Stirring the Pot: Can Dietary Modification Alleviate the Burden of CKD?
Abstract
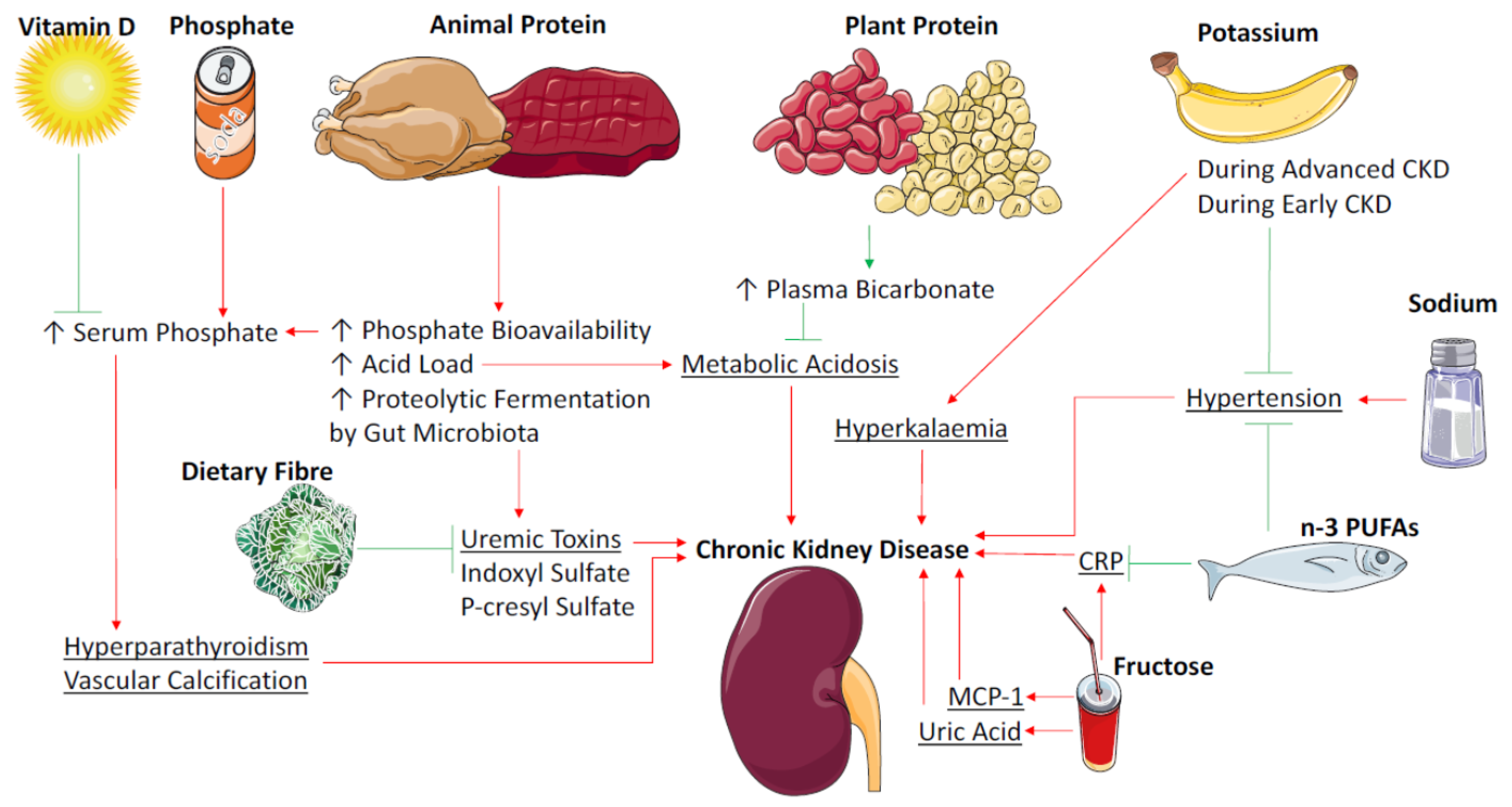
:1. Introduction
2. Protein
2.1. Protein Source and Metabolic Acidosis
2.2. Protein Fermentation by the Colonic Microbiota
3. Dietary Fibre/Non Digestible Carbohydrates
Human Studies—Intervention
4. Sodium
5. Potassium
6. Vitamin D
6.1. Low Vitamin D, CKD and Association with Mortality
6.2. Vitamin D Supplementation and Parathyroid Hormone
6.3. Vitamin D Supplementation and Proteinuria
6.4. Vitamin D Supplementation and Clinical Outcomes—Mortality
7. Phosphorus
7.1. Dietary Sources of Organic Phosphorus
7.2. Dietary Sources of Inorganic Phosphorous
7.3. Reducing Dietary Phosphorus and Serum Phosphate Levels
7.4. Phosphate to Protein Ratio
8. Omega-3 Polyunsaturated Fatty Acids (n-3 PUFAs)
8.1. n-3 PUFAs and Triglyceride Levels
8.2. n-3 PUFAs and Blood Pressure
8.3. n-3 PUFAs and Inflammation in HD Patients
8.4. n-3 PUFAs and Proteinuria/GFR in CKD
8.5. Membrane Levels of n3/n6 PUFAs Associated with Mortality Outcomes in HD
8.6. CVD Events and Mortality Outcomes
9. Sugars and Sugar-Derived Products
9.1. Dietary Fructose
9.2. Dietary Advanced Glycation End Products
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.-U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [PubMed]
- James, M.T.; Hemmelgarn, B.R.; Tonelli, M. Early recognition and prevention of chronic kidney disease. Lancet 2010, 375, 1296–1309. [Google Scholar] [CrossRef]
- Weiner, D.E.; Tighiouart, H.; Amin, M.G.; Stark, P.C.; MacLeod, B.; Griffith, J.L.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J. Am. Soc. Nephrol. 2004, 15, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.-U.; Coresh, J.; Devuyst, O.; Johnson, R.J.; Köttgen, A.; Levey, A.S.; Levin, A. Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 2013, 382, 158–169. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [PubMed]
- Odermatt, A. The Western-style diet: A major risk factor for impaired kidney function and chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2011, 301, F919–F931. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group: KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150.
