Elevated Serum Hepcidin Levels during an Intensified Training Period in Well-Trained Female Long-Distance Runners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
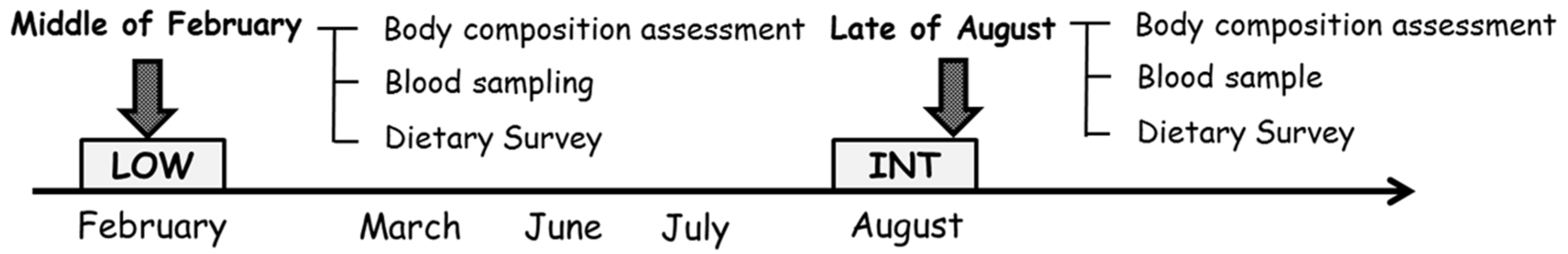
2.2. Experimental Design
2.3. Measurement Procedures
2.3.1. Body Composition
2.3.2. Blood Sampling and Analysis
2.3.3. Dietary Assessment
2.3.4. Statistical Analyses
3. Results
3.1. Body Composition
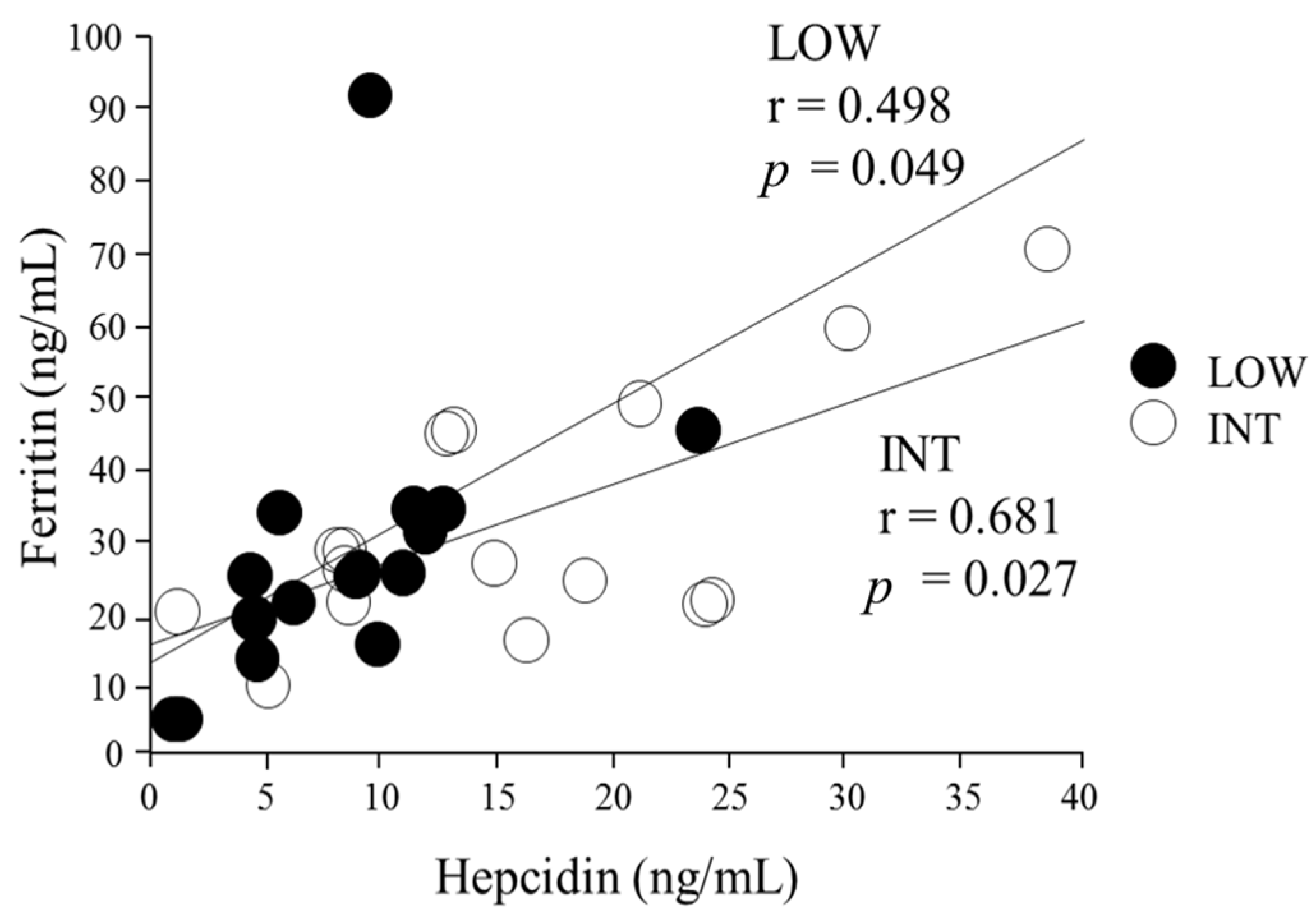
3.2. Iron Status Assessment
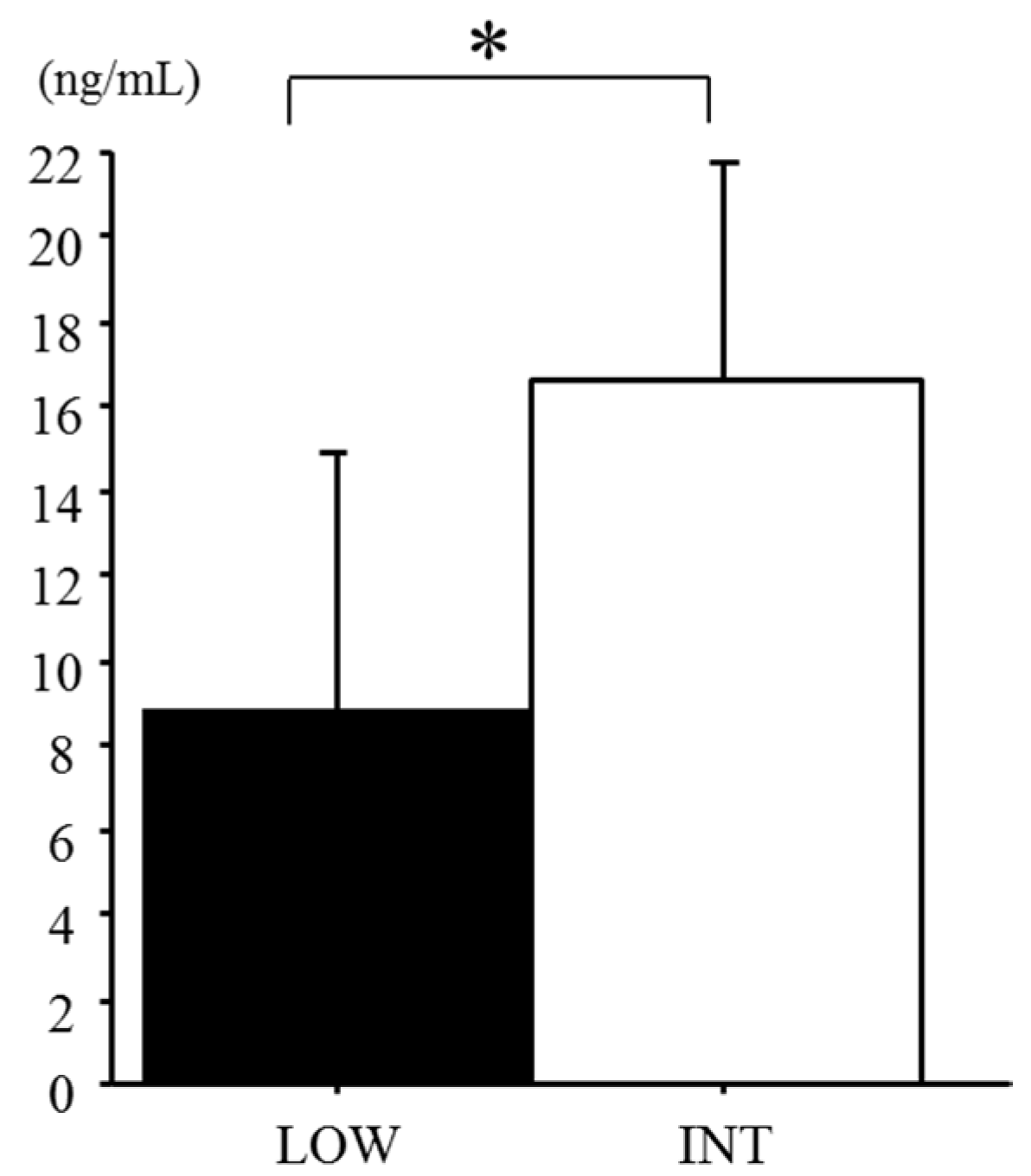
3.3. Serum Hepcidin Levels
3.4. Energy and Macronutrient Intake
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IL-6 | interleukin-6 |
| Hb | hemoglobin |
| MCV | mean corpuscular volume |
| MCH | mean corpuscular hemoglobin |
| MCHC | mean corpuscular hemoglobin concentration |
| TIBC | total iron-binding capacity |
| CK | creatine kinase |
| TSAT | transferrin saturation |
| CV | coefficients of variation |
References
- Lukaski, H.C. Vitamin and mineral status: Effects on physical performance. Nutrition 2004, 20, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Celsing, F.; Ekblom, B. Anemia causes a relative decrease in blood lactate concentration during exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.D.; Brownlie, T. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676–690. [Google Scholar]
- Beard, J.; Tobin, B. Iron status and exercise. Am. J. Clin. Nutr. 2000, 72, 594–597. [Google Scholar]
- Stewart, J.G.; Ahlquist, D.A.; McGill, D.B.; Ilstrup, D.M.; Schwartz, S.; Owen, R.A. Gastrointestinal blood loss and anemia in runners. Ann. Intern. Med. 1984, 100, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Pate, R.; Burgess, W. Foot Impact Force and Intravascular Hemolysis during Distance Running. Int. J. Sports Med. 1988, 9, 56–60. [Google Scholar] [CrossRef] [PubMed]
- King, N.; Fridlund, K.E.; Askew, E.W. Nutrition issues of military women. J. Am. Coll. Nutr. 1993, 12, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Brune, M.; Magnusson, B.; Persson, H.; Hallberg, L. Iron losses in sweat. Am. J. Clin. Nutr. 1986, 43, 438–443. [Google Scholar] [PubMed]
