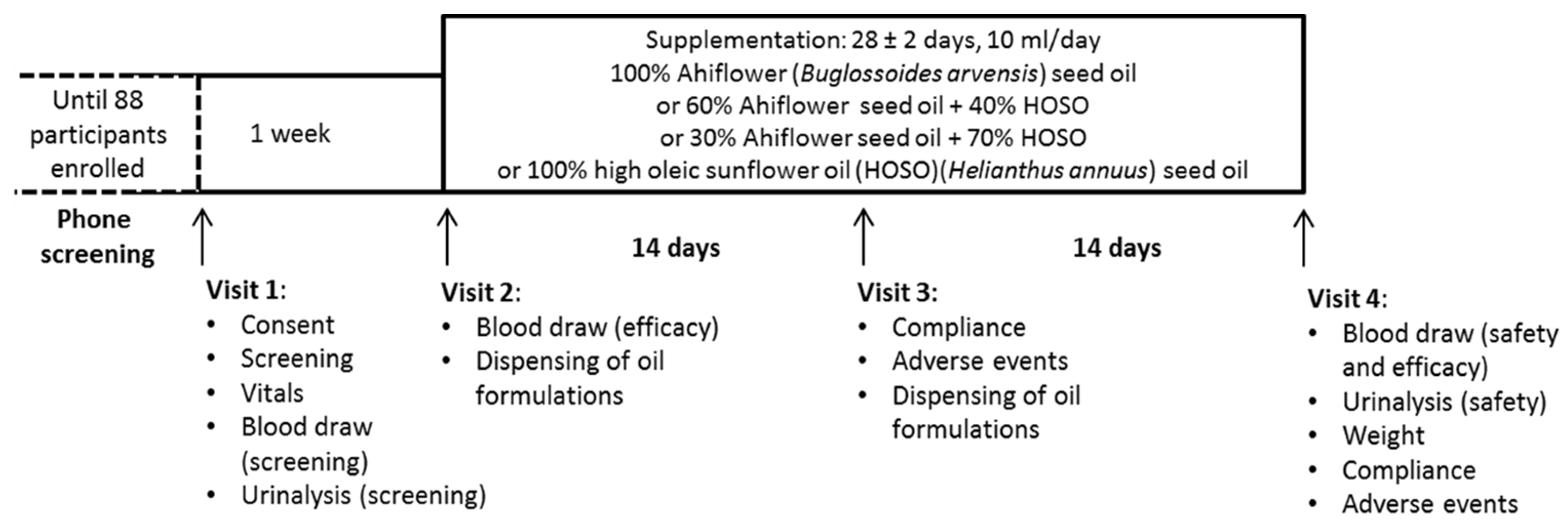
2. Materials and Methods
3. Results
3.1. Subject Characteristics
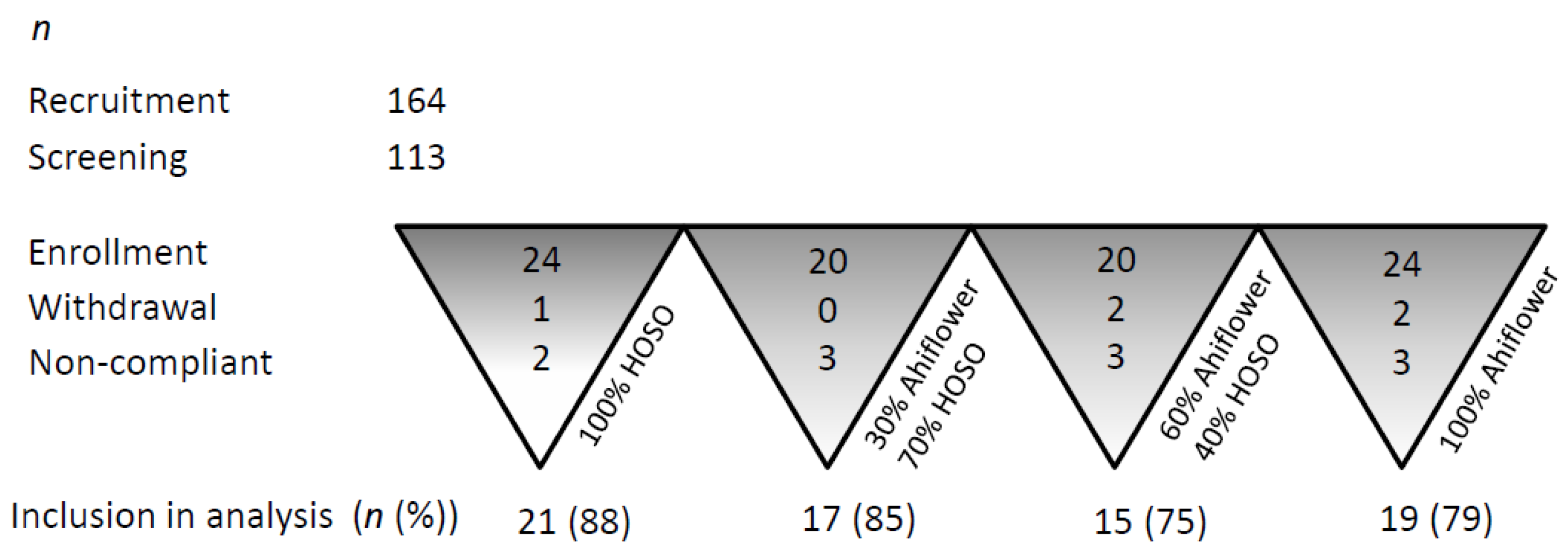
3.1.1. Retention and Adherence to Study Protocol
3.1.2. Safety Outcomes
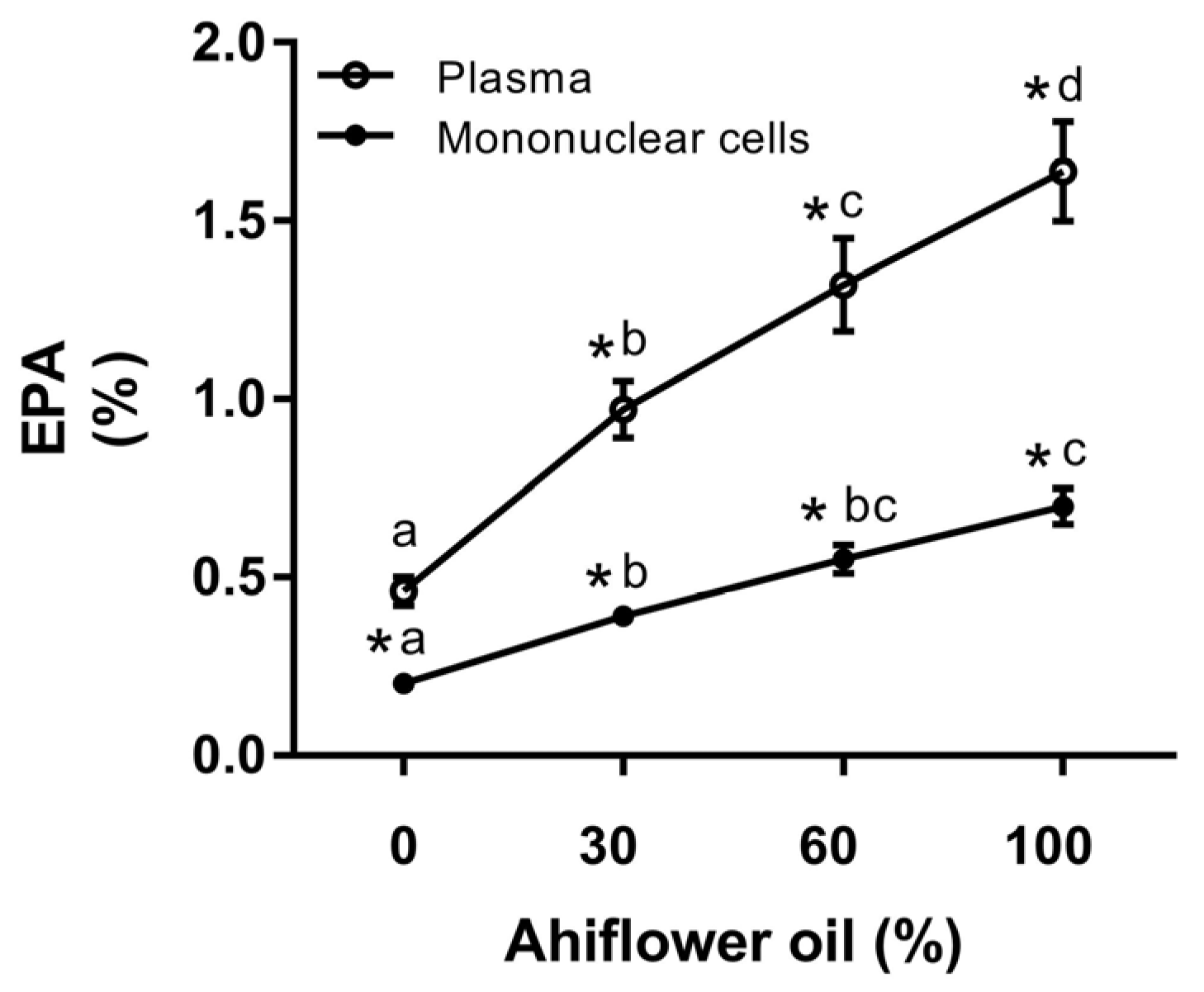
3.2. Fatty Acid Analyses
3.2.1. α-Linolenic Acid (18:3 n-3)
3.2.2. Stearidonic Acid (18:4 n-3) and Eicosatetraenoic Acid (20:4 n-3)
3.2.3. Eicosapentaenoic Acid (20:5 n-3)
3.2.4. Docosapentaenoic Acid (22:5 n-3) and Docosahexaenoic Acid (22:6 n-3)
3.2.5. Dihomo-γ-Linolenic Acid (20:3 n-6)
3.3. Cytokine Analyses
3.3.1. Whole Blood Cytokine and Chemokine Response to LPS
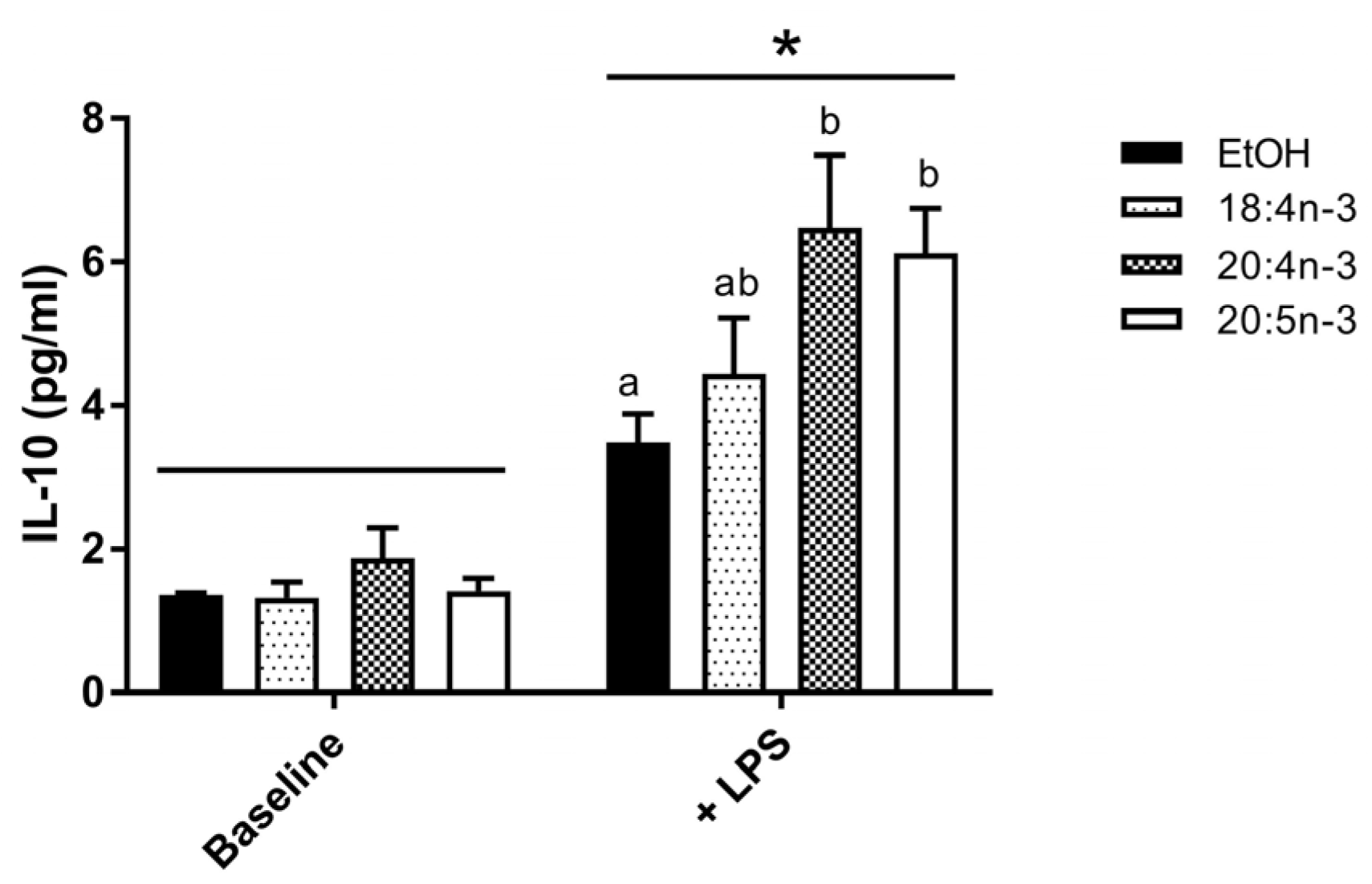
3.3.2. PUFA Modulation of IL-10 Production by M2-Like THP-1 Macrophages
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Calder, P.C. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [PubMed]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.J.; Katz, J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007, 129, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B.; Depner, C.M.; Tripathy, S. Omega-3 fatty acid supplementation and cardiovascular disease. J. Lipid Res. 2012. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lip, G.Y. The role of omega-3 fatty acids in the secondary prevention of cardiovascular disease. QJM 2003, 96, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Chen, J.; Evans, R.M. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. USA 1997, 94, 4312–4317. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A.; Southon, S.; Pinder, A.C. (n-3) polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes in vitro. J. Nutr. 1996, 126, 603–610. [Google Scholar] [PubMed]