- Dunkler, D.; Kohl, M.; Teo, K.K.; Heinze, G.; Dehghan, M.; Clase, C.M.; Gao, P.; Yusuf, S.; Mann, J.F.; Oberbauer, R. Dietary risk factors for incidence or progression of chronic kidney disease in individuals with type 2 diabetes in the European Union. Nephrol. Dial. Transplant. 2015, 30, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.R. Effects of dietary interventions on glomerular pathophysiology. Am. J. Physiol. Ren. Physiol. 1990, 258, F1–F8. [Google Scholar]
- Fouque, D.; Aparicio, M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat. Clin. Pract. Nephrol. 2007, 3, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Kaysen, G.A.; Gambertoglio, J.; Jimenez, I.; Jones, H.; Hutchison, F.N. Effect of dietary protein intake on albumin homeostasis in nephrotic patients. Kidney Int. 1986, 29, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A.; Parrett, A.; Khanna, S. Nondigestible Carbohydrates. In Carbohydrates in Food, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 273–303. [Google Scholar]
- Ash, S.; Campbell, K.L.; Bogard, J.; Millichamp, A. Nutrition prescription to achieve positive outcomes in chronic kidney disease: A systematic review. Nutrients 2014, 6, 416–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellizzi, V.; Di Iorio, B.R.; De Nicola, L.; Minutolo, R.; Zamboli, P.; Trucillo, P.; Catapano, F.; Cristofano, C.; Scalfi, L.; Conte, G.; on behalf of the ES-g. Very low protein diet supplemented with ketoanalogs improves blood pressure control in chronic kidney disease. Kidney Int. 2007, 71, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.R.; Bellizzi, V.; Bellasi, A.; Torraca, S.; D’Arrigo, G.; Tripepi, G.; Zoccali, C. Phosphate attenuates the anti-proteinuric effect of very low-protein diet in CKD patients. Nephrol. Dial. Transplant. 2013, 28, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Comparison of high vs. normal/low protein diets on renal function in subjects without chronic kidney disease: A systematic review and meta-analysis. PLoS ONE 2014, 9, e97656. [Google Scholar] [CrossRef] [PubMed]
- Jarusiripipat, C.; Shapiro, J.I.; Chan, L.; Schrier, R.W. Reduction of remnant nephron hypermetabolism by protein restriction. Am. J. Kidney Dis. 1991, 18, 367–374. [Google Scholar] [CrossRef]
- Kleinknecht, C.; Salusky, I.; Broyer, M.; Gubler, M.-C. Effect of various protein diets on growth, renal function, and survival of uremic rats. Kidney Int. 1979, 15, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Kenner, C.H.; Evan, A.P.; Blomgren, P.; Aronoff, G.R.; Luft, F.C. Effect of protein intake on renal function and structure in partially nephrectomized rats. Kidney Int. 1985, 27, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Hostetter, T.H.; Meyer, T.W.; Rennke, H.G.; Brenner, B.M.; Noddin, J.A.; Sandstrom, D.J. Chronic effects of dietary protein in the rat with intact and reduced renal mass. Kidney Int. 1986, 30, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.M.; Waugh, N.; Robertson, A. Protein restriction for diabetic renal disease. Cochrane Libr. 2007, 4, CD002181. [Google Scholar]
- Williams, P.; Stevens, M.; Fass, G.; Irons, L.; Bone, J. Failure of dietary protein and phosphate restriction to retard the rate of progression of chronic renal failure: A prospective, randomized, controlled trial. QJM 1991, 81, 837–855. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Levey, A.S.; Beck, G.J.; Caggiula, A.W.; Hunsicker, L.; Kusek, J.W.; Striker, G. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N. Engl. J. Med. 1994, 330, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.M.; et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement From the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, V.; Kopple, J.D.; Wang, X.; Beck, G.J.; Collins, A.J.; Kusek, J.W.; Greene, T.; Levey, A.S.; Sarnak, M.J. Effect of a very low-protein diet on outcomes: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am. J. Kidney Dis. 2009, 53, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Dobre, M.; Yang, W.; Chen, J.; Drawz, P.; Hamm, L.L.; Horwitz, E.; Hostetter, T.; Jaar, B.; Lora, C.M.; Nessel, L.; et al. Association of Serum Bicarbonate With Risk of Renal and Cardiovascular Outcomes in CKD: A Report From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2013, 62, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.N.; Abramowitz, M.; Hostetter, T.H.; Melamed, M.L. Serum Bicarbonate Levels and the Progression of Kidney Disease: A Cohort Study. Am. J. Kidney Dis. 2009, 54, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Anderson, J.E.; Kalantar-Zadeh, K. Association of serum bicarbonate levels with mortality in patients with non-dialysis-dependent CKD. Nephrol. Dial. Transplant. 2009, 24, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Adeva, M.M.; Souto, G. Diet-induced metabolic acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Alpern, R.J.; Sakhaee, K. The clinical spectrum of chronic metabolic acidosis: Homeostatic mechanisms produce significant morbidity. Am. J. Kidney Dis. 1997, 29, 291–302. [Google Scholar] [CrossRef]
- Kurtz, I. Role of Ammonia in the Induction of Renal Hypertrophy. Am. J. Kidney Dis. 1991, 17, 650–653. [Google Scholar] [CrossRef]
- Mitch, W.E.; Medina, R.; Grieber, S.; May, R.C.; England, B.K.; Price, S.R.; Bailey, J.L.; Goldberg, A.L. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J. Clin. Investig. 1994, 93, 2127–2133. [Google Scholar] [CrossRef] [PubMed]
- Scialla, J.J.; Appel, L.J.; Wolf, M.; Yang, W.; Zhang, X.; Sozio, S.M.; Miller, E.R.; Bazzano, L.A.; Cuevas, M.; Glenn, M.J.; et al. Plant Protein Intake Is Associated with Fibroblast Growth Factor 23 and Serum Bicarbonate in Patients with CKD: The Chronic Renal Insufficiency Cohort Study. J. Ren. Nutr. 2012, 22, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Atabak, S.; Esmaillzadeh, A. Soy Protein Intake, Cardiorenal Indices, and C-Reactive Protein in Type 2 Diabetes With Nephropathy: A longitudinal randomized clinical trial. Diabetes Care 2008, 31, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Kontessis, P.; Jones, S.; Dodds, R.; Trevisan, R.; Nosadini, R.; Fioretto, P.; Borsato, M.; Sacerdoti, D.; Viberti, G. Renal, metabolic and hormonal responses to ingestion of animal and vegetable proteins. Kidney Int. 1990, 38, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low–Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, B.; Di Micco, L.; Marzocco, S.; De Simone, E.; De Blasio, A.; Sirico, M.; Nardone, L.; Group oboUS. Very Low-Protein Diet (VLPD) Reduces Metabolic Acidosis in Subjects with Chronic Kidney Disease: The “Nutritional Light Signal” of the Renal Acid Load. Nutrients 2017, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014, 86, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Goraya, N.; Simoni, J.; Jo, C.-H.; Wesson, D.E. A Comparison of Treating Metabolic Acidosis in CKD Stage 4 Hypertensive Kidney Disease with Fruits and Vegetables or Sodium Bicarbonate. Clin. J. Am. Soc. Nephrol. 2013, 8, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.; Evenepoel, P. The gut–kidney axis: Indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrol. Dial. Transplant. 2011, 26, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T. Indoxyl sulfate is a nephro-vascular toxin. J. Ren. Nutr. 2010, 20, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Tumur, Z.; Shimizu, H.; Enomoto, A.; Miyazaki, H.; Niwa, T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-ĸB activation. Am. J. Nephrol. 2010, 31, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Bammens, B.; Evenepoel, P.; Keuleers, H.; Verbeke, K.; Vanrenterghem, Y. Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int. 2006, 69, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.P.; Luo, F.J.-G.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. The Production of p-Cresol Sulfate and Indoxyl Sulfate in Vegetarians versus Omnivores. Clin. J. Am. Soc. Nephrol. 2012, 7, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Kandouz, S.; Mohamed, A.S.; Zheng, Y.; Sandeman, S.; Davenport, A. Reduced protein bound uraemic toxins in vegetarian kidney failure patients treated by haemodiafiltration. Hemodial. Int. 2016, 20, 610–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Rampton, D.S.; Cohen, S.L.; Crammond, V.D.; Gibbons, J.; Lilburn, M.F.; Rabet, J.Y.; Vince, A.J.; Wager, J.D.; Wrong, O.M. Treatment of chronic renal failure with dietary fiber. Clin. Nephrol. 1984, 21, 159–163. [Google Scholar] [PubMed]