- Nicolas, G.; Chauvet, C.; Viatte, L.; Danan, J.L.; Bigard, X.; Devaux, I.; Beaumont, C.; Kahn, A.; Vaulont, S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J. Clin. Investig. 2002, 110, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Roecker, L.; Meier-Buttermilch, R.; Brechtel, L.; Nemeth, E.; Ganz, T. Iron-regulatory protein hepcidin is increased in female athletes after a marathon. Eur. J. Appl. Physiol. 2005, 95, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Effects of exercise on hepcidin response and iron metabolism during recovery. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Sim, M.; Badenhorst, C.E.; Dawson, B.; Govus, A.D.; Abbiss, C.R.; Swinkels, D.W.; Trinder, D. Iron Status and the Acute Post-Exercise Hepcidin Response in Athletes. PLoS ONE 2014, 9, e93002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, T.; Nemeth, E.; Iron imports, I.V. Hepcidin and regulation of body iron metabolism. Am. J. Physiol. 2006, 2, 199–203. [Google Scholar]
- Recalcati, S.; Minotti, G.; Cairo, G. Iron regulatory proteins: From molecular mechanisms to drug development. Antioxid. Redox 2010, 13, 1593–1616. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 2003, 101, 2461–2463. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 2003, 102, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron Sequestration and Anemia of Inflammation. Semin. Hematol. 2009, 46, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Ronsen, O.; Lea, T.; Bahr, R.; Pedersen, B.K.; Bahr, R.; Hostmark, A.; Newsholme, E.; Gronnerod, O.; Sejersted, O.; Bangsbo, J. Enhanced plasma IL-6 and IL-1ra responses to repeated vs. single bouts of prolonged cycling in elite athletes. J. Appl. Physiol. 2002, 92, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Training surface and intensity: Inflammation, hemolysis, and hepcidin expression. Med. Sci. Sports Exerc. 2009, 41, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Dawson, B.; Goodman, C.; Landers, G.; Wiegerinck, E.T.; Swinkels, D.W.; Trinder, D. Cumulative effects of consecutive running sessions on hemolysis, inflammation and hepcidin activity. Eur. J. Appl. Physiol. 2009, 106, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Newlin, M.; Williams, S. The effects of acute exercise bouts on hepcidin in women. Int. J. Sport Nutr. 2012, 22, 79–88. [Google Scholar] [CrossRef]
