Association between Circulating Vitamin D Level and Urolithiasis: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Data Extraction and Study Quality Assessment
2.3. Data Processing and Statistical Analysis
3. Results
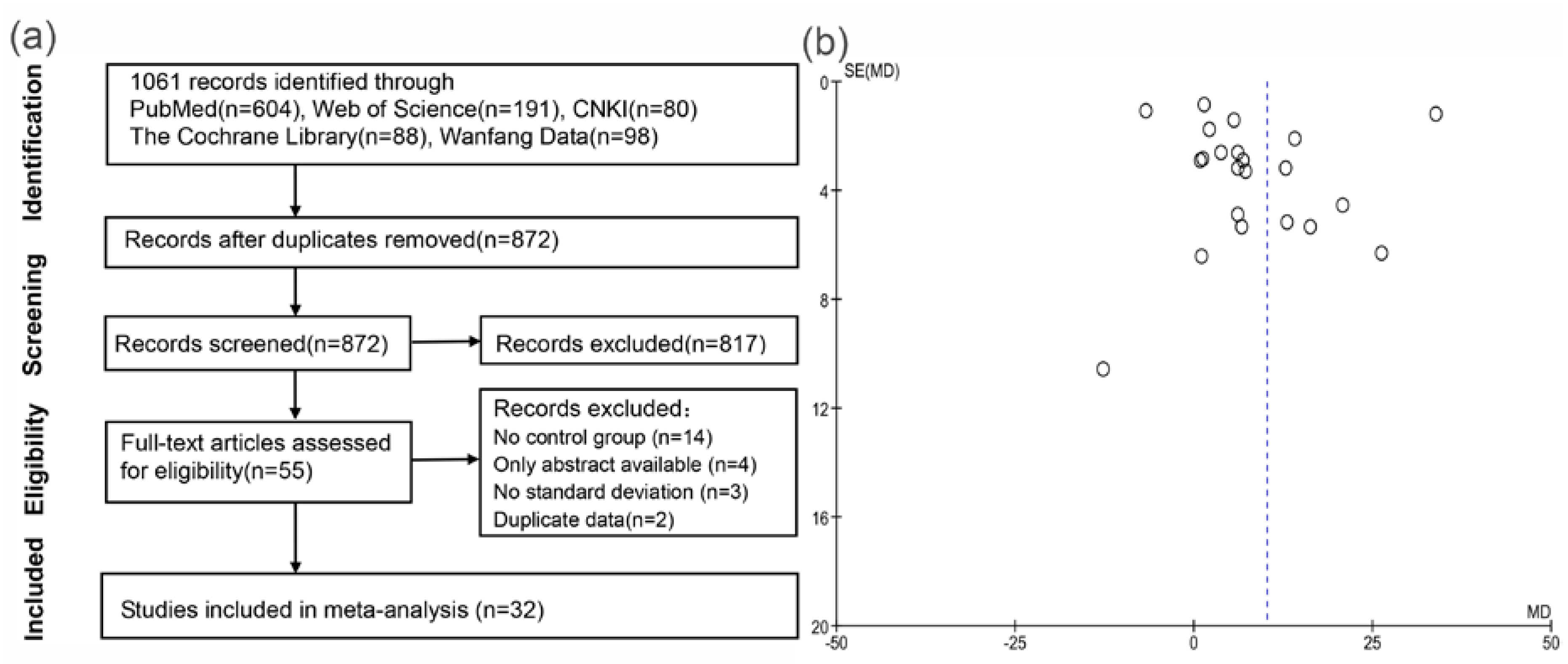
3.1. Literature Search and Study Selection
3.2. Systematic Reviews of Included Studies
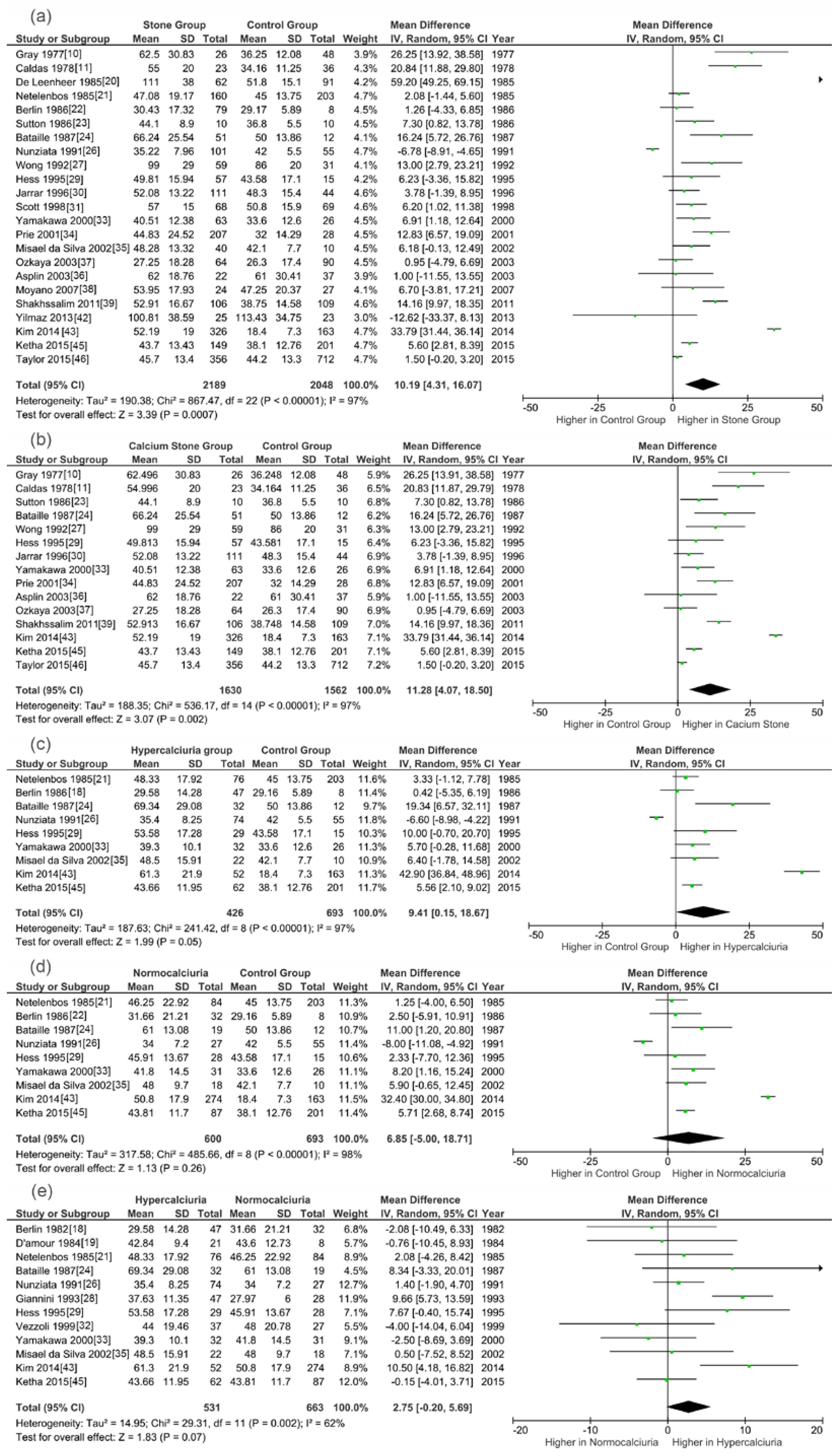
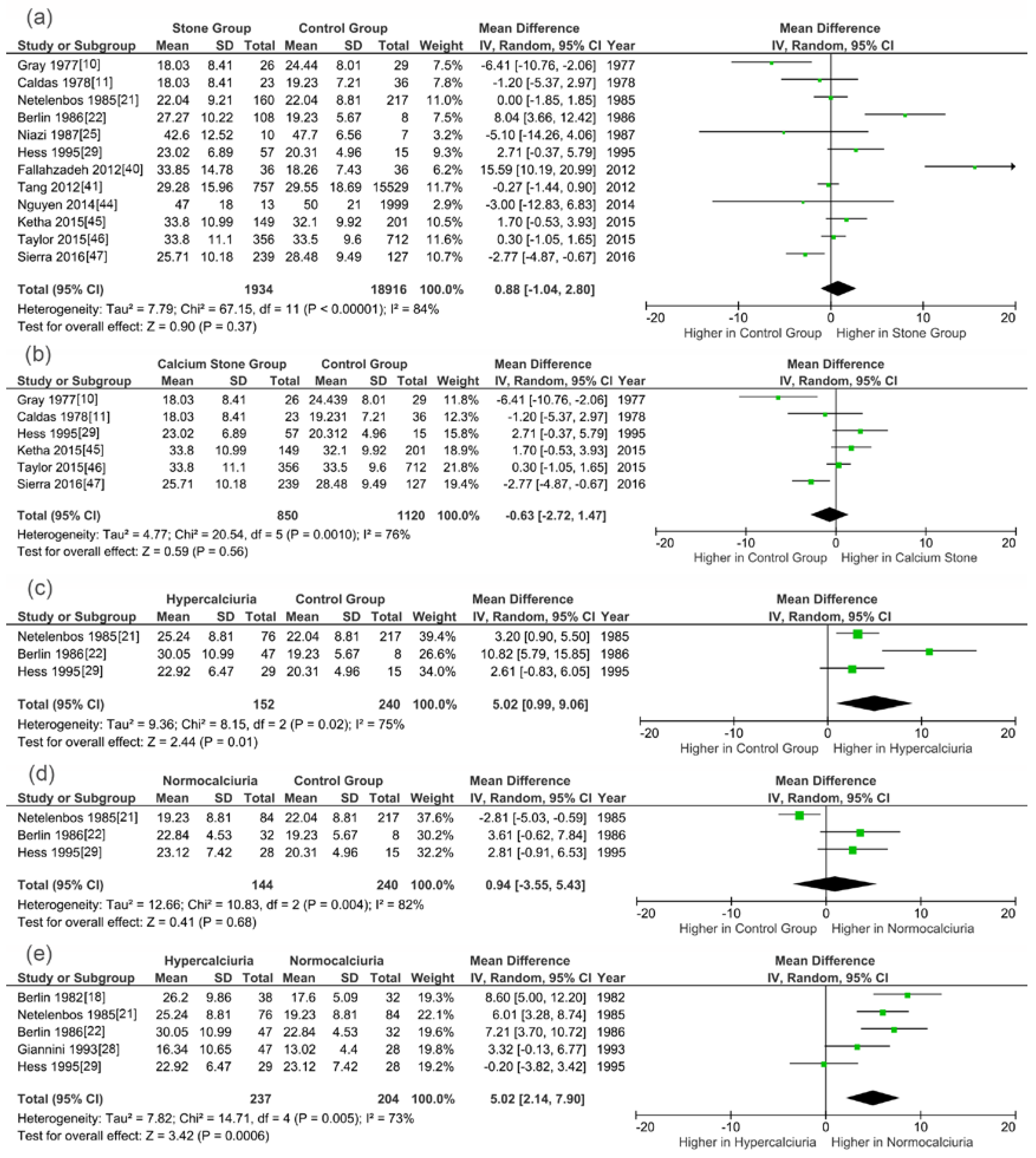
3.3. Meta-Analysis Results
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scales, C.D.; Smith, A.C.; Hanley, J.M.; Saigal, C.S. Prevalence of kidney stones in the United States. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Skolarikos, A.; Straub, M.; Knoll, T.; Sarica, K.; Seitz, C.; Petrik, A.; Turk, C. Metabolic evaluation and recurrence prevention for urinary stone patients: Eau guidelines. Eur. Urol. 2015, 67, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, J.A.; Maalouf, N.M.; Pearle, M.S.; Lotan, Y. Use of the national health and nutrition examination survey to calculate the impact of obesity and diabetes on cost and prevalence of urolithiasis in 2030. Eur. Urol. 2014, 66, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Sigurjonsdottir, V.K.; Runolfsdottir, H.L.; Indridason, O.S.; Palsson, R.; Edvardsson, V.O. Impact of nephrolithiasis on kidney function. BMC Nephrol. 