Lactational Stage of Pasteurized Human Donor Milk Contributes to Nutrient Limitations for Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design/Participants
2.2. Nutrient Analyses
2.3. Statistical Analysis
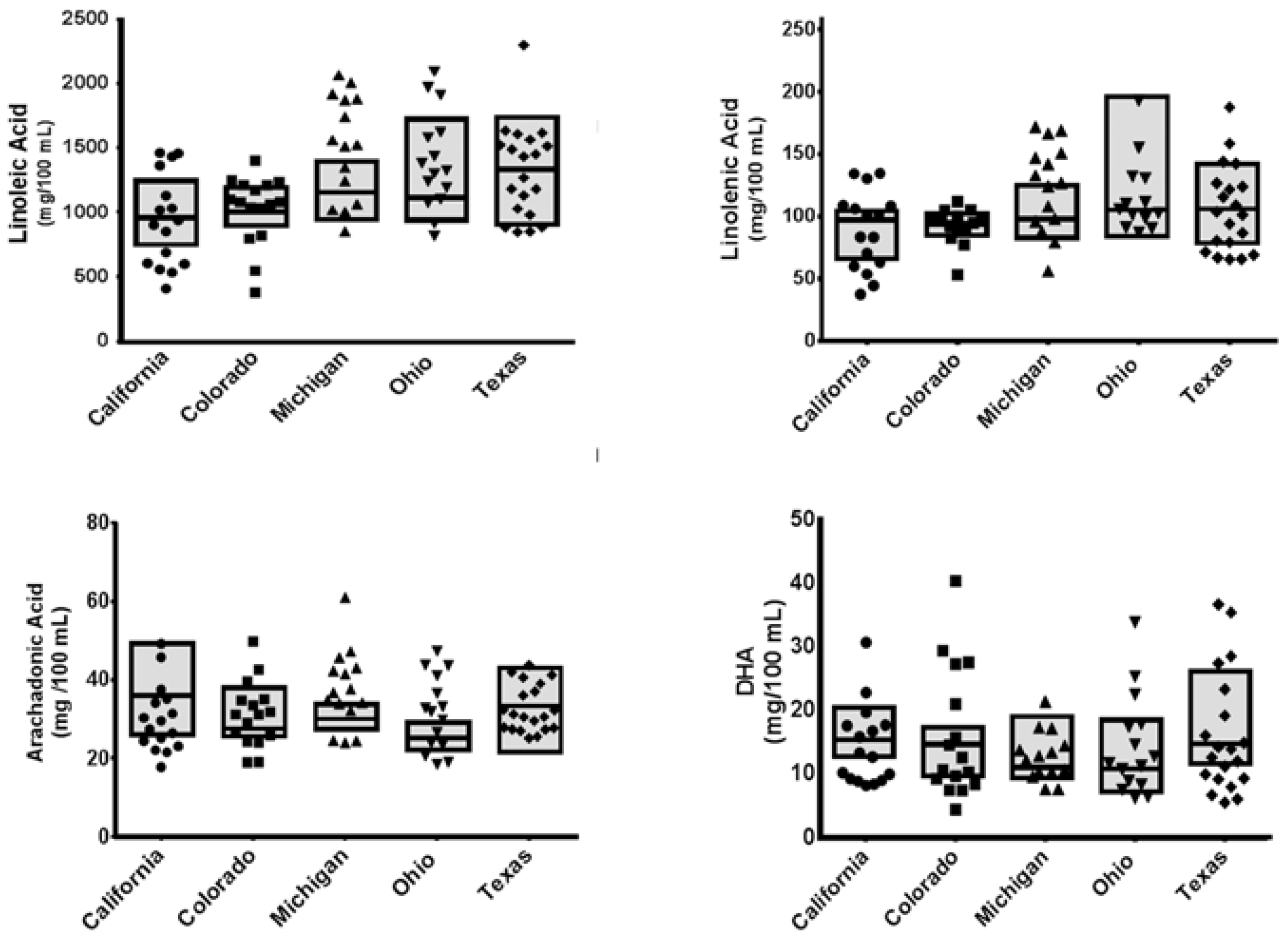
3. Results
3.1. Participants
3.2. Nutrient Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I.; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar]
- World Health Organization; United Nations Children’s Fund. Protecting, Promoting and Supporting Breast-Feeding. The Special Role of Maternity Services; World Health Organization and United Nations Children’s Fund: Geneva, Switzerland, 1989; p. 32. [Google Scholar]
- Schanler, R.J.; Lau, C.; Hurst, N.M.; Smith, E.O. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics 2005, 116, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Ahrabi, A.F.; Schanler, R.J. Human milk is the only milk for premies in the nicu! Early Hum. Dev. 2013, 89 (Suppl. 2), S51–S53. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Schanler, R.J.; Kim, J.H.; Patel, A.L.; Trawoger, R.; Kiechl-Kohlendorfer, U.; Chan, G.M.; Blanco, C.L.; Abrams, S.; Cotten, C.M.; et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J. Pediatr. 2010, 156, 562–567, e561. [Google Scholar] [CrossRef] [PubMed]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J. Pediatr. 2013, 163, 1592–1595.e1. [Google Scholar] [CrossRef] [PubMed]
- Younge, N.; Yang, Q.; Seed, P.C. Enteral high fat-polyunsaturated fatty acid blend alters the pathogen composition of the intestinal microbiome in premature infants with an enterostomy. J. Pediatr. 2016, 181, 93–101.e6. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Patel, A.L.; Bigger, H.R.; Engstrom, J.L.; Meier, P.P. Economic benefits and costs of human milk feedings: A strategy to reduce the risk of prematurity-related morbidities in very-low-birth-weight infants. Adv. Nutr. 2014, 5, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Brent, N. The risks and benefits of human donor breast milk. Pediatr. Ann. 2013, 42, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Ziegler, E.E. Preterm infants fed fortified human milk receive less protein than they need. J. Perinatol. 2009, 29, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Corpeleijn, W.; Moro, G.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; et al.; Espghan Committee on Nutrition Donor human milk for preterm infants: Current evidence and research directions. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Committee on Nutrition; Section on Breastfeeding; Committee on Fetus and Newborn. Donor human milk for the high-risk infant: Preparation, safety, and usage options in the united states. Pediatrics 2017, 139, e20163440. [Google Scholar]