- Harris, W.S. International recommendations for consumption of long-chain omega-3 fatty acids. J. Cardiovasc. Med. 2007, 8 (Suppl. 1), S50–S52. [Google Scholar] [CrossRef] [PubMed]
- Taber, L.; Chiu, C.H.; Whelan, J. Assessment of the arachidonic acid content in foods commonly consumed in the american diet. Lipids 1998, 33, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.; Rust, C. Innovative dietary sources of n-3 fatty acids. Annu. Rev. Nutr. 2006, 26, 75–103. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Sievenpiper, J.L.; Pauly, D.; Sumaila, U.R.; Kendall, C.W.; Mowat, F.M. Are dietary recommendations for the use of fish oils sustainable? CMAJ 2009, 180, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. N-3 oil sources for use in aquaculture—Alternatives to the unsustainable harvest of wild fish. Nutr. Res. Rev. 2008, 21, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Nichols, P.D.; Petrie, J.; Singh, S. Long-chain omega-3 oils-an update on sustainable sources. Nutrients 2010, 2, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Surette, M.E. Dietary omega-3 pufa and health: Stearidonic acid-containing seed oils as effective and sustainable alternatives to traditional marine oils. Mol. Nutr. Food Res. 2013, 57, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Lefort, N.; LeBlanc, R.; Giroux, M.A.; Surette, M.E. Consumption of Buglossoides arvensis seed oil is safe and increases tissue long-chain n-3 fatty acid content more than flax seed oil—Results of a phase i randomised clinical trial. J. Nutr. Sci. 2016, 5, e2. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Chilton-Lopez, T.; Surette, M.E.; Swan, D.D.; Fonteh, A.N.; Johnson, M.M.; Chilton, F.H. Metabolism of gammalinolenic acid in human neutrophils. J. Immunol. 1996, 156, 2941–2947. [Google Scholar] [PubMed]
- Surette, M.E.; Koumenis, I.L.; Edens, M.B.; Tramposch, K.M.; Chilton, F.H. Inhibition of leukotriene synthesis, pharmacokinetics, and tolerability of a novel dietary fatty acid formulation in healthy adult subjects. Clin. Ther. 2003, 25, 948–971. [Google Scholar] [CrossRef]
- Arm, J.P.; Boyce, J.A.; Wang, L.; Chhay, H.; Zahid, M.; Patil, V.; Govindarajulu, U.; Ivester, P.; Weaver, K.L.; Sergeant, S.; et al. Impact of botanical oils on polyunsaturated fatty acid metabolism and leukotriene generation in mild asthmatics. Lipids Health Dis. 2013, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, M.; Jahreis, G.; Bothor, K.; Drechsel, C.; Kiehntopf, M.; Bluher, M.; Dawczynski, C. Benefits of foods supplemented with vegetable oils rich in alpha-linolenic, stearidonic or docosahexaenoic acid in hypertriglyceridemic subjects: A double-blind, randomized, controlled trail. Eur. J. Nutr. 2015, 54, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Lemke, S.L.; Hansen, S.N.; Goldstein, D.A.; DiRienzo, M.A.; Su, H.; Nemeth, M.A.; Taylor, M.L.; Ahmed, G.; George, C. Stearidonic acid-enriched soybean oil increased the omega-3 index, an emerging cardiovascular risk marker. Lipids 2008, 43, 805–811. [Google Scholar] [CrossRef] [PubMed]
- James, M.J.; Ursin, V.M.; Cleland, L.G. Metabolism of stearidonic acid in human subjects: Comparison with the metabolism of other n-3 fatty acids. Am. J. Clin. Nutr. 2003, 77, 1140–1145. [Google Scholar] [PubMed]