2015, 16, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, R.T.; Hemmelgarn, B.R.; Wiebe, N.; Bello, A.; Morgan, C.; Samuel, S.; Klarenbach, S.W.; Curhan, G.C.; Tonelli, M.A. Kidney stones and kidney function loss: A cohort study. Br. Med. J. 2012, 345, e5287. [Google Scholar] [CrossRef] [PubMed]
- Turk, C.; Petrik, A.; Sarica, K.; Seitz, C.; Skolarikos, A.; Straub, M.; Knoll, T. Eau guidelines on interventional treatment for urolithiasis. Eur. Urol. 2016, 69, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Lu, Y.; He, D.; Cui, L.; Zhang, J.; Zhao, Z.; Qin, B.; Wang, Y.; Lin, F.; Wang, S. Comparison of minimally invasive percutaneous nephrolithotomy and flexible ureteroscopy for the treatment of intermediate proximal ureteral and renal stones in the elderly. Urolithiasis 2016, 44, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Pearle, M.S.; Antonelli, J.A.; Lotan, Y. Urinary lithiasis: Etiology, epidemiology, and pathogenesis. In Campbell-Walsh Urology, 11th ed.; Wein, A.J., Kavoussi, L.R., Partin, A.W., Peters, A.C., Eds.; Elsevier: Philadelphia, PA, USA, 2016; Volume 2, pp. 1170–1199. [Google Scholar]
- Daudon, M.; Bazin, D.; Letavernier, E. Randall's plaque as the origin of calcium oxalate kidney stones. Urolithiasis 2015, 43, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.W.; Wilz, D.R.; Caldas, A.E.; Lemann, J., Jr. The importance of phosphate in regulating plasma 1,25-(OH)2-vitamin D levels in humans: Studies in healthy subjects in calcium-stone formers and in patients with primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 1977, 45, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Caldas, A.E.; Gray, R.W.; Lemann, J., Jr. The simultaneous measurement of vitamin D metabolites in plasma: Studies in healthy adults and in patients with calcium nephrolithiasis. J. Lab. Clin. Med. 1978, 91, 840–849. [Google Scholar] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; RISMA Group. PPreferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: Http://www.Ohri.Ca/programs/clinical_epidemiology/oxford.Asp (accessed on 1 January 2017).
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). Available online: www.handbook.cochrane.org (accessed on 1 January 2017).