- Vasilis, F.; Dimosthenis, V. Inflammatory Syndrome in Chronic Kidney Disease: Pathogenesis and Influence on Outcomes. Inflamm. Allergy-Drug Targets 2009, 8, 369–382. [Google Scholar]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, P.; Meijers, B.K. Dietary fiber and protein: Nutritional therapy in chronic kidney disease and beyond. Kidney Int. 2012, 81, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Salmean, Y.A.; Segal, M.S.; Langkamp-Henken, B.; Canales, M.T.; Zello, G.A.; Dahl, W.J. Foods With Added Fiber Lower Serum Creatinine Levels in Patients With Chronic Kidney Disease. J. Ren. Nutr. 2013, 23, e29–e32. [Google Scholar] [CrossRef] [PubMed]
- Salmean, Y.A.; Segal, M.S.; Palii, S.P.; Dahl, W.J. Fiber Supplementation Lowers Plasma p-Cresol in Chronic Kidney Disease Patients. J. Ren. Nutr. 2015, 25, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Bliss, D.Z.; Stein, T.P.; Schleifer, C.R.; Settle, R.G. Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am. J. Clin. Nutr. 1996, 63, 392–398. [Google Scholar] [PubMed]
- Ali, A.A.; Ali, K.E.; Fadlalla, A.E.; Khalid, K.E. The effects of gum arabic oral treatment on the metabolic profile of chronic renal failure patients under regular haemodialysis in Central Sudan. Nat. Prod. Res. 2007, 22, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.A.; Darling, P.B. Dietary fiber effects in chronic kidney disease: A systematic review and meta-analysis of controlled feeding trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.K.I.; De Preter, V.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol. Dial. Transplant. 2010, 25, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, I.; Nakamura, M.; Kawakami, K.; Ohta, T.; Kato, I.; Uchida, K.; Yoshida, M. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: A preliminary study. Nephrol. Dial. Transplant. 2011, 26, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of Increasing Dietary Fiber on Plasma Levels of Colon-Derived Solutes in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; Atai, E.; Chan, M.; Phoon, R.K.S.; Scott, C.; Toussaint, N.D.; Turner, G.L.; Usherwood, T.; Wiggins, K.J. KHA-CARI Guideline: Early chronic kidney disease: Detection, prevention and management. Nephrology 2013, 18, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; D’Alessandro, C.; Valeri, A.; Capitanini, A.; Meola, M.; Betti, G.; Barsotti, G. Food Intake and Nutritional Status in Stable Hemodialysis Patients. Ren. Fail. 2010, 32, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalantar-Zadeh, K.; Kopple, J.D.; Deepak, S.; Block, D.; Block, G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J. Ren. Nutr. 2002, 12, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Khoueiry, G.; Waked, A.; Goldman, M.; El-Charabaty, E.; Dunne, E.; Smith, M.; Kleiner, M.; Lafferty, J.; Kalantar-Zadeh, K.; El-Sayegh, S. Dietary Intake in Hemodialysis Patients Does Not Reflect a Heart Healthy Diet. J. Ren. Nutr. 2011, 21, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Klein, K.; Johnson, D.W.; Campbell, K.L. Pre-, Pro-, and Synbiotics: Do They Have a Role in Reducing Uremic Toxins? A Systematic Review and Meta-Analysis. Int. J. Nephrol. 2012, 2012, 673631. [Google Scholar] [CrossRef] [PubMed]
- Salmean, Y.A.; Zello, G.A.; Dahl, W.J. Foods with added fiber improve stool frequency in individuals with chronic kidney disease with no impact on appetite or overall quality of life. BMC Res. Notes 2013, 6, 510. [Google Scholar] [CrossRef] [PubMed]
- Grabitske, H.A.; Slavin, J.L. Gastrointestinal Effects of Low-Digestible Carbohydrates. Crit. Rev. Food Sci. Nutr. 2009, 49, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Hultström, M. Development of structural kidney damage in spontaneously hypertensive rats. J. Hypertens. 2012, 30, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.M.; Garcia, D.L.; Anderson, S. Glomeruli and blood pressure Less of one, more the other? Am. J. Hypertens. 1988, 1, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Jones-Burton, C.; Mishra, S.I.; Fink, J.C.; Brown, J.; Gossa, W.; Bakris, G.L.; Weir, M.R. An in-depth review of the evidence linking dietary salt intake and progression of chronic kidney disease. Am. J. Nephrol. 2006, 26, 268–275. [Google Scholar] [CrossRef] [PubMed]
- McMahon, E.J.; Bauer, J.D.; Hawley, C.M.; Isbel, N.M.; Stowasser, M.; Johnson, D.W.; Campbell, K.L. A randomized trial of dietary sodium restriction in CKD. J. Am. Soc. Nephrol. 2013, 24, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Slagman, M.C.J.; Waanders, F.; Hemmelder, M.H.; Woittiez, A.-J.; Janssen, W.M.T.; Lambers Heerspink, H.J.; Navis, G.; Laverman, G.D. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: Randomised controlled trial. BMJ 2011, 343, d4366. [Google Scholar] [CrossRef] [PubMed]
- Adrogué, H.J.; Madias, N.E. Sodium and Potassium in the Pathogenesis of Hypertension. N. Engl. J. Med. 2007, 356, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Tyson, C.C.; Kuchibhatla, M.; Patel, U.D.; Pun, P.H.; Chang, A.; Nwankwo, C.; Joseph, M.A.; Svetkey, L.P. Impact of Kidney Function on Effects of the Dietary Approaches to Stop Hypertension (Dash) Diet. J. Hypertens. 2014, 3, 1000168. [Google Scholar]
- Noori, N.; Kalantar-Zadeh, K.; Kovesdy, C.P.; Murali, S.B.; Bross, R.; Nissenson, A.R.; Kopple, J.D. Dietary potassium intake and mortality in long-term hemodialysis patients. Am. J. Kidney Dis. 2010, 56, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhang, W.; Zhang, X.; Li, X.; Chen, J. Efficacy and Safety of Paricalcitol Therapy for Chronic Kidney Disease: A Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2012, 7, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, S.P.B. Secondary Hyperparathyroidism and Chronic Kidney Disease. Diabetes Spectr. 2008, 21, 19–25. [Google Scholar] [CrossRef]
- Saravanan, P.; Davidson, N.C. Risk Assessment for Sudden Cardiac Death in Dialysis Patients. Circ. Arrhythm. Electrophysiol. 2010, 3, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Pilz, S.; Obermayer-Pietsch, B.; Verduijn, M.; Tomaschitz, A.; Krane, V.; Espe, K.; Dekker, F.; Brandenburg, V.; März, W.; et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur. Heart J. 2010, 31, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Iodice, S.; Zittermann, A.; Grant, W.B.; Gandini, S. Vitamin D Status and Mortality Risk in CKD: A Meta-analysis of Prospective Studies. Am. J. Kidney Dis. 2011, 58, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.G.; Goldin, J.; Kuizon, B.D.; Yoon, C.; Gales, B.; Sider, D.; Wang, Y.; Chung, J.; Emerick, A.; Greaser, L.; et al. Coronary-Artery Calcification in Young Adults with End-Stage Renal Disease Who Are Undergoing Dialysis. N. Engl. J. Med. 2000, 342, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Hulbert-Shearon, T.E.; Levin, N.W.; Port, F.K. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am. J. Kidney Dis. 1998, 31, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Keyzer, C.A.; Lambers-Heerspink, H.J.; Joosten, M.M.; Deetman, P.E.; Gansevoort, R.T.; Navis, G.; Kema, I.P.; de Zeeuw, D.; Bakker, S.J.; de Borst, M.H.; Group, P.S. Plasma Vitamin D Level and Change in Albuminuria and eGFR According to Sodium Intake. Clin. J. Am. Soc. Nephrol. 2015, 10, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wan, X.; Huang, Z.; Zeng, F.; Wei, G.; Fang, D.; Deng, W.; Li, Y. Impact of Vitamin D on Chronic Kidney Diseases in Non-Dialysis Patients: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e61387. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Sachs, M.; Hoofnagle, A.N.; Utzschneider, K.M.; Kahn, S.E.; Kestenbaum, B.; Himmelfarb, J. Paricalcitol does not improve glucose metabolism in patients with stage 3–4 chronic kidney disease. Kidney Int. 2013, 83, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Chittineni, H.; Packman, M.; Dutka, P.; Ali, N.; Durie, N. Oral Paricalcitol in the Treatment of Patients With CKD and Proteinuria: A Randomized Trial. Am. J. Kidney Dis. 2009, 54, 647–652. [Google Scholar] [CrossRef] [PubMed]