- De Halleux, V.; Pieltain, C.; Senterre, T.; Rigo, J. Use of donor milk in the neonatal intensive care unit. Semin. Fetal Neonatal Med. 2017, 22, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Valentine, C.J.; Dumm, M. Pasteurized donor human milk use in the neonatal intensive care unit. NeoReviews 2015, 16, e152–e159. [Google Scholar] [CrossRef]
- Assad, M.; Elliott, M.J.; Abraham, J.H. Decreased cost and improved feeding tolerance in vlbw infants fed an exclusive human milk diet. J. Perinatol. 2016, 36, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Patel, A.; Esquerra-Zwiers, A. Donor human milk update: Evidence, mechanisms, and priorities for research and practice. J. Pediatr. 2016, 180, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Valentine, C.J.; Wagner, C.L. Nutritional management of the breastfeeding dyad. Pediatr. Clin. N. Am. 2013, 60, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Colaizy, T.T.; Carlson, S.; Saftlas, A.F.; Morriss, F.H., Jr. Growth in vlbw infants fed predominantly fortified maternal and donor human milk diets: A retrospective cohort study. BMC Pediatr. 2012, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Schanler, R.J. Post-discharge nutrition for the preterm infant. Acta Paediatr. Suppl. 2005, 94, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Schanler, R.J.; Shulman, R.J.; Lau, C.; Smith, E.O.; Heitkemper, M.M. Feeding strategies for premature infants: Randomized trial of gastrointestinal priming and tube-feeding method. Pediatrics 1999, 103, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Das, A.; Wrage, L.A.; Poindexter, B.B.; Higgins, R.D.; Stoll, B.J.; Oh, W.; The Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Early nutrition mediates the influence of severity of illness on extremely LBW infants. Pediatr. Res. 2011, 69, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Demerath, E.W.; Gray, H.L.; Younge, N.; Boys, C.; Georgieff, M.K. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology 2012, 102, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Georgieff, M.K. Preterm nutrition and the brain. World Rev. Nutr. Diet. 2014, 110, 190–200. [Google Scholar] [PubMed]
- O’Connor, D.L.; Gibbins, S.; Kiss, A.; Bando, N.; Brennan-Donnan, J.; Ng, E.; Campbell, D.M.; Vaz, S.; Fusch, C.; Asztalos, E.; et al. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: A randomized clinical trial. JAMA 2016, 316, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G. What is the optimal age for introduction of complementary foods? Nestle Nutr. Workshop Ser. Pediatr. Progr. 2006, 58, 161–170. [Google Scholar]