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Lu, Y.; Cui, L.; Zhang, J.; Zhao, Z.; Qin, B.; Wang, Y.; Wang, Q.; Wang, S. Impact of previous open renal surgery on the outcomes of subsequent percutaneous nephrolithotomy: A meta-analysis. BMJ Open 2016, 6, e010627. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Qin, B.; He, D.; Lu, Y.; Zhao, Z.; Zhang, J.; Wang, Y.; Wang, S. Regional versus general anesthesia for percutaneous nephrolithotomy: A meta-analysis. PLoS ONE 2015, 10, e0126587. [Google Scholar] [CrossRef] [PubMed]
- Berlin, T.; Bjorkhem, I.; Collste, L.; Holmberg, I.; Wijkstrom, H. Relation between hypercalciuria and vitamin D3-status in patients with urolithiasis. Scand. J. Urol. Nephrol. 1982, 16, 269–273. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, P.; Gascon-Barre, M.; Dufresne, L.; Perreault, J.P. Influence of dietary calcium on serum 1,25-dihydroxyvitamin D concentrations in renal stone formers. Clin. Endocrinol. (Oxf.) 1984, 21, 549–562. [Google Scholar] [CrossRef] [PubMed]
- De Leenheer, A.P.; Bauwens, R.M. Radioimmunoassay for 1,25-dihydroxyvitamin D in serum or plasma. Clin. Chem. 1985, 31, 142–146. [Google Scholar] [PubMed]
- Netelenbos, J.C.; Jongen, M.J.; van der Vijgh, W.J.; Lips, P.; van Ginkel, F.C. Vitamin D status in urinary calcium stone formation. Arch. Intern. Med. 1985, 145, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Berlin, T.; Holmberg, I.; Bjorkhem, I. High circulating levels of 25-hydroxyvitamin D3 in renal stone formers with hyperabsorptive hypercalciuria. Scand. J. Clin. Lab. Investig. 1986, 46, 367–374. [Google Scholar] [CrossRef]
- Sutton, R.A.; Walker, V.R. Bone resorption and hypercalciuria in calcium stoneformers. Metabolism 1986, 35, 485–488. [Google Scholar] [CrossRef]
- Bataille, P.; Bouillon, R.; Fournier, A.; Renaud, H.; Gueris, J.; Idrissi, A. Increased plasma concentrations of total and free 1,25-(OH)2D3 in calcium stone formers with idiopathic hypercalciuria. Contrib. Nephrol. 1987, 58, 137–142. [Google Scholar] [PubMed]
- Niazi, M.K.; Khanum, A.; Sheikh, M.A.; Naqvi, S.A. Study of 25-hydroxy vitamin D3, calcium, phosphorus in normal subjects and patients with calculi. J. Pak. Med. Assoc. 1987, 37, 198–199. [Google Scholar] [PubMed]
- Nunziata, V.; di Giovanni, G.; Giannattasio, R.; Lettera, A.M.; Mancini, M. Recurrent kidney stones: Causes and diagnostic criteria in patients from Campania (Southern Italy). Br. J. Urol. 1991, 68, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.; Slater, S.R.; Evans, R.A.; Mason, R.; Lancaster, E.K.; Acland, S.M.; Eade, Y.; Hills, E.; Dunstan, C.R. Metabolic studies in kidney stone disease. Q. J. Med. 1992, 82, 247–258. [Google Scholar] [PubMed]
- Giannini, S.; Nobile, M.; Castrignano, R.; Pati, T.; Tasca, A.; Villi, G.; Pellegrini, F.; D’Angelo, A. Possible link between vitamin D and hyperoxaluria in patients with renal stone disease. Clin. Sci. 1993, 84, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; Ackermann, D.; Essig, M.; Takkinen, R.; Jaeger, P. Renal mass and serum calcitriol in male idiopathic calcium renal stone formers: Role of protein intake. J. Clin. Endocrinol. Metab. 1995, 80, 1916–1921. [Google Scholar] [PubMed]
- Jarrar, K.; Amasheh, R.A.; Graef, V.; Weidner, W. Relationship between 1,25-dihydroxyvitamin-D, calcium and uric acid in urinary stone formers. Urol. Int. 1996, 56, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Ouimet, D.; Proulx, Y.; Trouve, M.L.; Guay, G.; Gagnon, B.; Valiquette, L.; Bonnardeaux, A. The 1 alpha-hydroxylase locus is not linked to calcium stone formation or calciuric phenotypes in French-Canadian families. J. Am. Soc. Nephrol. 1998, 9, 425–432. [Google Scholar] [PubMed]