- de Zeeuw, D.; Agarwal, R.; Amdahl, M.; Audhya, P.; Coyne, D.; Garimella, T.; Parving, H.-H.; Pritchett, Y.; Remuzzi, G.; Ritz, E.; et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet 2010, 376, 1543–1551. [Google Scholar] [CrossRef]
- Alborzi, P.; Patel, N.A.; Peterson, C.; Bills, J.E.; Bekele, D.M.; Bunaye, Z.; Light, R.P.; Agarwal, R. Paricalcitol Reduces Albuminuria and Inflammation in Chronic Kidney Disease: A Randomized Double-Blind Pilot Trial. Hypertension 2008, 52, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Kandula, P.; Dobre, M.; Schold, J.D.; Schreiber, M.J.; Mehrotra, R.; Navaneethan, S.D. Vitamin D Supplementation in Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Observational Studies and Randomized Controlled Trials. Clin. J. Am. Soc. Nephrol. 2011, 6, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Rong, G.; Quan, D.; Shu, Y.; Liang, Z.; She, N.; Liu, M.; Yang, B.; Cheng, G.; Lv, Y.; et al. Meta-Analysis: The Efficacy and Safety of Paricalcitol for the Treatment of Secondary Hyperparathyroidism and Proteinuria in Chronic Kidney Disease. BioMed Res. Int. 2013, 2013, 320560. [Google Scholar] [CrossRef] [PubMed]
- de Zeeuw, D.; Remuzzi, G.; Parving, H.-H.; Keane, W.F.; Zhang, Z.; Shahinfar, S.; Snapinn, S.; Cooper, M.E.; Mitch, W.E.; Brenner, B.M. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int. 2004, 65, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Acharya, M.; Tian, J.; Hippensteel, R.L.; Melnick, J.Z.; Qiu, P.; Williams, L.; Batlle, D. Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int. 2005, 68, 2823–2828. [Google Scholar] [CrossRef] [PubMed]
- de Borst, M.H.; Hajhosseiny, R.; Tamez, H.; Wenger, J.; Thadhani, R.; Goldsmith, D.J.A. Active Vitamin D Treatment for Reduction of Residual Proteinuria: A Systematic Review. J. Am. Soc. Nephrol. 2013, 24, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Ruggenenti, P.; Perna, A.; Gherardi, G.; Garini, G.; Zoccali, C.; Salvadori, M.; Scolari, F.; Schena, F.P.; Remuzzi, G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999, 354, 359–364. [Google Scholar] [CrossRef]
- Brenner, B.M.; Cooper, M.E.; de Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.-H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2001, 345, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Shi, H.; Jia, J.; Li, D.; Lin, S. Vitamin D supplementation and mortality risk in chronic kidney disease: A meta-analysis of 20 observational studies. BMC Nephrol. 2013, 14, 199. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.C.; Hobbs, A.J.; Hemmelgarn, B.R.; Roberts, D.J.; Ahmed, S.B.; Rabi, D.M. Effect of oral vitamin D analogus on mortality and cardiovascular outcomes among adults with chronic kidney disease: A meta-analysis. Clin. Kidney J. 2015, 8, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Morrone, L.F.; Cozzolino, M. The beneficial impact of vitamin D treatment in CKD patients: What’s next? Clin. Kidney J. 2015, 8, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Theodoratou, E.; Tzoulaki, I.; Zgaga, L.; Ioannidis, J.P.A. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 2014, 348, g2035. [Google Scholar] [CrossRef] [PubMed]
- Blaine, J.; Chonchol, M.; Levi, M. Renal Control of Calcium, Phosphate, and Magnesium Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Bover, J.; Andrés, E.; Lloret, M.J.; Aguilar, A.; Ballarín, J. Dietary and Pharmacological Control of Calcium and Phosphate Metabolism in Dialysis Patients. Blood Purif. 2009, 27, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Bakris, G.L.; Molitch, M.; Smulders, M.; Tian, J.; Williams, L.A.; Andress, D.L. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int. 2006, 71, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Achinger, S.G.; Ayus, J.C. Left Ventricular Hypertrophy: Is Hyperphosphatemia among Dialysis Patients a Risk Factor? J. Am. Soc. Nephrol. 2006, 17, S255–S261. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Saab, G.; Mathew, S.; Lund, R. Phosphorus Metabolism and Management in Chronic Kidney Disease: Renal Osteodystrophy, Phosphate Homeostasis, and Vascular Calcification. Semin. Dial. 2007, 20, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Voormolen, N.; Noordzij, M.; Grootendorst, D.C.; Beetz, I.; Sijpkens, Y.W.; van Manen, J.G.; Boeschoten, E.W.; Huisman, R.M.; Krediet, R.T.; Dekker, F.W.; group tPs. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol. Dial. Transplant. 2007, 22, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Kestenbaum, B.; Sampson, J.N.; Rudser, K.D.; Patterson, D.J.; Seliger, S.L.; Young, B.; Sherrard, D.J.; Andress, D.L. Serum Phosphate Levels and Mortality Risk among People with Chronic Kidney Disease. J. Am. Soc. Nephrol. 2005, 16, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Eddington, H.; Hoefield, R.; Sinha, S.; Chrysochou, C.; Lane, B.; Foley, R.N.; Hegarty, J.; New, J.; O’Donoghue, D.J.; Middleton, R.J.; et al. Serum Phosphate and Mortality in Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral Metabolism, Mortality, and Morbidity in Maintenance Hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef] [PubMed]
- Young, E.W.; Albert, J.M.; Satayathum, S.; Goodkin, D.A.; Pisoni, R.L.; Akiba, T.; Akizawa, T.; Kurokawa, K.; Bommer, J.; Piera, L.; et al. Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005, 67, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.K.; Stack, A.G.; Levin, N.W.; Hulbert-Shearon, T.E.; Port, F.K. Association of Elevated Serum PO4, Ca × PO4 Product, and Parathyroid Hormone with Cardiac Mortality Risk in Chronic Hemodialysis Patients. J. Am. Soc. Nephrol. 2001, 12, 2131–2138. [Google Scholar] [PubMed]