- Schanler, R.J.; Oh, W. Composition of breast milk obtained from mothers of premature infants as compared to breast milk obtained from donors. J. Pediatr. 1980, 96, 679–681. [Google Scholar] [CrossRef]
- Schanler, R.J. Mother’s own milk, donor human milk, and preterm formulas in the feeding of extremely premature infants. J. Pediatr. Gastroenterol. Nutr. 2007, 45 (Suppl. 3), S175–S177. [Google Scholar] [CrossRef] [PubMed]
- Valentine, C.J.; Morrow, G.; Fernandez, S.; Gulati, P.; Bartholomew, D.; Long, D.; Welty, S.E.; Morrow, A.L.; Rogers, L.K. Docosahexaenoic acid and amino acid contents in pasteurized donor milk are low for preterm infants. J. Pediatr. 2010, 157, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Kuschel, C.A.; Harding, J.E. Protein supplementation of human milk for promoting growth in preterm infants. Cochrane Libr. 2000. [Google Scholar] [CrossRef]
- Kuschel, C.A.; Harding, J.E. Multicomponent fortified human milk for promoting growth in preterm infants. Cochrane Libr. 2004. [Google Scholar] [CrossRef]
- Kuschel, C.A.; Harding, J.E. Fat supplementation of human milk for promoting growth in preterm infants. Cochrane Libr. 2000. [Google Scholar] [CrossRef]
- Kuschel, C.A.; Harding, J.E. Carbohydrate supplementation of human milk to promote growth in preterm infants. Cochrane Libr. 1999. [Google Scholar] [CrossRef]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.Y. Factors affecting human milk composition. Pediatr. Neonatol. 2014, 55, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Tudehope, D.I. Human milk and the nutritional needs of preterm infants. J. Pediatr. 2013, 162, S17–S25. [Google Scholar] [CrossRef] [PubMed]
- Human Milk Banking Association of North America. Guidelines for the Establishment and Operation of a Donor Human Milk Bank; Human Milk Banking Association of North America, Inc.: Raliegh, NC, USA, 2009. [Google Scholar]
- Human Milk Banking Association of North America. Best practice for expressing, storing and handling human milk in hospitals, homes, and child care settings, 3rd ed.; Human Milk Banking Association of North America, Inc.: Fort Worth, Texas, USA, 2011. [Google Scholar]
- Koletzko, B.; Agostoni, C.; Carlson, S.E.; Clandinin, T.; Hornstra, G.; Neuringer, M.; Uauy, R.; Yamashiro, Y.; Willatts, P. Long chain polyunsaturated fatty acids (LC-PUFA) and perinatal development. Acta Paediatr. 2001, 90, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Hay, W.W., Jr.; Brown, L.D.; Denne, S.C. Energy requirements, protein-energy metabolism and balance, and carbohydrates in preterm infants. World Rev. Nutr. Diet. 2014, 110, 64–81. [Google Scholar] [PubMed]
- Valentine, C.J.; Morrow, A.L. Chapter 13: Human Milk Feeding of the High Risk NeonateGastroenterology and Nutrition, 2nd ed.; Elsevier: Philidephia, PA, USA, 2012. [Google Scholar]