- Vezzoli, G.; Caumo, A.; Baragetti, I.; Zerbi, S.; Bellinzoni, P.; Centemero, A.; Rubinacci, A.; Moro, G.; Adamo, D.; Bianchi, G.; et al. Study of calcium metabolism in idiopathic hypercalciuria by strontium oral load test. Clin. Chem. 1999, 45, 257–261. [Google Scholar] [PubMed]
- Yamakawa, K.; Kawamura, J. Analysis of hypophosphatemia in calcium nephrolithiasis. Mol. Urol. 2000, 4, 365–370. [Google Scholar] [PubMed]
- Prie, D.; Ravery, V.; Boccon-Gibod, L.; Friedlander, G. Frequency of renal phosphate leak among patients with calcium nephrolithiasis. Kidney Int. 2001, 60, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Misael da Silva, A.M.; dos Reis, L.M.; Pereira, R.C.; Futata, E.; Branco-Martins, C.T.; Noronha, I.L.; Wajchemberg, B.L.; Jorgetti, V. Bone involvement in idiopathic hypercalciuria. Clin. Nephrol. 2002, 57, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Asplin, J.R.; Bauer, K.A.; Kinder, J.; Muller, G.; Coe, B.J.; Parks, J.H.; Coe, F.L. Bone mineral density and urine calcium excretion among subjects with and without nephrolithiasis. Kidney Int. 2003, 63, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, O.; Soylemezoglu, O.; Misirlioglu, M.; Gonen, S.; Buyan, N.; Hasanoglu, E. Polymorphisms in the vitamin d receptor gene and the risk of calcium nephrolithiasis in children. Eur. Urol. 2003, 44, 150–154. [Google Scholar] [CrossRef]
- Moyano, M.J.; de Tejada, M.J.G.; Lozano, R.G.; Moruno, R.; Ortega, R.; Marti, V.; Palencia, R.S.; Miranda, M.J.; Palma, A.; Cano, R.P. Alterations in bone mineral metabolism in patients with calcium kidney stone disease and polymorphism of vitamin D receptor. Preliminary results. Nefrologia 2007, 27, 694–703. [Google Scholar] [PubMed]
- Shakhssalim, N.; Gilani, K.R.; Parvin, M.; Torbati, P.M.; Kashi, A.H.; Azadvari, M.; Golestan, B.; Basiri, A. An assessment of parathyroid hormone, calcitonin, 1,25 (OH)(2) vitamin D3, estradiol and testosterone in men with active calcium stone disease and evaluation of its biochemical risk factors. Urol. Res. 2011, 39, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fallahzadeh, M.H.; Zare, J.; Al-Hashemi, G.H.; Derakhshan, A.; Basiratnia, M.; Arasteh, M.M.; Fallahzadeh, M.A.; Fallahzadeh, M.K. Elevated serum levels of vitamin D in infants with urolithiasis. Iran. J. Kidney Dis. 2012, 6, 186–191. [Google Scholar] [PubMed]
- Tang, J.; McFann, K.K.; Chonchol, M.B. Association between serum 25-hydroxyvitamin D and nephrolithiasis: The national health and nutrition examination survey III, 1988–1994. Nephrol. Dial. Transplant. 2012, 27, 4385–4389. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, D.; Sonmez, F.; Yenisey, C.; Girisgen, I. The role of active vitamin D on stone formation and hypercalciuria. Nobel Med. 2013, 9, 88–91. [Google Scholar]
- Kim, W.T.; Kim, Y.-J.; Yun, S.J.; Shin, K.-S.; Choi, Y.D.; Lee, S.C.; Kim, W.-J. Role of 1,25-dihydroxy vitamin D-3 and parathyroid hormone in urinary calcium excretion in calcium stone formers. Yonsei Med. J. 2014, 55, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Baggerly, L.; French, C.; Heaney, R.P.; Gorham, E.D.; Garland, C.F. 25-hydroxyvitamin D in the range of 20 to 100 ng/mL and incidence of kidney stones. Am. J. Public Health 2014, 104, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Ketha, H.; Singh, R.J.; Grebe, S.K.; Bergstralh, E.J.; Rule, A.D.; Lieske, J.C.; Kumar, R. Altered calcium and vitamin D homeostasis in first-time calcium kidney stone-formers. PLoS ONE 2015, 10, e0137350. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Hoofnagle, A.N.; Curhan, G.C. Calcium and phosphorus regulatory hormones and risk of incident symptomatic kidney stones. Clin. J. Am. Soc. Nephrol. 2015, 10, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Sierra Giron-Prieto, M.; del Carmen Cano-Garcia, M.; Angel Arrabal-Polo, M.; Poyatos-Andujar, A.; Quesada-Charneco, M.; de Haro-Munoz, T.; Arias-Santiago, S.; Arrabal-Martin, M. Analysis of vitamin D deficiency in calcium stone-forming patients. Int. Urol. Nephrol. 