- Kovesdy, C.; Kalantar-Zadeh, K. Bone and mineral disorders in pre-dialysis CKD. Int. Urol. Nephrol. 2008, 40, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Trivedi, B.K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of Disorders in Mineral Metabolism with Progression of Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2006, 1, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Ruggenenti, P.; Perna, A.; Leonardis, D.; Tripepi, R.; Tripepi, G.; Mallamaci, F.; Remuzzi, G. Phosphate May Promote CKD Progression and Attenuate Renoprotective Effect of ACE Inhibition. J. Am. Soc. Nephrol. 2011, 22, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Chue, C.D.; Edwards, N.C.; Davis, L.J.; Steeds, R.P.; Townend, J.N.; Ferro, C.J. Serum phosphate but not pulse wave velocity predicts decline in renal function in patients with early chronic kidney disease. Nephrol. Dial. Transplant. 2011, 26, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.J.; Bhandari, S.K.; Smith, N.; Chung, J.; Liu, I.L.A.; Jacobsen, S.J.; Kalantar-Zadeh, K. Phosphorus and Risk of Renal Failure in Subjects with Normal Renal Function. Am. J. Med. 2013, 126, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Gutekunst, L.; Mehrotra, R.; Kovesdy, C.P.; Bross, R.; Shinaberger, C.S.; Noori, N.; Hirschberg, R.; Benner, D.; Nissenson, A.R.; et al. Understanding Sources of Dietary Phosphorus in the Treatment of Patients with Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, W.D.; Stegeman, C.A.; Kremer Hovinga, T.K.; Vastenburg, G.; Vos, P.; de Jong, P.E.; Huisman, R.M. Effect of prescribing a high protein diet and increasing the dose of dialysis on nutrition in stable chronic haemodialysis patients: A randomized, controlled trial. Nephrol. Dial. Transplant. 2004, 19, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Kalantar-Zadeh, K. Management of Natural and Added Dietary Phosphorus Burden in Kidney Disease. Semin. Nephrol. 2013, 33, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Calvo, M.S. Hidden Sources of Phosphorus in the Typical American Diet: Does it Matter in Nephrology? Semin. Dial. 2003, 16, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Moshfegh, A.J.; Tucker, K.L. Assessing the Health Impact of Phosphorus in the Food Supply: Issues and Considerations. Adv. Nutr. Int. Rev. J. 2014, 5, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Prasad, N. Dietary management of hyperphosphatemia in chronic kidney disease. Clin. Queries Nephrol. 2014, 3, 38–45. [Google Scholar] [CrossRef]
- Nadkarni, G.N.; Uribarri, J. Phosphorus and the Kidney: What Is Known and What Is Needed. Adv. Nutr. Int. Rev. J. 2014, 5, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Takeda, E.; Yamamoto, H.; Yamanaka-Okumura, H.; Taketani, Y. Increasing Dietary Phosphorus Intake from Food Additives: Potential for Negative Impact on Bone Health. Adv. Nutr. Int. Rev. J. 2014, 5, 92–97. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, J.; Campbell, K.; Ferguson, M.; Day, S.; Rossi, M. Prevalence of Phosphorus-Based Additives in the Australian Food Supply: A Challenge for Dietary Education? J. Ren. Nutr. 2015, 25, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Guida, B.; Piccoli, A.; Trio, R.; Laccetti, R.; Nastasi, A.; Paglione, A.; Memoli, A.; Memoli, B. Dietary phosphate restriction in dialysis patients: A new approach for the treatment of hyperphosphataemia. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 879–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, C.; Sayre, S.S.; Leon, J.B.; Machekano, R.; Love, T.E.; Porter, D.; Marbury, M.; Sehgal, A.R. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: A randomized controlled trial. JAMA 2009, 301, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Shinaberger, C.S.; Greenland, S.; Kopple, J.D.; Van Wyck, D.; Mehrotra, R.; Kovesdy, C.P.; Kalantar-Zadeh, K. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am. J. Clin. Nutr. 2008, 88, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.E.; Lynch, R.; Curhan, G.C.; Brunelli, S.M. Prescribed Dietary Phosphate Restriction and Survival among Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.A. Dietary Phosphate Restriction and Protein Intake in Dialysis Patients: A Misdirected Focus. Semin. Dial. 2007, 20, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Benini, O.; D’Alessandro, C.; Gianfaldoni, D.; Cupisti, A. Extra-Phosphate Load from Food Additives in Commonly Eaten Foods: A Real and Insidious Danger for Renal Patients. J. Ren. Nutr. 2011, 21, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.A.; Mehta, O. Phosphorus and Potassium Content of Enhanced Meat and Poultry Products: Implications for Patients Who Receive Dialysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.G.; Sun, J.W.; Yang, Y.; Ma, X.; Wang, Y.Y.; Xiang, Y.B. Fish consumption and all-cause mortality: A meta-analysis of cohort studies. Eur. J. Clin. Nutr. 2016, 70, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Theilla, M.; Attal-Singer, J.; Singer, P. Effects of polyunsaturated fatty acid consumption in diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Gobe, G.C.; Peake, J.M.; Coombes, J.S. Omega-3 Polyunsaturated Fatty Acids in the Treatment of Kidney Disease. Am. J. Kidney Dis. 2010, 56, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.H.; Schmidt, E.B.; Svensson, M. n-3 polyunsaturated fatty acids, lipids and lipoproteins in end-stage renal disease. Clin. Lipidol. 2011, 6, 563–576. [Google Scholar] [CrossRef]
- Eslick, G.D.; Howe, P.R.C.; Smith, C.; Priest, R.; Bensoussan, A. Benefits of fish oil supplementation in hyperlipidemia: A systematic review and meta-analysis. Int. J. Cardiol. 2009, 136, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Burke, V.; Puddey, I.; Irish, A.; Cowpland, C.A.; Beilin, L.; Dogra, G.; Watts, G.F. The effects of [omega]3 fatty acids and coenzyme Q10 on blood pressure and heart rate in chronic kidney disease: A randomized controlled trial. J. Hypertens. 2009, 27, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Christensen, J.H.; Sølling, J.; Schmidt, E.B. The effect of n-3 fatty acids on plasma lipids and lipoproteins and blood pressure in patients with CRF. Am. J. Kidney Dis. 2004, 44, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Guebre-Egziabher, F.; Debard, C.; Drai, J.; Denis, L.; Pesenti, S.; Bienvenu, J.; Vidal, H.; Laville, M.; Fouque, D. Differential dose effect of fish oil on inflammation and adipose tissue gene expression in chronic kidney disease patients. Nutrition 2013, 29, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Schmidt, E.B.; Jørgensen, K.A.; Christensen, J.H. The effect of n-3 fatty acids on lipids and lipoproteins in patients treated with chronic haemodialysis: A randomized placebo-controlled intervention study. Nephrol. Dial. Transplant. 2008, 23, 2918–2924. [Google Scholar] [CrossRef] [PubMed]
- Khajehdehi, P. Lipid-lowering effect of polyunsaturated fatty acids in hemodialysis patients. J. Ren. Nutr. 2000, 10, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Sanaka, T.; Nihei, H. Eicosapentanoic Acid Reduces Plasma Levels of Remnant Lipoproteins and Prevents in Vivo Peroxidation of LDL in Dialysis Patients. J. Am. Soc. Nephrol. 1999, 10, 2177–2184. [Google Scholar] [PubMed]
- Kooshki, A.; Taleban, F.A.; Tabibi, H.; Hedayati, M. Effects of Omega-3 Fatty Acids on Serum Lipids, Lipoprotein (a), and Hematologic Factors in Hemodialysis Patients. Ren. Fail. 2011, 33, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Saifullah, A.; Watkins, B.A.; Saha, C.; Li, Y.; Moe, S.M.; Friedman, A.N. Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients—A pilot study. Nephrol. Dial. Transplant. 2007, 22, 3561–3567. [Google Scholar] [CrossRef] [PubMed]
- Perunicic-Pekovic, G.B.; Rasic, Z.R.; Pljesa, S.I.; Sobajic, S.S.; Djuricic, I.; Maletic, R.; Ristic-Medic, D.K. Effect of n-3 fatty acids on nutritional status and inflammatory markers in haemodialysis patients. Nephrology 2007, 12, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Taziki, O.; Lessan-Pezeshki, M.; Akha, O.; Vasheghani, F. The Effect of Low Dose Omega-3 on Plasma Lipids in Hemodialysis Patients. Saudi J. Kidney Dis. Transpl. 2007, 18, 571. [Google Scholar] [PubMed]
- Poulia, K.-A.; Panagiotakos, D.B.; Tourlede, E.; Rezou, A.; Stamatiadis, D.; Boletis, J.; Zampelas, A. Omega-3 Fatty Acids Supplementation Does Not Affect Serum Lipids in Chronic Hemodialysis Patients. J. Ren. Nutr. 2011, 21, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.M.; Ali, M.A.; Churchill, D.N. Effect of n-3 fatty acids from fish oil on hemostasis, blood pressure, and lipid profile of dialysis patients. J. Am. Soc. Nephrol. 1992, 2, 1634–1639. [Google Scholar] [PubMed]