- Fairey, A.K.; Butte, N.F.; Mehta, N.; Thotathuchery, M.; Schanler, R.J.; Heird, W.C. Nutrient accretion in preterm infants fed formula with different protein:Energy ratios. J. Pediatr. Gastroenterol. Nutr. 1997, 25, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Makrides, M.; Smithers, L.G.; Gibson, R.A. Role of long-chain polyunsaturated fatty acids in neurodevelopment and growth. Nestle Nutr. Workshop Ser. Pediatr. Progr. 2010, 65, 123–133. [Google Scholar]
- McNamara, R.K.; Carlson, S.E. Role of omega-3 fatty acids in brain development and function: Potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fat. Acids 2006, 75, 329–349. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Dasilva, D.A.; Cluette-Brown, J.E.; Dimonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 2011, 159, 743–749, e741–e742. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Essential fatty acid transfer and fetal development. Placenta 2005, 26 (Suppl. A), S70–S75. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [PubMed]
- Valentine, C.J.; Morrow, G.; Pennell, M.; Morrow, A.L.; Hodge, A.; Haban-Bartz, A.; Collins, K.; Rogers, L.K. Randomized controlled trial of docosahexaenoic acid supplementation in midwestern U.S. Human milk donors. Breastfeed. Med. 2013, 8, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.P.; Courtney-Martin, G.; Moore, A.M.; Langer, J.C.; Tomlinson, C.; Ball, R.O.; Pencharz, P.B. Lysine requirement in parenterally fed postsurgical human neonates. Am. J. Clin. Nutr. 2010, 91, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hogewind-Schoonenboom, J.E.; de Groof, F.; Twisk, J.W.; Voortman, G.J.; Dorst, K.; Schierbeek, H.; Boehm, G.; Huang, Y.; Chen, C.; et al. Lysine requirement of the enterally fed term infant in the first month of life. Am. J. Clin. Nutr. 2011, 94, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Radmacher, P.G.; Adamkin, D.H. Fortification of human milk for preterm infants. Semin. Fetal Neonatal Med. 2016, 22, 30–35. [Google Scholar] [CrossRef] [PubMed]

| Region | n | Age | Lactational Stage (months) * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Range | Mean | Std. Dev. | Median | Range | Mean | Std. Dev. | ||
| California | 16 | 33.5 | 24–41 | 32.9 | 5.3 | 4.0 | 1–13 | 4.8 | 3.4 |
| Colorado | 16 | 31.5 | 25–38 | 31.7 | 3.6 | 1.0 # | 1–8 | 2.2 | 1.9 |
| Michigan | 15 | 32.0 | 26–41 | 33.5 | 4.9 | 3.0 | 1–12 | 4.4 | 3.8 |
| Ohio | 15 | 30.0 | 20–42 | 31.7 | 5.9 | 2.0 # | 0–11 | 2.7 | 2.8 |
| Texas-Ft Worth | 16 | 32.0 | 25–37 | 31.2 | 4.4 | 5.5 | 2–11 | 6.1 | 2.7 |
| mg/100 mL | California | Colorado | Michigan | Ohio | Texas | r2 (Adjusted) | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean ± SD | med. | mean ± SD | med. | mean ± SD | med. | mean ± SD | med. | mean ± SD | med. | |||