- Krul, E.S.; Lemke, S.L.; Mukherjea, R.; Taylor, M.L.; Goldstein, D.A.; Su, H.; Liu, P.; Lawless, A.; Harris, W.S.; Maki, K.C. Effects of duration of treatment and dosage of eicosapentaenoic acid and stearidonic acid on red blood cell eicosapentaenoic acid content. Prostaglandins Leukot. Essent. Fatty Acids 2012, 86, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kuhnt, K.; Fuhrmann, C.; Kohler, M.; Kiehntopf, M.; Jahreis, G. Dietary echium oil increases long-chain n-3 pufas, including docosapentaenoic acid, in blood fractions and alters biochemical markers for cardiovascular disease independently of age, sex, and metabolic syndrome. J. Nutr. 2014, 144, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Kuhnt, K.; Weiss, S.; Kiehntopf, M.; Jahreis, G. Consumption of echium oil increases epa and dpa in blood fractions more efficiently compared to linseed oil in humans. Lipids Health Dis. 2016, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Ivester, P.; Hester, A.G.; Sergeant, S.; Case, L.D.; Morgan, T.; Kouba, E.O.; Chilton, F.H. The impact of polyunsaturated fatty acid-based dietary supplements on disease biomarkers in a metabolic syndrome/diabetes population. Lipids Health Dis. 2014, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Lemke, S.L.; Vicini, J.L.; Su, H.; Goldstein, D.A.; Nemeth, M.A.; Krul, E.S.; Harris, W.S. Dietary intake of stearidonic acid-enriched soybean oil increases the omega-3 index: Randomized, double-blind clinical study of efficacy and safety. Am. J. Clin. Nutr. 2010, 92, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Banerjee, T.; Calder, P.C. The influence of different combinations of gamma-linolenic, stearidonic and eicosapentaenoic acids on the fatty acid composition of blood lipids and mononuclear cells in human volunteers. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Pieters, D.J.; Mensink, R.P. Effects of stearidonic acid on serum triacylglycerol concentrations in overweight and obese subjects: A randomized controlled trial. Eur. J. Clin. Nutr. 2015, 69, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Surette, M.E.; Edens, M.; Chilton, F.H.; Tramposch, K.M. Dietary echium oil increases plasma and neutrophil long-chain (n-3) fatty acids and lowers serum triacylglycerols in hypertriglyceridemic humans. J. Nutr. 2004, 134, 1406–1411. [Google Scholar] [PubMed]
- Duffy, D.; Rouilly, V.; Libri, V.; Hasan, M.; Beitz, B.; David, M.; Urrutia, A.; Bisiaux, A.; Labrie, S.T.; Dubois, A.; et al. Functional analysis via standardized whole-blood stimulation systems defines the boundaries of a healthy immune response to complex stimuli. Immunity 2014, 40, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Huerta, O.D.; Aguilera, C.M.; Mesa, M.D.; Gil, A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: A systematic review of randomised clinical trials. Br. J. Nutr. 2012, 107 (Suppl. 2), S159–S170. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). NIH Publication No. 09-5410; US Department of Health and Human Services: Bethesda, MD, USA. Available online: Http://evs.Nci.Nih.Gov/ftp1/ctcae/ctcae_4.03_2010-06-14_quickreference_5x7.Pdf (accessed on 15 November 2015).