2016, 48, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Coe, F.L.; Worcester, E.M.; Evan, A.P. Idiopathic hypercalciuria and formation of calcium renal stones. Nat. Rev. Nephrol. 2016, 12, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Kuo, R.L.; Lingeman, J.E.; Evan, A.P.; Paterson, R.F.; Parks, J.H.; Bledsoe, S.B.; Munch, L.C.; Coe, F.L. Urine calcium and volume predict coverage of renal papilla by randall’s plaque. Kidney Int. 2003, 64, 2150–2154. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, T.O. Cyp24a1 loss of function: Clinical phenotype of monoallelic and biallelic mutations. J. Steroid Biochem. Mol. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.L.; Wang, S.G.; Ye, Z.Q.; Zhu, Z.W.; Li, C.; Bai, J.; Yu, X.; Liu, J.H. Effect of silencing VDR gene in kidney on renal epithelial calcium transporter proteins and urinary calcium excretion in genetic hypercalciuric stone-forming rats. Urology 2011, 78. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, S.; Tang, J.; He, D.; Cui, L.; Liu, Z.; Guo, B.; Huang, L.; Lu, Y.; Hu, H. Does crystal deposition in genetic hypercalciuric rat kidney tissue share similarities with bone formation? Urology 2014, 83. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.K.; Krieger, N.S.; Bushinsky, D.A. Modeling hypercalciuria in the genetic hypercalciuric stone-forming rat. Curr. Opin. Nephrol. Hypertens. 2015, 24, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, M.; Li, M.; Ma, H.; Tong, S.; Lei, Y.; Qi, L. Vitamin d receptor gene (VDR) polymorphisms and the urolithiasis risk: An updated meta-analysis based on 20 case-control studies. Urolithiasis 2014, 42, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Malihi, Z.; Wu, Z.; Stewart, A.W.; Lawes, C.M.M.; Scragg, R. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 104, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Letavernier, E.; Verrier, C.; Goussard, F.; Perez, J.; Huguet, L.; Haymann, J.-P.; Baud, L.; Bazin, D.; Daudon, M. Calcium and vitamin D have a synergistic role in a rat model of kidney stone disease. Kidney Int. 2016, 90, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Man, L.; Li, G.; Huang, G.; Liu, N. Association between serum vitamin D levels and the risk of kidney stone: Evidence from a meta-analysis. Nutr. J. 2016, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Karras, S.N.; Anagnostis, P.; Beauchet, O.; Goulis, D.G.; Annweiler, C. Vitamin D supplements and bone mineral density. Lancet 2014, 383, 1292–1293. [Google Scholar] [CrossRef]
- Reid, I.R.; Bolland, M.J.; Grey, A. Effects of vitamin d supplements on bone mineral density: A systematic review and meta-analysis. Lancet 2014, 383, 146–155. [Google Scholar] [CrossRef]
- Grober, U.; Reichrath, J.; Holick, M.F. Live longer with vitamin D? Nutrients 2015, 7, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.N.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Vitamin D status and efficacy of vitamin D supplementation in atopic dermatitis: A systematic review and meta-analysis. Nutrients 2016, 8, 789. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.M.; Ekwaru, J.P.; Setayeshgar, S.; Veugelers, P.J. The effect of changing serum 25-hydroxyvitamin D concentrations on metabolic syndrome: A longitudinal analysis of participants of a preventive health program. Nutrients 2015, 7, 7271–7284. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.F. Associations between vitamin D status, supplementation, outdoor work and risk of parkinson's disease: A meta-analysis assessment. Nutrients 2015, 7, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Jaeger, P.; Ferraro, P.M.; Shavit, L.; Nair, D.; Robertson, W.G.; Gambaro, G.; Unwin, R.J. Vitamin D deficiency is prevalent among idiopathic stone formers, but does correction pose any risk? Urolithiasis 2016. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, M.C.; Rycyna, K.J.; Averch, T.D.; Semins, M.J. Vitamin D repletion in kidney stone formers: A randomized controlled trial. J. Urol. 2016. [Google Scholar] [CrossRef] [PubMed]