- Nickerson, H.J.; Holubets, M.; Tripp, A.D.; Pierce, W.E. Decreased iron stores in high school female runners. Am. J. Dis. Child. 1985, 139, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Demura, S.; Sato, S.; Kitabayashi, T. Percentage of total body fat as estimated by three automatic bioelectrical impedance analyzers. J. Physiol. Anthropol. Appl. Hum. Sci. 2004, 23, 93–99. [Google Scholar] [CrossRef]
- Deurenberg, P.; van der Kooy, K.; Leenen, R.; Schouten, F.J. Body impedance is largely dependent on the intra- and extra-cellular water distribution. Eur. J. Clin. Nutr. 1989, 43, 845–853. [Google Scholar] [PubMed]
- Asobayire, F,S.; Adou, P.; Davidsson, L.; Cook, J. D.; Hurrell, R. F. Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: a study in Côte d ‘Ivoire. Am J Clin Nutr. 2001, 74, 776–782. [Google Scholar] [PubMed]
- Reinke, S.; Taylor, W.R.; Duda, G.N.; von Haehling, S.; Reinke, P.; Volk, H.D.; Anker, S.D.; Doehner, W. Absolute and functional iron deficiency in professional athletes during training and recovery. Int. J. Cardiol. 2012, 156, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Dawson, B.; Landers, G.; Swinkels, D.W.; Tjalsma, H.; Trinder, D.; Peeling, P. Effect of exercise modality and intensity on post-exercise interleukin-6 and hepcidin levels. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, E.; Kasprowicz, K.; Kasperska, A.; Zembroń-Lacny, A.; Antosiewicz, J.; Laskowski, R. Do high blood hepcidin concentrations contribute to low ferritin levels in young tennis players at the end of tournament season? J. Sports Sci. Med. 2013, 12, 249–258. [Google Scholar] [PubMed]
- Dzedzej, A.; Ignatiuk, W.; Jaworska, J.; Grzywacz, T.; Lipińska, P.; Antosiewicz, J.; Korek, A.; Ziemann, E. The effect of the competitive season in professional basketball on inflammation and iron metabolism. Biol. Sport 2016, 33, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.; Dawson, B.; Landers, G.J.; Swinkels, D.W.; Tjalsma, H.; Wiegerinck, E.T.; Trinder, D.; Peeling, P. A seven day running training period increases basal urinary hepcidin levels as compared to cycling. J. Int. Soc. Sports Nutr. 2014, 11, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemna, E. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood 2005, 106, 1864–1866. [Google Scholar] [CrossRef] [PubMed]