- Schmitz, P.G.; McCloud, L.K.; Reikes, S.T.; Leonard, C.L.; Gellens, M.E. Prophylaxis of Hemodialysis Graft Thrombosis with Fish Oil: Double-Blind, Randomized, Prospective Trial. J. Am. Soc. Nephrol. 2002, 13, 184–190. [Google Scholar] [PubMed]
- Bowden, R.G.; Jitomir, J.; Wilson, R.L.; Gentile, M. Effects of Omega-3 Fatty Acid Supplementation on Lipid Levels in Endstage Renal Disease Patients. J. Ren. Nutr. 2009, 19, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Tayebi-Khosroshahi, H.; Dehgan, R.; Habibi Asl, B.; Safaian, A.; Panahi, F.; Estakhri, R.; Purasgar, B. Effect of omega-3 supplementation on serum level of homocysteine in hemodialysis patients. Iran J. Kidney Dis. 2013, 7, 479–484. [Google Scholar] [PubMed]
- Rasic-Milutinovic, Z.; Perunicic, G.; Pljesa, S.; Gluvic, Z.; Sobajic, S.; Djuric, I.; Ristic, D. Effects of N-3 PUFAs Supplementation on Insulin Resistance and Inflammatory Biomarkers in Hemodialysis Patients. Ren. Fail. 2007, 29, 321–329. [Google Scholar] [CrossRef] [PubMed]
- An, W.S.; Lee, S.M.; Son, Y.K.; Kim, S.E.; Kim, K.H.; Han, J.Y.; Bae, H.R.; Rha, S.H.; Park, Y. Omega-3 fatty acid supplementation increases 1,25-dihydroxyvitamin D and fetuin-A levels in dialysis patients. Nutr. Res. 2012, 32, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Bowden, R.G.; Wilson, R.L.; Deike, E.; Gentile, M. Fish Oil Supplementation Lowers C-Reactive Protein Levels Independent of Triglyceride Reduction in Patients With End-Stage Renal Disease. Nutr. Clin. Pract. 2009, 24, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.E.; Moist, L.; Hemmelgarn, B.R.; Tonelli, M.; Vazquez, M.A.; Dorval, M.; Oliver, M.; Donnelly, S.; Allon, M.; Stanley, K. Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts: A randomized trial. JAMA 2012, 307, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Bessell, E.; Jose, M.D.; McKercher, C. Associations of fish oil and vitamin B and E supplementation with cardiovascular outcomes and mortality in people receiving haemodialysis: A review. BMC Nephrol. 2015, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Dong, C.; Du, H.; Zhang, H.; Chen, J.; Hu, X.; Hu, F. Effects of fish oil on serum lipid profile in dialysis patients: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2014, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, O.A.; Gaziano, J.M.; Djoussé, L. N-3 Fatty Acids for Prevention of Cardiovascular Disease. Curr. Atheroscler. Rep. 2014, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Lin, X.; Huang, H.; Zheng, X.; Li, T.; Zou, Y. Omega-3 Fatty Acid Supplementation on Lipid Profiles in Dialysis Patients: Meta-analysis. Arch. Med. Res. 2014, 45, 469–477. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, M.-S.; Lin, M.; Zhao, T.-Y.; Gao, P. Effect of fish oil supplement in maintenance hemodialysis patients: A systematic review and meta-analysis of published randomized controlled trials. Eur. J. Clin. Pharmacol. 2016, 72, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Hypertension in chronic kidney disease. Clin. Queries Nephrol. 2013, 2, 15–22. [Google Scholar] [CrossRef]
- Miller, E.R.; Juraschek, S.P.; Anderson, C.A.; Guallar, E.; Henoch-Ryugo, K.; Charleston, J.; Turban, S.; Bennett, M.R.; Appel, L.J. The Effects of n-3 Long-Chain Polyunsaturated Fatty Acid Supplementation on Biomarkers of Kidney Injury in Adults with Diabetes: Results of the GO-FISH trial. Diabetes Care 2013, 36, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-Chain Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid and Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N. Omega-3 Fatty Acid Supplementation in Advanced Kidney Disease. Semin. Dial. 2010, 23, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Moe, S. Review of the Effects of Omega-3 Supplementation in Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2006, 1, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Noori, N.; Dukkipati, R.; Kovesdy, C.P.; Sim, J.J.; Feroze, U.; Murali, S.B.; Bross, R.; Benner, D.; Kopple, J.D.; Kalantar-Zadeh, K. Dietary Omega-3 Fatty Acid, Ratio of Omega-6 to Omega-3 Intake, Inflammation, and Survival in Long-term Hemodialysis Patients. Am. J. Kidney Dis. 2011, 58, 248–256. [Google Scholar] [CrossRef] [PubMed]
- An, W.S.; Lee, S.M.; Son, Y.K.; Kim, S.E.; Kim, K.H.; Han, J.Y.; Bae, H.R.; Park, Y. Effect of omega-3 fatty acids on the modification of erythrocyte membrane fatty acid content including oleic acid in peritoneal dialysis patients. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Gharekhani, A.; Khatami, M.-R.; Dashti-khavidaki, S.; Razeghi, E.; Noorbala, A.-A.; Hashemi-nazari, S.-S.; Mansournia, M.-A. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: A randomized, placebo-controlled clinical trial. Eur. J. Clin. Pharmacol. 2014, 70, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.M.; Booker, C.; Ellis, C.D.; Siew, E.D.; Graves, A.J.; Shintani, A.; Abumrad, N.N.; Himmelfarb, J.; Ikizler, T.A. Omega-3 fatty acids inhibit the up-regulation of endothelial chemokines in maintenance hemodialysis patients. Nephrol. Dial. Transplant. 2015, 30, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Bazeley, J.; Bieber, B.; Li, Y.; Morgenstern, H.; de Sequera, P.; Combe, C.; Yamamoto, H.; Gallagher, M.; Port, F.K.; Robinson, B.M. C-Reactive Protein and Prediction of 1-Year Mortality in Prevalent Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2452–2461. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Li, Y.-J.; Wu, H.-H.; Lee, C.-C.; Lin, C.-Y.; Weng, C.-H.; Chen, Y.-C.; Chang, M.-Y.; Hsu, H.-H.; Fang, J.-T.; et al. High-Sensitivity C-Reactive Protein Predicts Mortality and Technique Failure in Peritoneal Dialysis Patients. PLoS ONE 2014, 9, e93063. [Google Scholar] [CrossRef] [PubMed]
- Möllsten, A.V.; Dahlquist, G.G.; Stattin, E.-L.; Rudberg, S. Higher Intakes of Fish Protein Are Related to a Lower Risk of Microalbuminuria in Young Swedish Type 1 Diabetic Patients. Diabetes Care 2001, 24, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R.; Juraschek, S.P.; Appel, L.J.; Madala, M.; Anderson, C.A.; Bleys, J.; Guallar, E. The effect of n–3 long-chain polyunsaturated fatty acid supplementation on urine protein excretion and kidney function: Meta-analysis of clinical trials. Am. J. Clin. Nutr. 2009, 89, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Chiou, Y.Y.; Hung, P.H.; Chiang, P.C.; Wang, S.T. Omega-3 Fatty Acids Ameliorate Proteinuria but Not Renal Function in IgA Nephropathy: A Meta-Analysis of Randomized Controlled Trials. Nephron Clin. Pract. 2012, 121, c30–c35. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Chung, S.H.; Park, Y.; Park, M.K.; Son, Y.K.; Kim, S.E.; An, W.S. Effect of Omega-3 Fatty Acid on the Fatty Acid Content of the Erythrocyte Membrane and Proteinuria in Patients with Diabetic Nephropathy. Int. J. Endocrinol. 2015, 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Ohtani, K.-I.; Tanaka, Y.; Sato, N.; Mori, M.; Shimomura, Y. Long-term effect of eicosapentaenoic acid ethyl (EPA-E) on albuminuria of non-insulin dependent diabetic patients. Diabetes Res. Clin. Pract. 1995, 28, 35–40. [Google Scholar] [CrossRef]