| Surname of First Author | Year | Country | NOS Score | Sample Type | Measurement Method for 1,25(OH)2D | Measurement Method For 25(OH)D | Stone Component | Groups | Participant Number | Age (Mean ± SD) | Sex Ratio (M/F) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gray [10] | 1977 | USA | 7/9 | plasma | Chromatin binding assay | competitive protein binding assay | calcium oxalate/apatite | SG | 26 | 48 | 24/2 |
| CG | 48 | 25 | 27/21 | ||||||||
| Caldas [11] | 1978 | USA | 7/9 | plasma | Cytosol binding assay | competitive protein binding assay | calcium oxalate/apatite | SG | 23 | NA | 20/3 |
| CG | 36 | NA | 22/14 | ||||||||
| Berlin [18] | 1982 | Sweden | 8/9 | serum | - | Isotope dilution-mass spectrometry | calcium oxalate/phosphate | HSG | 38 | NA | 16/6 |
| NSG | 32 | NA | 34/4 | ||||||||
| D’Amour [19] | 1984 | Canada | 7/9 | serum | Competitive binding assay | - | NA | HSG | 21 | 36.16 ± 31.39 | 17/4 |
| NSG | 8 | 31.8 ± 18.38 | 3/5 | ||||||||
| De Leenheer [20] | 1985 | Belgium | 7/9 | serum | Radioimmunoassay | - | NA | SG | 62 | NA | NA |
| CG | 91 | NA | NA | ||||||||
| Netelenbos [21] | 1985 | Netherlands | 8/9 | serum | Competitive protein binding assay | Competitive protein binding assay | NA | SG | 160 | 43 ± 14 | 106/54 |
| CG | 203 | 39 ± 11 | 147/70 | ||||||||
| Berlin [22] | 1986 | Sweden | 8/9 | serum | Radioreceptor assay | Isotope dilution-mass spectrometry | NA | SG | 79 | 43 ± 3.33 | NA |
| CG | 8 | 31 ± 6.83 | NA | ||||||||
| Sutton [23] | 1986 | Canada | 9/9 | serum | Cytosol receptor assay | - | Calcium | SG | 10 | 47 ± 11 | 10/0 |
| CG | 10 | 47 ± 10 | 10/0 | ||||||||
| Bataille [24] | 1987 | France | 8/9 | plasma | Radioimmunoassay | - | Calcium | SG | 51 | NA | 29/22 |
| CG | 12 | NA | 7/5 | ||||||||
| Niazi [25] | 1987 | Parkistan | 7/9 | serum | - | NA | NA | SG | 10 | 34 | NA |
| CG | 7 | 26 | NA | ||||||||
| Nunziata [26] | 1991 | Italy | 7/9 | serum | Competitive binding assay | - | NA | SG | 101 | NA | NA |
| CG | 55 | NA | NA | ||||||||
| Wong [27] | 1992 | Australia | 8/9 | serum | Microassay | - | Calcium | SG | 59 | 46.59 ± 13.92 | 51/8 |
| CG | 31 | 43.52 ± 13.55 | 20/11 | ||||||||
| Giannini [28] | 1993 | Italy | 8/9 | serum | Competitive protein binding assay | - | Calcium | HSG | 47 | 40.5 ± 2.8 | NA |
| NSG | 28 | 48.8 ± 2.6 | NA | ||||||||
| Hess [29] | 1995 | Switzerland | 8/9 | serum | Radioimmunoassay | Radioimmunoassay | Calcium | SG | 57 | NA | NA |
| CG | 15 | NA | NA | ||||||||
| Jarrar [30] | 1996 | Germany | 9/9 | serum | Radioreceptor assay | - | Calcium | SG | 111 | 54.92 ± 23.36 | 64/47 |
| CG | 44 | 53.34 ± 18.66 | 22/22 | ||||||||
| Scott [31] | 1998 | Canada | 7/9 | serum | Radioimmunoassay | - | Mixed | SG | 68 | NA | 45/23 |
| CG | 69 | NA | 26/43 | ||||||||
| Vezzoli [32] | 1999 | Italy | 7/9 | plasm | Radioreceptor assay | - | Calcium oxalate | HSG | 37 | NA | NA |
| NSG | 27 | NA | NA | ||||||||
| Yamakawa [33] | 2000 | Japan | 9/9 | serum | Radioreceptor assay | - | Calcium | SG | 63 | 55.7 ± 12.5 | 47/16 |
| CG | 26 | 55.9 ± 15.9 | 21/5 | ||||||||
| Prie [34] | 2001 | France | 7/9 | serum | Radioimmunoassay | - | Calcium | HSG | 207 | NA | NA |
| NSG | 28 | NA | NA | ||||||||
| Misael da Silva [35] | 2002 | Brazil | 9/9 | serum | Radioisotopic assay | - | NA | SG | 40 | 34.77 ± 11.73 | 19/21 |
| CG | 10 | 32.4 ± 8.4 | 5/5 | ||||||||
| Asplin [36] | 2003 | USA | 7/9 | serum | Radioreceptor assay | - | Calcium | SG | 22 | NA | 15/7 |