- Banzet, S.; Sanchez, H.; Chapot, R.; Bigard, X.; Vaulont, S.; Koulmann, N. Interleukin-6 contributes to hepcidin mRNA increase in response to exercise. Cytokine 2012, 58, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Heisterberg, M.F.; Fahrenkrug, J.; Krustrup, P.; Storskov, A.; Kjær, M; Andersen, J.L. Extensive Monitoring Through Multiple Blood Samples in Professional Soccer Players. J. Strength Cond. Res. 2013, 27, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Thurnham, D.I.; McCabe, L.D.; Haldar, S.; Wieringa, F.T.; Northrop-Clewes, C.A.; McCabe, G.P. Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. Am. J. Clin. Nutr. 2010, 92, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Borrione, P.; Spaccamiglio, A.; Rizzo, M. Urinary hepcidin identifies a serum ferritin cut-off for iron supplementation in young athletes: A pilot study. J. Biol. 2011, 25, 427–434. [Google Scholar]
- Nuviala, R.J.; Lapieza, M.G. Disparity between diet and serum ferritin in elite sportswomen. Nutr. Res. 1997, 17, 451–461. [Google Scholar] [CrossRef]
- Zotter, H.; Robinson, N.; Zorzoli, M.; Schattenberg, L.; Saugy, M.; Mangin, P. Abnormally high serum ferritin levels among professional road cyclists. Br. J. Sports Med. 2004, 38, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Immermann, M.B.; Troesch, B.; Biebinger, R.; Egli, I.; Zeder, C.; Hurrell, R.F. Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am. J. Clin. Nutr. 2009, 90, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Samuelson, G.; Bratteby, L.; Berggren, K. Dietary iron intake and iron status in adolescents. Acta Paediatr. 1996, 85, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Kabasakalis, A.; Kalitsis, K.; Tsalis, G.; Mougios, V. Imbalanced Nutrition of Top-Level Swimmers. Int. J. Sports Med. 2007, 28, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Deldicque, L.; Francaux, M. Recommendations for Healthy Nutrition in Female Endurance Runners: An Update. Front. Nutr. 2015, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Pasiakos, S.M.; Margolis, L.M.; Murphy, N.E.; McClung, H.L.; Martini, S.; Gundersen, Y.; Castellani, J.W.; Karl, J.P.; Teien, H.K.; Madslien, E.H. Effects of exercise mode, energy, and macronutrient interventions on inflammation during military training. Physiol. Rep. 2016, 4, e12820. [Google Scholar] [CrossRef] [PubMed]