- Madsen, T.; Christensen, J.H.; Svensson, M.; Witt, P.M.; Toft, E.; Schmidt, E.B. Marine n-3 polyunsaturated fatty acids in patients with end-stage renal failure and in subjects without kidney disease: A comparative study. J. Ren. Nutr. 2011, 21, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N.; Saha, C.; Watkins, B.A. A Feasbility Study of Erythrocyte Long Chain Omega-3 Polyunsaturated Fatty Acid Content and Mortality Risk in Hemodialysis Patients. J. Ren. Nutr. 2008, 18, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Terashima, Y.; Hamazaki, K.; Itomura, M.; Tomita, S.; Kuroda, M.; Hirata, H.; Hamazaki, T.; Inadera, H. Inverse association between docosahexaenoic acid and mortality in patients on hemodialysis during over 10 years. Hemodial. Int. 2014, 18, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, K.; Terashima, Y.; Itomura, M.; Sawazaki, S.; Inagaki, H.; Kuroda, M.; Tomita, S.; Hirata, H.; Inadera, H.; Hamazaki, T. Docosahexaenoic Acid Is an Independent Predictor of All-Cause Mortality in Hemodialysis Patients. Am. J. Nephrol. 2011, 33, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N.; Yu, Z.; Tabbey, R.; Denski, C.; Tamez, H.; Wenger, J.; Thadhani, R.; Li, Y.; Watkins, B.A. Inverse relationship between long-chain n-3 fatty acids and risk of sudden cardiac death in patients starting hemodialysis. Kidney Int. 2013, 83, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Kakiya, R.; Hayashi, T.; Tsujimoto, Y.; Sonoda, M.; Shima, H.; Mori, K.; Fukumoto, S.; Tahara, H.; Shioi, A.; et al. Serum n-3 and n-6 Polyunsaturated Fatty Acid Profile as an Independent Predictor of Cardiovascular Events in Hemodialysis Patients. Am. J. Kidney Dis. 2013, 62, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, R.G.; James, M.J.; Gibson, R.A.; Edwards, J.R.; Stubberfield, J.; Stuklis, R.; Roberts-Thomson, K.; Young, G.D.; Cleland, L.G. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am. J. Clin. Nutr. 2007, 85, 1222–1228. [Google Scholar] [PubMed]
- Kutner, N.G.; Clow, P.W.; Zhang, R.; Aviles, X. Association of fish intake and survival in a cohort of incident dialysis patients. Am. J. Kidney Dis. 2002, 39, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Okano, K.; Tsuruta, Y.; Tsuruta, Y.; Tsuchiya, K.; Akiba, T.; Nitta, K. Eicosapentaenoic Acid (EPA) Decreases the All-Cause Mortality in Hemodialysis Patients. Intern. Med. 2015, 54, 3133–3137. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Schmidt, E.B.; Jørgensen, K.A.; Christensen, J.H.; Group obotOS. N-3 Fatty Acids as Secondary Prevention against Cardiovascular Events in Patients Who Undergo Chronic Hemodialysis: A Randomized, Placebo-Controlled Intervention Trial. Clin. J. Am. Soc. Nephrol. 2006, 1, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Zabel, R.; Ash, S.; King, N.; Bauer, J. Adherence to Fish Oil Intervention in Patients with Chronic Kidney Disease. J. Ren. Nutr. 2010, 20, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Stevens, S.; Gorman, D.; Pan, A.; Warnakula, S.; Chowdhury, S.; Ward, H.; Johnson, L.; Crowe, F.; Hu, F.B.; et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: Systematic review and meta-analysis. BMJ 2012, 345, e6698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visioli, F.; Risé, P.; Barassi, M.C.; Marangoni, F.; Galli, C. Dietary intake of fish vs. formulations leads to higher plasma concentrations of n-3 fatty acids. Lipids 2003, 38, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.M.; Pan, A.; Rexrode, K.M.; Stampfer, M.; Hu, F.B.; Mozaffarian, D.; Willett, W.C. Dietary Protein Sources and the Risk of Stroke in Men and Women. Stroke 2012, 43, 637. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Sanchez-Lozada, L.G.; Nakagawa, T. The effect of fructose on renal biology and disease. J. Am. Soc. Nephrol. 2010, 21, 2036–2039. [Google Scholar] [CrossRef] [PubMed]
- Singh AKab, Kari JAc: Metabolic syndrome and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2013, 22, 198–203.
- Bomback, A.S.; Derebail, V.K.; Shoham, D.A.; Anderson, C.A.; Steffen, L.M.; Rosamond, W.D.; Kshirsagar, A.V. Sugar-sweetened soda consumption, hyperuricemia, and kidney disease. Kidney Int. 2010, 77, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Kosugi, T.; Gersch, M.; Connor, T.; Sanchez-Lozada, L.G.; Lanaspa, M.A.; Roncal, C.; Perez-Pozo, S.E.; Johnson, R.J.; Nakagawa, T. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am. J. Physiol. Ren. Physiol. 2010, 298, F712–F720. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, P.; Gersch, M.S.; Mu, W.; Scherer, P.M.; Kim, K.M.; Gesualdo, L.; Henderson, G.N.; Johnson, R.J.; Sautin, Y.Y. Ketohexokinase-Dependent Metabolism of Fructose Induces Proinflammatory Mediators in Proximal Tubular Cells. J. Am. Soc. Nephrol. 2009, 20, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Shoham, D.A.; Durazo-Arvizu, R.; Kramer, H.; Luke, A.; Vupputuri, S.; Kshirsagar, A.; Cooper, R.S. Sugary soda consumption and albuminuria: Results from the National Health and Nutrition Examination Survey, 1999–2004. PLoS ONE 2008, 3, e3431. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.J.; Ford, E.S.; Gao, X.; Choi, H.K. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: The third national health and nutrition examination survey. Arthritis Care Res. 2008, 59, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Qi, L.; Qiao, N.; Choi, H.K.; Curhan, G.; Tucker, K.L.; Ascherio, A. Intake of Added Sugar and Sugar-Sweetened Drink and Serum Uric Acid Concentration in US Men and Women. Hypertension 2007, 50, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Saldana, T.M.; Basso, O.; Darden, R.; Sandler, D.P. Carbonated beverages and chronic kidney disease. Epidemiology 2007, 18, 501. [Google Scholar] [CrossRef] [PubMed]
- Yuzbashian, E.; Asghari, G.; Mirmiran, P.; Zadeh-Vakili, A.; Azizi, F. Sugar-sweetened beverage consumption and risk of incident chronic kidney disease: Tehran Lipid and Glucose Study. Nephrology 2016, 21, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Curhan, G.C. Associations of Sugar and Artificially Sweetened Soda with Albuminuria and Kidney Function Decline in Women. Clin. J. Am. Soc. Nephrol. 2011, 6, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; O’Corragain, O.A.; Edmonds, P.J.; Kittanamongkolchai, W.; Erickson, S.B. Associations of sugar-sweetened and artificially sweetened soda with chronic kidney disease: A systematic review and meta-analysis. Nephrology 2014, 19, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Karalius, V.P.; Shoham, D.A. Dietary sugar and artificial sweetener intake and chronic kidney disease: A review. Adv. Chronic Kidney Dis. 2013, 20, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Gersch, M.S.; Mu, W.; Cirillo, P.; Reungjui, S.; Zhang, L.; Roncal, C.; Sautin, Y.Y.; Johnson, R.J.; Nakagawa, T. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2007, 293, F1256–F1261. [Google Scholar] [CrossRef] [PubMed]
- Tapia, E.; Cristóbal, M.; García-Arroyo, F.E.; Soto, V.; Monroy-Sánchez, F.; Pacheco, U.; Lanaspa, M.A.; Roncal-Jiménez, C.A.; Cruz-Robles, D.; Ishimoto, T.; et al. Synergistic effect of uricase blockade plus physiological amounts of fructose-glucose on glomerular hypertension and oxidative stress in rats. Am. J. Physiol. Ren. Physiol. 2013, 304, F727–F736. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Sievenpiper, J.L.; de Souza, R.J.; Chiavaroli, L.; Ha, V.; Cozma, A.I.; Mirrahimi, A.; Matthew, E.Y.; Carleton, A.J.; Di Buono, M. The effects of fructose intake on serum uric acid vary among controlled dietary trials. J. Nutr. 2012, 142, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Brymora, A.; Flisiński, M.; Johnson, R.J.; Goszka, G.; Stefańska, A.; Manitius, J. Low-fructose diet lowers blood pressure and inflammation in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Penfold, S.A.; Coughlan, M.T.; Patel, S.K.; Srivastava, P.M.; Sourris, K.C.; Steer, D.; Webster, D.E.; Thomas, M.C.; MacIsaac, R.J.; Jerums, G.; et al. Circulating high-molecular-weight RAGE ligands activate pathways implicated in the development of diabetic nephropathy. Kidney Int. 2010, 78, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kratochvilová, M.; Zakiyanov, O.; Kalousová, M.; Kříha, V.; Zima, T.; Tesař, V. Associations of Serum Levels of Advanced Glycation end Products with Nutrition Markers and Anemia in Patients with Chronic Kidney Disease. Ren. Fail. 2011, 33, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sato, E.; Fujiwara, N.; Kawagoe, Y.; Ueda, Y.; Suzuki, T.; Yamada, S.; Takeuchi, M.; Fukami, K.; Ueda, S. Positive association of serum levels of advanced glycation end products and high mobility group box–1 with asymmetric dimethylarginine in nondiabetic chronic kidney disease patients. Metabolism 2009, 58, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Henle, T.; Miyata, T. Advanced glycation end products in uremia. Adv. Ren. Replace. Ther. 2003, 10, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Koschinsky, T.; He, C.-J.; Mitsuhashi, T.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Peppa, M.; Cai, W.; Goldberg, T.; Lu, M.; Baliga, S.; Vassalotti, J.A.; Vlassara, H. Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am. J. Kidney Dis. 2003, 42, 532–538. [Google Scholar] [CrossRef]