| CG | 37 | NA | 14/23 | ||||||||
| Ozkaya [37] | 2003 | Turkey | 8/9 | serum | Radioimmunoassay | - | Calcium | SG | 64 | 6.7 ± 3.5 | 26/38 |
| CG | 90 | 7.2 ± 2.3 | 47/43 | ||||||||
| Moyano [38] | 2007 | Spain | 9/9 | serum | Radioimmunoassay | - | NA | SG | 24 | 45.5 ± 13.5 | 22/29 |
| CG | 27 | 48.6 ± 15.4 | 9/12 | ||||||||
| Shakhssalim [39] | 2011 | Iran | 9/9 | serum | Enzyme Immunoassay | - | Calcium | SG | 106 | 43.4 ± 6.9 | 106/0 |
| CG | 109 | 38.4 ± 6.9 | 109/0 | ||||||||
| Fallahzadeh [40] | 2012 | Iran | 9/9 | serum | - | Electrochemiluminescence | NA | SG | 36 | 0.7 ± 0.39 | 24/12 |
| CG | 36 | 0.7 ± 0.39 | 22/14 | ||||||||
| Tang [41] | 2012 | USA | 6/9 | serum | - | Radioimmunoassay | NA | SG | 757 | 54 ± 22.29 | 453/304 |
| CG | 15529 | 43 ± 23.68 | 7175/8354 | ||||||||
| Yilmaz [42] | 2013 | Turkey | 8/9 | serum | Enzyme linked immunosorbent assay | - | NA | SG | 25 | 8.08 ± 5.18 | 13/12 |
| CG | 23 | 10.2 ± 3.64 | 11/12 | ||||||||
| Kim [43] | 2014 | Korea | 9/9 | serum | Radioimmunoassay | - | Calcium | SG | 326 | 45.8 ± 12.3 | 204/122 |
| CG | 163 | NA | NA | ||||||||
| Nguyen [44] | 2014 | USA | 7/9 | serum | - | Liquid chromatography and mass spectrometry | NA | SG | 13 | 60 ± 10 | 8/5 |
| CG | 1999 | 53 ± 14 | 767/1232 | ||||||||
| Ketha [45] | 2015 | USA | 9/9 | serum | Mass spectrometry | Mass spectrometry | Calcium | SG | 149 | NA | NA |
| CG | 201 | NA | NA | ||||||||
| Taylor [46] | 2015 | USA | 9/9 | plasma | Liquid chromatography–tandem mass spectrometry | Liquid chromatography–tandem mass spectrometry | Calcium | SG | 356 | 57.4 ± 8.1 | 356/0 |
| CG | 712 | 57.4 ± 8.1 | 712/0 | ||||||||
| Sierra [47] | 2016 | Spain | 8/9 | Serum | - | NA | Calcium | SG | 239 | 49.61 ± 13.64 | NA |
| CG | 127 | 52.09 ± 11.02 | NA |
| Items | Comparisons | Sample Size | Tests for Heterogeneity | Analysis Model | Test for Overall Effect | WWD pg/mL or ng/mL | Higher in | ||
|---|---|---|---|---|---|---|---|---|---|
| I2 | p * | Z | p * | 95% CI | |||||
| 1,25(OH)2D | SG vs. CG | 1601/1676 | 97% | <0.0001 | Random | 2.22 | 0.03 | 7.92 (0.93,14.91) | SG |
| CSG vs. CG | 1352/1413 | 98% | <0.0001 | Random | 2.26 | 0.02 | 9.94 (1.34,18.56) | CSG | |
| HSG vs. CG | 305/630 | 95% | <0.0001 | Random | 2.39 | 0.02 | 13.21 (2.38,24.04) | HSG | |
| NSG vs. CG | 541/630 | 98% | <0.0001 | Random | 1.55 | 0.12 | 9.67 (−2.55,21.89) | NSG | |
| HSG vs. NSG | 420/609 | 65% | 0.002 | Random | 1.88 | 0.06 | 3.39 (−0.13,6.91) | HSG | |
| 25(OH)D | SG vs. CG | 997/1308 | 98% | <0.0001 | Random | 1.48 | 0.14 | 2.02 (−0.66,4.69) | SG |
| CSG vs. CG | 801/1055 | 75% | 0.007 | Random | 0.31 | 0.75 | 0.33 (−1.76,2.43) | CSG | |
| HSG vs. CG | 105/232 | 0% | 0.78 | Fixed | 3.09 | 0.002 | 3.02 (1.10,4.93) | HSG | |
| NSG vs. CG | 112/232 | 85% | 0.01 | Random | 0.07 | 0.94 | −0.21 (−5.70,5.29) | CG | |
| HSG vs. NSG | 190/172 | 77% | 0.005 | Random | 2.53 | 0.01 | 4.48 (1.01,7.95) | HSG | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Zhang, J.; Lu, Y.; Zhang, Z.; Qin, B.; Gao, H.; Wang, Y.; Zhu, J.; Wang, Q.; Zhu, Y.; et al. Association between Circulating Vitamin D Level and Urolithiasis: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 301. https://doi.org/10.3390/nu9030301
Hu H, Zhang J, Lu Y, Zhang Z, Qin B, Gao H, Wang Y, Zhu J, Wang Q, Zhu Y, et al. Association between Circulating Vitamin D Level and Urolithiasis: A Systematic Review and Meta-Analysis. Nutrients. 2017; 9(3):301. https://doi.org/10.3390/nu9030301
Chicago/Turabian StyleHu, Henglong, Jiaqiao Zhang, Yuchao Lu, Zongbiao Zhang, Baolong Qin, Hongbin Gao, Yufeng Wang, Jianning Zhu, Qing Wang, Yunpeng Zhu, and et al. 2017. "Association between Circulating Vitamin D Level and Urolithiasis: A Systematic Review and Meta-Analysis" Nutrients 9, no. 3: 301. https://doi.org/10.3390/nu9030301