| Variables | LOW | INT | p |
|---|---|---|---|
| Height (cm) | 160.5 ± 3.9 | - | - |
| Body weight (kg) | 49.7 ± 5.1 | 49.1 ± 4.0 | N.S. |
| Lean body mass (kg) | 41.4 ± 3.4 | 41.7 ± 2.0 | N.S. |
| Body fat (kg) | 8.0 ± 2.9 | 7.4 ± 2.3 | 0.015 |
| % Body fat (%) | 15.8 ± 4.9 | 15.0 ± 4.3 | 0.023 |
| Variables | LOW | INT | p |
|---|---|---|---|
| Hb (g/dL) | 12.9 ± 0.8 | 13.4 ± 0.1 | N.S. |
| MCV (μm3) | 90.0 ± 2.8 | 92.7 ± 3.0 | N.S. |
| MCH (pg) | 30.6 ± 2.6 | 30.7 ± 1.0 | N.S. |
| MCHC (g/dL) | 32.1 ± 0.7 | 33.1 ± 0.7 | N.S. |
| Ferritin (ng/mL) | 30.9 ± 22.2 | 28.1 ± 11.8 | N.S. |
| Total Protein (g/dL) | 7.2 ± 0.4 | 7.1 ± 0.3 | N.S. |
| Iron (μg/dL) | 55 ± 24 | 65 ± 8 | N.S. |
| TIBC (μg/dL) | 340.7 ± 44.6 | 323.0 ± 6.9 | N.S. |
| TSAT (%) | 16.4 ± 7.5 | 20.1 ± 2.4 | N.S. |
| CK (IU) | 227 ± 110 | 369 ± 66 | 0.09 |
| IL-6 (pg/mL) | 0.35 ± 0.23 | 0.33 ± 0.23 | N.S. |
| Variables | LOW | INT | p | |
|---|---|---|---|---|
| Energy | (kcal) | 2140 ± 130 | 2318 ± 343 | 0.052 |
| (KJ) | 8958 ± 544 | 9703 ± 1437 | 0.052 | |
| (kcal/BWkg) | 44 ± 5 | 48 ± 4 | 0.034 | |
| Protein | (g) | 115.8 ± 9.7 | 103.5 ± 17.9 | 0.002 |
| (g/BWkg) | 2.4 ± 0.4 | 2.1 ± 0.5 | <0.001 | |
| Fat | (g) | 64.2 ± 13.5 | 54.2 ± 0.6 | 0.010 |
| Carbohydrate | (g) | 275.8 ± 31.2 | 353.0 ± 75.4 | 0.002 |
| (g/BWkg) | 5.6 ± 1.0 | 7.2 ± 1.5 | <0.001 | |
| Iron | (mg) | 14.4 ± 1.6 | 17.3 ± 5.4 | 0.047 |
| Vitamin C | (mg) | 228 ± 53 | 243 ± 94 | N.S. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishibashi, A.; Maeda, N.; Sumi, D.; Goto, K. Elevated Serum Hepcidin Levels during an Intensified Training Period in Well-Trained Female Long-Distance Runners. Nutrients 2017, 9, 277. https://doi.org/10.3390/nu9030277
Ishibashi A, Maeda N, Sumi D, Goto K. Elevated Serum Hepcidin Levels during an Intensified Training Period in Well-Trained Female Long-Distance Runners. Nutrients. 2017; 9(3):277. https://doi.org/10.3390/nu9030277
Chicago/Turabian StyleIshibashi, Aya, Naho Maeda, Daichi Sumi, and Kazushige Goto. 2017. "Elevated Serum Hepcidin Levels during an Intensified Training Period in Well-Trained Female Long-Distance Runners" Nutrients 9, no. 3: 277. https://doi.org/10.3390/nu9030277