| C10:0 | 19.0 ± 8.5 | 17.6 | 26.1 ± 10.8 | 23.7 | 22.2 ± 7.3 | 21.1 | 15.3 ± 5.0 | 12.2 | 28.9 ± 13.6 | 31.6 | 0.055 | 0.314 |
| C12:0 | 118 ± 31.0 | 117 | 130 ± 60.0 | 108 | 134 ± 28.5 | 125 | 123 ± 35.1 | 116 | 151 ± 45.8 | 134 | 0.151 | 0.864 |
| C14:0 | 144 ± 32.5 | 142 | 156 ± 63.8 | 135 | 182 ± 33.6 | 184 | 162 ± 43.6 | 147 | 172 ± 37.8 | 153 | 0.140 | 0..835 |
| C16:0 | 553 ± 86.8 | 580 | 614 ± 159 | 577 | 666 ± 1125 | 651 | 570 ± 79.6 | 569 | 677 ± 179 | 601 | 0.005 | 0.456 |
| C16:1ω7 | 68.4 ± 14.4 | 71.3 | 68.9 ± 20.3 | 59.3 | 76.1 ± 13.8 | 75.1 | 54.1 ± 8.21 | 58.3 | 74.0 ± 26.0 | 68.1 | 0.047 | 0.331 |
| C18:0 | 184 ± 34.1 | 191 | 193 ± 36.8 | 178 | 214 ± 55.4 | 195 | 193 ± 32.0 | 197 | 239 ± 67.7 | 202 | 0.051 | 0.322 |
| C18:1ω9 | 935 ± 189 | 983 | 923 ± 157 | 880 | 1019 ± 173 | 1004 | 1008 ± 216 | 975 | 1134 ± 275 | 1033 | 0.019 | 0.396 |
| C18:1ω7 | 69.5 ± 15.8 | 63.3 | 81.9 ± 18.0 | 72.8 | 85.6 ± 20.9 | 75.4 | 74.1 ± 16.9 | 79.2 | 89.7 ± 35.7 | 81.3 | 0.050 | 0.581 |
| C18:2ω6 | 494 ± 90 | 483 | 510 ± 57 | 506 | 593 ± 82 | 584 | 661 ± 171 | 626 | 603 ± 156 | 562 | 0.127 | 0.184 |
| C18:3ω6 | 6.64 ± 0.68 | 6.49 | 6.63 ± 0.62 | 6.58 | 7.16 ± 0.82 | 7.13 | 4.42 ± 1.04 | 4.55 | 6.49 ± 2.45 | 5.73 | 0.241 | 0.065 |
| C18:3ω3 | 44.2 ± 7.47 | 46.8 | 44.9 ± 3.26 | 45.3 | 48.5 ± 7.46 | 47.3 | 52.1 ± 12.8 | 51.7 | 57.8 ± 21.4 | 51.1 | 0.044 | 0.338 |
| C20:4ω6 | 18.1 ± 4.97 | 18.1 | 14.9 ± 2.50 | 13.9 | 15.4 ± 1.41 | 15.1 | 16.3 ± 4.23 | 15.8 | 12.7 ± 1.56 | 12.7 | 0.074 | 0.274 |
| C20:5ω3 | 3.74 ± 0.47 | 3.55 | 3.80 ± 0.53 | 3.53 | 3.15 ± 0.82 | 2.94 | 2.20 ± 0.44 | 2.35 | 3.56 ± 2.51 | 2.36 | 0.016 | 0.403 |
| C22:6ω3 | 7.81 ± 1.62 | 7.82 | 7.29 ± 1.58 | 7.46 | 6.17 ± 2.00 | 5.62 | 8.20 ± 3.02 | 6.89 | 6.09 ± 2.63 | 5.48 | 0.066 | 0.627 |
| mg/100 mL | California | Colorado | Michigan | Ohio | Texas | r2 (Adjusted) | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean ± SD | med. | mean ± SD | med. | mean ± SD | med. | mean ± SD | med. | mean ± SD | med. | |||
| Phosphoserine | 97.8 ± 41.4 | 80.4 | 85.5 ± 28.0 | 84.4 | 80.2 ± 13.4 | 73.3 | 82.2 ± 23.1 | 81.1 | 83.1 ± 17.2 | 74.9 | 0.050 | 0.333 |
| Taurine | 8.44 ± 3.63 | 8.17 | 7.00 ± 4.72 | 8.06 | 4.89 ± 3.42 | 2.59 | 5.5 ± 2.6 | 9.00 | 8.3 ± 4.9 | 6.57 | 0.051 | 0.332 |
| Aspartic Acid | 102 ± 24.4 | 94.4 | 88.2 ± 26.1 | 82.6 | 83.6 ± 13.0 | 79.9 | 83.4 ± 8.8 | 74.6 | 87.5 ± 22.8 | 85.9 | 0.097 | 0.240 |
| Threonine | 44.1 ± 13.0 | 39.8 | 33.4 ± 10.0 | 29.3 | 35.9 ± 3.25 | 34.8 | 35.0 ± 6.58 | 34.0 | 34.4 ± 7.96 | 32.5 | 0.130 | 0.185 |
| Serine | 56.9 ± 14.4 | 52.7 | 46.2 ± 14.1 | 44.4 | 44.8 ± 8.21 | 41.1 | 44.8 ± 5.02 | 38.4 | 47.3 ± 14.4 | 43.5 | 0.105 | 0.227 |