- Semba, R.D.; Fink, J.C.; Sun, K.; Windham, B.G.; Ferrucci, L. Serum Carboxymethyl-lysine, a Dominant Advanced Glycation End Product, is Associated with Chronic Kidney Disease: The Baltimore Longitudinal Study of Aging. J. Ren. Nutr. 2010, 20, 74–81. [Google Scholar] [CrossRef] [PubMed]
- D’Agati, V.; Schmidt, A.M. RAGE and the pathogenesis of chronic kidney disease. Nat. Rev. Nephrol. 2010, 6, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Scivittaro, V.; Ganz, M.B.; Weiss, M.F. AGEs induce oxidative stress and activate protein kinase C-βII in neonatal mesangial cells. Am. J. Physiol. Ren. Physiol. 2000, 278, F676–F683. [Google Scholar]
- Li, J.; Schmidt, A.M. Characterization and Functional Analysis of the Promoter of RAGE, the Receptor for Advanced Glycation End Products. J. Biol. Chem. 1997, 272, 16498–16506. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, M.T.; Thorburn, D.R.; Penfold, S.A.; Laskowski, A.; Harcourt, B.E.; Sourris, K.C.; Tan, A.L.; Fukami, K.; Thallas-Bonke, V.; Nawroth, P.P.; et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 2009, 20, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.F.; Ren, H.; Owen, W.F.; Guo, Z.J.; Chen, P.Y.; Schmidt, A.M.; Miyata, T.; Zhang, X. Enhanced Expression of Receptor for Advanced Glycation End Products in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2004, 15, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Franke, S.; Muller, A.; Wolf, M.; Gerth, J.; Ott, U.; Niwa, T.; Stein, G. Potential cardiovascular risk factors in chronic kidney disease: AGEs, total homocysteine and metabolites, and the C-reactive protein. Kidney Int. 2004, 66, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Busch, M.; Franke, S.; Wolf, G.; Brandstädt, A.; Ott, U.; Gerth, J.; Hunsicker, L.G.; Stein, G. The Advanced Glycation End Product Nε-Carboxymethyllysine Is Not a Predictor of Cardiovascular Events and Renal Outcomes in Patients With Type 2 Diabetic Kidney Disease and Hypertension. Am. J. Kidney Dis. 2006, 48, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Schwedler, S.B.; Metzger, T.; Schinzel, R.; Wanner, C. Advanced glycation end products and mortality in hemodialysis patients. Kidney Int. 2002, 62, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.; Busch, M.; Muller, A.; Wendt, T.; Franke, C.; Niwa, T.; Franke, S. Are advanced glycation end products cardiovascular risk factors in patients with CRF? Am. J. Kidney Dis. 2003, 41, S52–S56. [Google Scholar] [CrossRef] [PubMed]
- Suliman, M.E.; Heimburger, O.; Barany, P.; Anderstam, B.; Pecoits-Filho, R.; Rodriguez Ayala, E.; Qureshi, A.R.; Fehrman-Ekholm, I.; Lindholm, B.; Stenvinkel, P. Plasma pentosidine is associated with inflammation and malnutrition in end-stage renal disease patients starting on dialysis therapy. J. Am. Soc. Nephrol. 2003, 14, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Wagner, Z.; Molnár, M.; Molnár, G.A.; Tamaskó, M.; Laczy, B.; Wagner, L.; Csiky, B.; Heidland, A.; Nagy, J.; Wittmann, I. Serum Carboxymethyllysine Predicts Mortality in Hemodialysis Patients. Am. J. Kidney Dis. 2006, 47, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Hartog, J.W.L.; Graaff, R.; Huisman, R.J.; Links, T.P.; den Hollander, N.C.; Thorpe, S.R.; Baynes, J.W.; Navis, G.; Gans, R.O.B.; et al. Skin Autofluorescence, a Measure of Cumulative Metabolic Stress and Advanced Glycation End Products, Predicts Mortality in Hemodialysis Patients. J. Am. Soc. Nephrol. 2005, 16, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.X.; Hou, F.F.; Liang, M.; Wang, G.B.; Zhang, X.; Li, H.Y.; Xie, D.; Tian, J.W.; Liu, Z.Q. Restricted intake of dietary advanced glycation end products retards renal progression in the remnant kidney model. Kidney Int. 2007, 71, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Šebeková, K.; Faist, V.; Hofmann, T.; Schinzel, R.; Heidland, A. Effects of a diet rich in advanced glycation end products in the rat remnant kidney model. Am. J. Kidney Dis. 2003, 41, S48–S51. [Google Scholar] [CrossRef] [PubMed]
- ŠEbeková, K.; Hofmann, T.; Boor, P.; ŠEbeková, K.; Ulicná, O.G.; Erbersdobler, H.F.; Baynes, J.W.; Thorpe, S.R.; Heidland, A.; Somoza, V. Renal Effects of Oral Maillard Reaction Product Load in the Form of Bread Crusts in Healthy and Subtotally Nephrectomized Rats. Ann. N. Y. Acad. Sci. 2005, 1043, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; He, C.; Cai, W.; Hattori, M.; Steffes, M.; Vlassara, H. Prevention of diabetic nephropathy in mice by a diet low in glycoxidation products. Diabetes Metab. Res. Rev. 2002, 18, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Somoza, V.; Lindenmeier, M.; Hofmann, T.; Frank, O.; Erbersdobler, H.F.; Baynes, J.W.; Thorpe, S.R.; Heidland, A.; Zill, H.; Bek, S.; et al. Dietary bread crust advanced glycation end products bind to the receptor for AGEs in HEK-293 kidney cells but are rapidly excreted after oral administration to healthy and subtotally nephrectomized rats. Ann. N. Y. Acad. Sci. 2005, 1043, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.L.Y.; Sourris, K.C.; Harcourt, B.E.; Thallas-Bonke, V.; Penfold, S.; Andrikopoulos, S.; Thomas, M.C.; O’Brien, R.C.; Bierhaus, A.; Cooper, M.E.; et al. Disparate effects on renal and oxidative parameters following RAGE deletion, AGE accumulation inhibition, or dietary AGE control in experimental diabetic nephropathy. Am. J. Physiol.-Ren. Physiol. 2010, 298, F763–F770. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Peppa, M.; Cai, W.; Goldberg, T.; Lu, M.; He, C.; Vlassara, H. Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. Int. J. Am. Soc. Nephrol. 2003, 14, 728–731. [Google Scholar] [CrossRef]
- Vlassara, H.; Cai, W.; Goodman, S.; Pyzik, R.; Yong, A.; Chen, X.; Zhu, L.; Neade, T.; Beeri, M.; Silverman, J.M.; et al. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: Role of the antiinflammatory age receptor-1. J. Clin. Endocrinol. Metab. 2009, 94, 4483–4491. [Google Scholar] [CrossRef] [PubMed]
- Harcourt, B.E.; Sourris, K.C.; Coughlan, M.T.; Walker, K.Z.; Dougherty, S.L.; Andrikopoulos, S.; Morley, A.L.; Thallas-Bonke, V.; Chand, V.; Penfold, S.A.; et al. Targeted reduction of advanced glycation improves renal function in obesity. Kidney Int. 2011, 80, 190–198. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snelson, M.; Clarke, R.E.; Coughlan, M.T. Stirring the Pot: Can Dietary Modification Alleviate the Burden of CKD? Nutrients 2017, 9, 265. https://doi.org/10.3390/nu9030265
Snelson M, Clarke RE, Coughlan MT. Stirring the Pot: Can Dietary Modification Alleviate the Burden of CKD? Nutrients. 2017; 9(3):265. https://doi.org/10.3390/nu9030265
Chicago/Turabian StyleSnelson, Matthew, Rachel E. Clarke, and Melinda T. Coughlan. 2017. "Stirring the Pot: Can Dietary Modification Alleviate the Burden of CKD?" Nutrients 9, no. 3: 265. https://doi.org/10.3390/nu9030265