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. Suppl. 1968, 97, 77–89. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Caughey, G.E.; Mantzioris, E.; Gibson, R.A.; Cleland, L.G.; James, M.J. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am. J. Clin. Nutr. 1996, 63, 116–122. [Google Scholar] [PubMed]
- Endres, S.; Ghorbani, R.; Kelley, V.E.; Georgilis, K.; Lonnemann, G.; van der Meer, J.W.; Cannon, J.G.; Rogers, T.S.; Klempner, M.S.; Weber, P.C.; et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 1989, 320, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; DiRienzo, M.A.; Sands, S.A.; George, C.; Jones, P.G.; Eapen, A.K. Stearidonic acid increases the red blood cell and heart eicosapentaenoic acid content in dogs. Lipids 2007, 42, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Richez, C.; Yasuda, K.; Watkins, A.A.; Akira, S.; Lafyatis, R.; van Seventer, J.M.; Rifkin, I.R. Tlr4 ligands induce ifn-alpha production by mouse conventional dendritic cells and human monocytes after ifn-beta priming. J. Immunol. 2009, 182, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Nile, C.J.; Barksby, E.; Jitprasertwong, P.; Preshaw, P.M.; Taylor, J.J. Expression and regulation of interleukin-33 in human monocytes. Immunology 2010, 130, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.H.; Britton, G.J.; Hill, E.V.; Verhagen, J.; Burton, B.R.; Wraith, D.C. Regulation of adaptive immunity; the role of interleukin-10. Front. Immunol. 2013, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Boraschi, D. From monocytes to m1/m2 macrophages: Phenotypical vs. Functional differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, J.; Bourgeois-Daigneault, M.C.; Huppe, G.; Tremblay, J.; Aumont, A.; Houde, M.; Bartee, E.; Brunet, A.; Gauvreau, M.E.; de Gassart, A.; et al. Interleukin-10-induced march1 mediates intracellular sequestration of mhc class ii in monocytes. Eur. J. Immunol. 2008, 38, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Buelens, C.; Willems, F.; Delvaux, A.; Pierard, G.; Delville, J.P.; Velu, T.; Goldman, M. Interleukin-10 differentially regulates b7-1 (cd80) and b7-2 (cd86) expression on human peripheral blood dendritic cells. Eur. J. Immunol. 1995, 25, 2668–2672. [Google Scholar] [CrossRef] [PubMed]
- Crawley, E.; Kay, R.; Sillibourne, J.; Patel, P.; Hutchinson, I.; Woo, P. Polymorphic haplotypes of the interleukin-10 5′ flanking region determine variable interleukin-10 transcription and are associated with particular phenotypes of juvenile rheumatoid arthritis. Arthritis Rheum. 1999, 42, 1101–1108. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Zhang, F.; Pan, L.; Li, N.; Li, J. Fish oil-supplemented parenteral nutrition in severe acute pancreatitis patients and effects on immune function and infectious risk: A randomized controlled trial. Inflammation 2009, 32, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Asahara, N.; Shimatsu, A.; Sasaki, Y.; Nakaoka, H.; Himeno, A.; Tochiya, M.; Kono, S.; Takaya, T.; Ono, K.; Wada, H.; et al. Highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care 2012, 35, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Jaudszus, A.; Gruen, M.; Watzl, B.; Ness, C.; Roth, A.; Lochner, A.; Barz, D.; Gabriel, H.; Rothe, M.; Jahreis, G. Evaluation of suppressive and pro-resolving effects of epa and dha in human primary monocytes and t-helper cells. J. Lipid Res. 2013, 54, 923–935. [Google Scholar] [CrossRef] [PubMed]