| Glutamic Acid | 206 ± 43.4 | 185 | 173.8 ± 37.0 | 162 | 171 ± 14.2 | 164 | 179 ± 23.46 | 151 | 164 ± 23.7 | 175 | 0.152 | 0.156 |
| Proline | 101 ± 25.8 | 83.5 | 78.9 ± 25.2 | 66.2 | 81.4 ± 5.71 | 79.7 | 79.3 ± 20.1 | 67.1 | 66.6 ± 6.81 | 70.3 | 0.205 | 0.096 |
| Glycine | 25.2 ± 5.9 | 23.0 | 22.3 ± 5.85 | 20.5 | 21.3 ± 2.26 | 21.0 | 20.5 ± 3.5 | 20.0 | 23.4 ± 7.17 | 21.0 | 0.027 | 0.385 |
| Alanine | 47.1 ± 11.4 | 44.7 | 39.5 ± 13.1 | 37.9 | 38.1 ± 7.51 | 35.0 | 37.4 ± 3.87 | 31.6 | 40.3 ± 13.5 | 36.4 | 0.066 | 0.299 |
| Valine | 41.1 ± 12.6 | 36.9 | 30.9 ± 9.45 | 30.7 | 32.2 ± 4.32 | 33.0 | 27.2 ± 9.96 | 23.4 | 25.9 ± 7.56 | 28.1 | 0.140 | 0.172 |
| Methionine | 19.7 ± 5.8 | 17.6 | 15.1 ± 6.48 | 14.6 | 14.2 ± 3.86 | 11.9 | 14.4 ± 1.38 | 11.5 | 13.8 ± 4.59 | 14.3 | 0.212 | 0.090 |
| Isoleucine | 37.4 ± 12.7 | 32.9 | 27.0 ± 8.69 | 25.1 | 28.8 ± 3.08 | 27.7 | 28.4 ± 6.71 | 23.6 | 23.8 ± 3.35 | 25.5 | 0.199 | 0.102 |
| Leucine | 99.1 ± 24.0 | 88.1 | 76.8 ± 21.2 | 68.6 | 79.4 ± 7.85 | 77.5 | 76.5 ± 16.8 | 67.8 | 72.6 ± 10.8 | 71.2 | 0.221 | 0.082 |
| Tyrosine | 55.6 ± 20.9 | 52.1 | 44.1 ± 19.5 | 44.6 | 40.4 ± 11.6 | 33.1 | 44.6 ± 6.6 | 32.2 | 42.1 ± 17.0 | 45.1 | 0.006 | 0.469 |
| Phenylalanine | 38.1 ± 11.4 | 34.4 | 29.7 ± 8.90 | 26.5 | 30.0 ± 3.65 | 29.3 | 29.8 ± 5.10 | 25.1 | 30.4 ± 9.03 | 29.8 | 0.098 | 0.238 |
| Tryptophan | 188.7 ± 39.6 | 176 | 172 ± 28.6 | 169 | 173 ± 9.39 | 173 | 173 ± 29.8 | 161 | 157 ± 16.4 | 158 | 0.064 | 0.624 |
| Lysine | 66.3 ± 23.2 | 62.9 | 54.8 ± 16.7 | 50.1 | 54.0 ± 7.41 | 51.1 | 54.5 ± 8.8 | 45.3 | 52.1 ± 11.4 | 53.2 | 0.022 | 0.399 |
| Histidine | 22.4 ± 6.3 | 18.7 | 18.1 ± 4.78 | 16.4 | 53.2 ± 78.2 | 18.6 | 19.0 ± 4.14 | 16.1 | 17.5 ± 3.31 | 17.5 | 0.042 | 0.564 |
| Arginine | 38.7 ± 9.8 | 36.0 | 31.7 ± 10.3 | 29.2 | 31.3 ± 7.6 | 27.6 | 33.8 ± 3.85 | 29.3 | 37.3 ± 15.5 | 34.1 | 0.038 | 0.360 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentine, C.J.; Morrow, G.; Reisinger, A.; Dingess, K.A.; Morrow, A.L.; Rogers, L.K. Lactational Stage of Pasteurized Human Donor Milk Contributes to Nutrient Limitations for Infants. Nutrients 2017, 9, 302. https://doi.org/10.3390/nu9030302
Valentine CJ, Morrow G, Reisinger A, Dingess KA, Morrow AL, Rogers LK. Lactational Stage of Pasteurized Human Donor Milk Contributes to Nutrient Limitations for Infants. Nutrients. 2017; 9(3):302. https://doi.org/10.3390/nu9030302
Chicago/Turabian StyleValentine, Christina J., Georgia Morrow, Amanda Reisinger, Kelly A. Dingess, Ardythe L. Morrow, and Lynette K. Rogers. 2017. "Lactational Stage of Pasteurized Human Donor Milk Contributes to Nutrient Limitations for Infants" Nutrients 9, no. 3: 302. https://doi.org/10.3390/nu9030302