| 0% Ahiflower 100% HOSO | 30% Ahiflower 70% HOSO | 60% Ahiflower 40% HOSO | 100% Ahiflower 0% HOSO | |
|---|---|---|---|---|
| Fatty acid 1 | % | |||
| 16:0 | 3.9 | 4.2 | 4.4 | 4.7 |
| 16:1n-7 | 0.1 | 0.1 | 0.1 | 0.1 |
| 18:0 | 2.6 | 2.3 | 2.1 | 1.8 |
| 18:1n-9 | 78.5 | 57.8 | 36.2 | 9.4 |
| 18:1n-7 | 0.8 | 0.8 | 0.7 | 0.6 |
| 18:2n-6 | 11.9 | 11.8 | 11.7 | 11.4 |
| 18:3n-6 (GLA) | 0.0 | 1.5 | 3.0 | 4.9 |
| 18:3n-3 (ALA) | 0.2 | 14.6 | 29.4 | 47.9 |
| 18:4n-3 (SDA) | 0.0 | 5.2 | 10.6 | 17.3 |
| 20:0 | 0.3 | 0.2 | 0.1 | 0.1 |
| 20:1n-9 | 0.3 | 0.4 | 0.6 | 0.8 |
| 20:5n-3 | ND | ND | ND | ND |
| 22:0 | 0.8 | 0.6 | 0.4 | 0.1 |
| 22:1n-9 | 0.0 | 0.1 | 0.1 | 0.2 |
| 22:5n-3 | ND | ND | ND | ND |
| 22:6n-3 | ND | ND | ND | ND |
| 24:0 | 0.3 | 0.2 | 0.1 | 0.0 |
| 24:1n-9 | 0.0 | 0.0 | 0.1 | 0.1 |
| ∑ n-3 PUFA | 0.2 | 19.8 | 40.0 | 65.2 |
| ∑ | 99.7 | 99.8 | 99.6 | 99.4 |
| Fatty acid | mg per 10 mL | |||
| SDA | 0 | 437 | 893 | 1469 |
| GLA | 0 | 130 | 260 | 428 |
| ALA | 17 | 1302 | 2613 | 4287 |
| 0% Ahiflower 100% HOSO | 30% Ahiflower 70% HOSO | 60% Ahiflower 40% HOSO | 100% Ahiflower 0% HOSO | |||||
|---|---|---|---|---|---|---|---|---|
| SEM | SEM | SEM | SEM | |||||
| n | 24 | 20 | 20 | 24 | ||||
| Gender | ||||||||
| Female | 12 | 13 | 14 | 10 | ||||
| Male | 12 | 7 | 6 | 14 | ||||
| Age (years) | 27.4 | 2.0 | 32.2 | 2.8 | 36.0 | 2.7 | 33.6 | 3.0 |
| Weight (kg) | 72.4 | 3.2 | 71.0 | 3.2 | 76.7 | 3.1 | 77.0 | 3.3 |
| BMI (kg/m2) | 25.1 | 1.0 | 24.7 | 1.1 | 27.1 | 1.3 | 25.9 | 1.0 |
| Blood pressure (mmHg) | ||||||||
| Systolic | 111 | 2 | 116 | 3 | 117 | 4 | 118 | 4 |
| Diastolic | 67 | 2 | 68 | 2 | 70 | 3 | 74 | 2 |
| HR (beats/min) | 67 | 3 | 69 | 3 | 72 | 3 | 66 | 3 |
| Triglycerides (mmol/L) | 0.98 | 0.11 | 1.08 | 0.11 | 1.18 | 0.14 | 1.13 | 0.13 |
| Total cholesterol (mmol/L) | 4.25 | 0.17 | 4.39 | 0.19 | 4.48 | 0.18 | 4.50 | 0.20 |
| LDL-C (mmol/L) | 2.31 | 0.15 | 2.52 | 0.17 | 2.62 | 0.16 | 2.64 | 0.15 |
| Non HDL-C (mmol/L) | 2.74 | 0.18 | 3.01 | 0.18 | 3.16 | 0.20 | 3.16 | 0.18 |
| HDL-C (mmol/L) | 1.50 | 0.08 | 1.39 | 0.08 | 1.32 | 0.08 | 1.35 | 0.05 |
| Glucose (mmol/L) | 4.97 | 0.07 | 4.95 | 0.07 | 5.06 | 0.07 | 5.25 | 0.07 |
| 0% Ahiflower 100% HOSO | 30% Ahiflower 70% HOSO | 60% Ahiflower 40% HOSO | 100% Ahiflower 0% HOSO | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Day 28 | Baseline | Day 28 | Baseline | Day 28 | Baseline | Day 28 | |
| Fatty acid | (g/100 g fatty acids) | |||||||
| 18:3 n-3 | 0.82 ± 0.04 | 0.80 ± 0.04 a | 0.80 ± 0.06 | 0.97 ± 0.08 *,b | 0.90 ± 0.07 | 1.45 ± 0.08 *,c | 0.89 ± 0.05 | 1.88 ± 0.14 *,d |
| 18:4 n-3 1 | 0.03 ± 0.02 | 0 a | 0.02 ± 0.01 | 0.10 ± 0.02 b | 0.01 ± 0.01 | 0.21 ± 0.03 b,c | 0 | 0.28 ± 0.05 c |
| 20:4 n-3 1 | 0.08 ± 0.02 | 0.09 ± 0.01 a | 0.08 ± 0.02 | 0.18 ± 0.02 a | 0.11 ± 0.03 | 0.38 ± 0.07 b | 0.09 ± 0.02 | 0.43 ± 0.04 b |
| 20:5 n-3 | 0.49 ± 0.05 | 0.46 ± 0.04 a | 0.60 ± 0.05 | 0.97 ± 0.08 *,b | 0.63 ± 0.06 | 1.32 ± 0.13 *,b,c | 0.67 ± 0.05 | 1.64 ± 0.14 *,c |
| 22:5 n-3 | 0.92 ± 0.08 | 0.87 ± 0.08 a | 0.91 ± 0.07 | 1.02 ± 0.08 a,b | 0.94 ± 0.09 | 1.12 ± 0.09 *,b | 1.15 ± 0.10 | 1.45 ± 0.07 *,c |
| 22:6 n-3 | 1.34 ± 0.10 | 1.33 ± 0.09 a | 1.73 ± 0.09 | 1.75 ± 0.09 a | 1.46 ± 0.07 | 1.44 ± 0.06 a | 1.54 ± 0.11 | 1.54 ± 0.10 a |
| 20:3 n-6 | 1.87 ± 0.08 | 1.80 ± 0.08 | 1.85 ± 0.11 | 1.76 ± 0.11 | 2.02 ± 0.15 | 1.98 ± 0.17 | 1.91 ±0.01 | 1.88 ± 0.07 |
| 0% Ahiflower 100% HOSO | 30% Ahiflower 70% HOSO | 60% Ahiflower 40% HOSO | 100% Ahiflower 0% HOSO | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Day 28 | Baseline | Day 28 | Baseline | Day 28 | Baseline | Day 28 | |
| Fatty acid | (g/100 g fatty acids) | |||||||
| 18:3 n-3 | 0.22 ± 0.02 | 0.23 ± 0.01 a | 0.23 ± 0.02 | 0.26 ± 0.03 a | 0.24 ± 0.03 | 0.31 ± 0.03 *,a,b | 0.27 ± 0.02 | 0.40 ± 0.02 *,b |
| 18:4 n-3 1 | 0.01 ± 0.00 | 0 a | 0 | 0 a | 0 | 0.02 ± 0.01 a | 0 | 0.07 ± 0.04 b |
| 20:4 n-3 1 | 0.07 ± 0.01 | 0.06 ± 0.01 a | 0.06 ± 0.01 | 0.10 ± 0.02 a | 0.08 ± 0.01 | 0.18 ± 0.0 b | 0.04 ± 0.01 | 0.25 ± 0.02 c |
| 20:5 n-3 | 0.25 ± 0.02 | 0.20 ± 0.02 *,a | 0.29 ± 0.02 | 0.39 ± 0.02 *,b | 0.29 ± 0.02 | 0.55 ± 0.04 *,b,c | 0.30 ± 0.01 | 0.70 ± 0.05 *,c |
| 22:5 n-3 | 2.36 ± 0.09 | 2.15 ± 0.07 *,a | 2.35 ± 0.08 | 2.68 ± 0.10 *b | 2.51 ± 0.12 | 3.21 ± 0.12 *,c | 2.46 ± 0.09 | 3.30 ± 0.15 *,c |
| 22:6 n-3 | 1.75 ± 0.09 | 1.64 ± 0.08 *,a | 1.98 ± 0.11 | 1.90 ± 0.11 b | 1.90 ± 0.08 | 1.71 ± 0.07 *,a | 1.82 ± 0.09 | 1.63 ± 0.08 *,a |
| 20:3 n-6 | 2.20 ± 0.08 | 2.16 ± 0.09 a | 1.84 ± 0.12 | 1.94 ± 0.14 a | 2.22 ± 0.16 | 2.33 ± 0.16 a,b | 1.92 ± 0.08 | 2.14 ± 0.09 *,b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lefort, N.; LeBlanc, R.; Surette, M.E. Dietary Buglossoides Arvensis Oil Increases Circulating n-3 Polyunsaturated Fatty Acids in a Dose-Dependent Manner and Enhances Lipopolysaccharide-Stimulated Whole Blood Interleukin-10—A Randomized Placebo-Controlled Trial. Nutrients 2017, 9, 261. https://doi.org/10.3390/nu9030261
Lefort N, LeBlanc R, Surette ME. Dietary Buglossoides Arvensis Oil Increases Circulating n-3 Polyunsaturated Fatty Acids in a Dose-Dependent Manner and Enhances Lipopolysaccharide-Stimulated Whole Blood Interleukin-10—A Randomized Placebo-Controlled Trial. Nutrients. 2017; 9(3):261. https://doi.org/10.3390/nu9030261
Chicago/Turabian StyleLefort, Natalie, Rémi LeBlanc, and Marc E. Surette. 2017. "Dietary Buglossoides Arvensis Oil Increases Circulating n-3 Polyunsaturated Fatty Acids in a Dose-Dependent Manner and Enhances Lipopolysaccharide-Stimulated Whole Blood Interleukin-10—A Randomized Placebo-Controlled Trial" Nutrients 9, no. 3: 261. https://doi.org/10.3390/nu9030261




