A Specific Mixture of Nutrients Suppresses Ovarian Cancer A-2780 Tumor Incidence, Growth, and Metastasis to Lungs
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vivo Studies
2.1.1. Animals
2.1.2. Experimental Design
2.1.3. Histology
2.2. In Vitro Studies
2.2.1. Cell Culture
2.2.2. MTT Assay
2.2.3. Gelatinase Zymography
2.2.4. Matrigel Invasion
2.2.5. Morphology: H & E
2.2.6. Statistical Analysis
3. Results
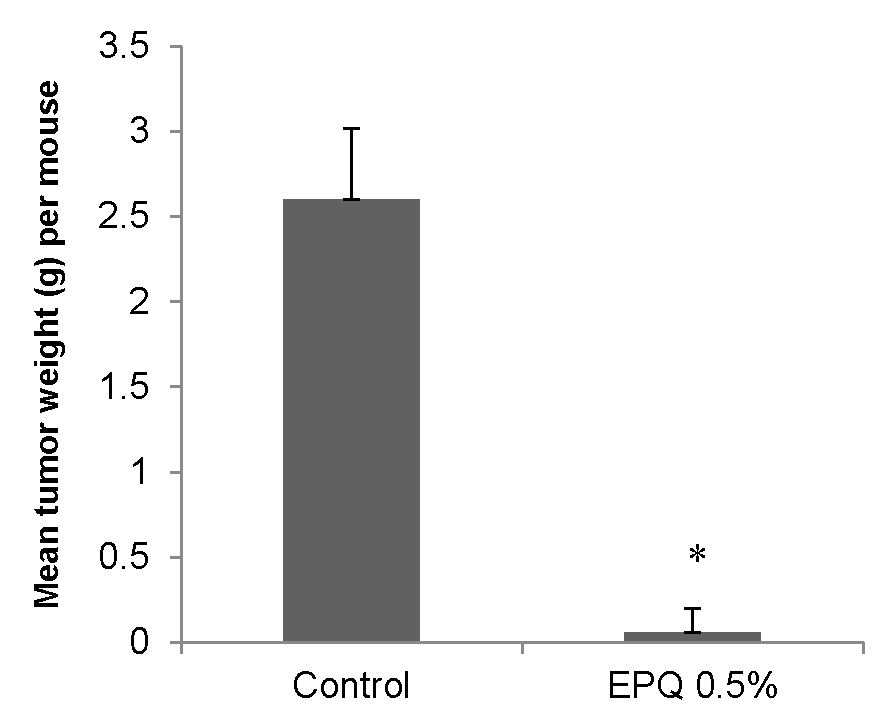
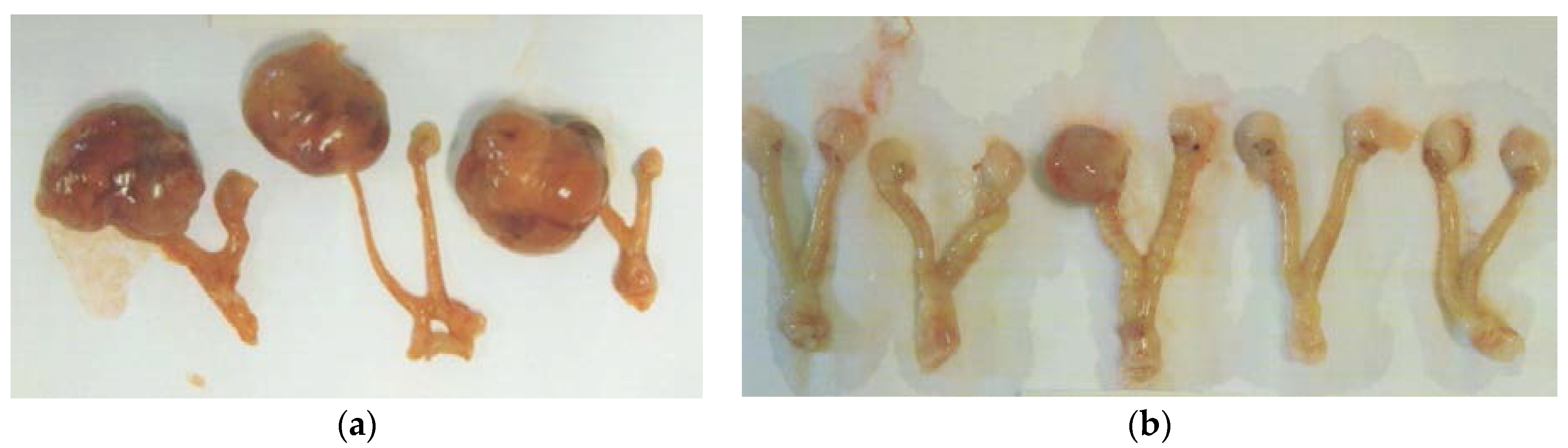
3.1. In Vivo
3.1.1. Tumor Incidence and Growth
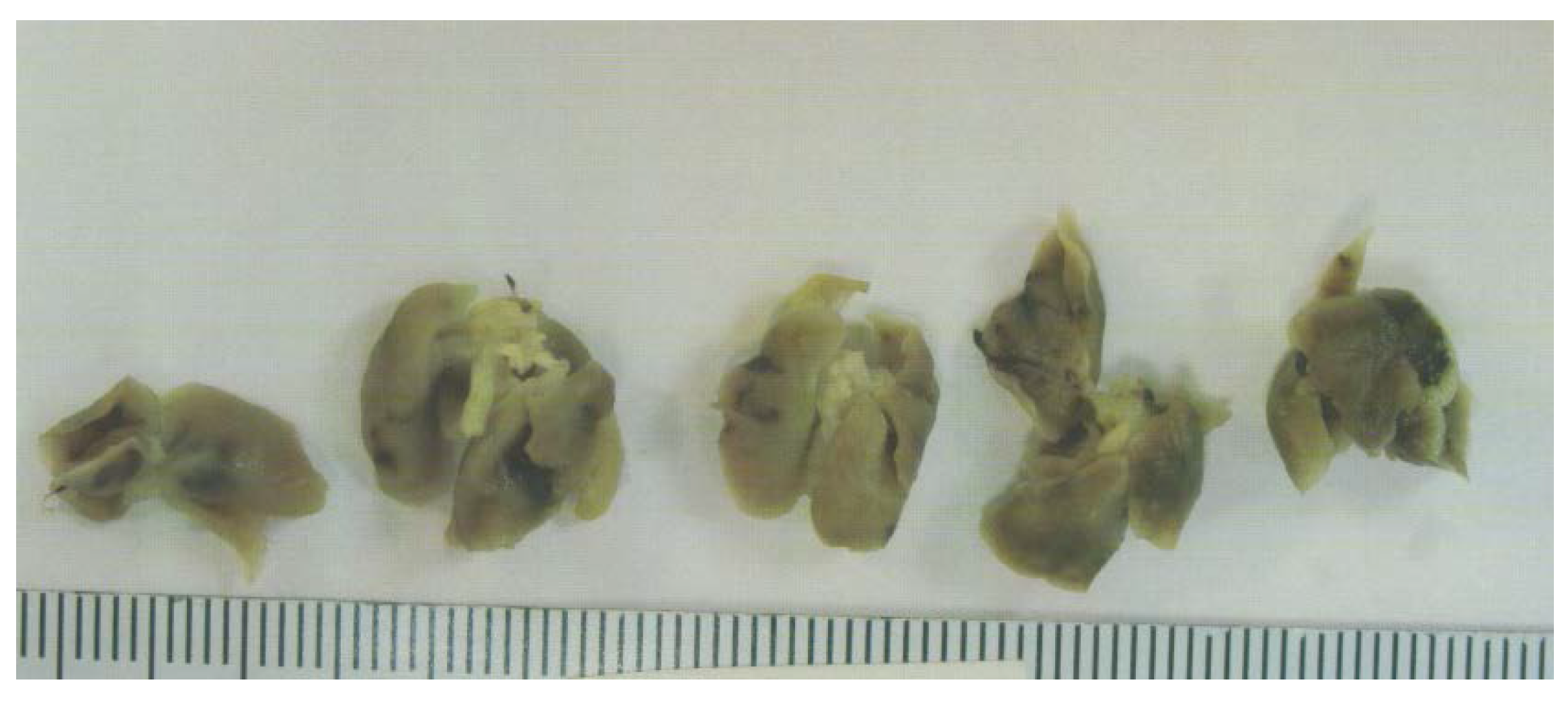
3.1.2. Tumor Metastasis to Lungs
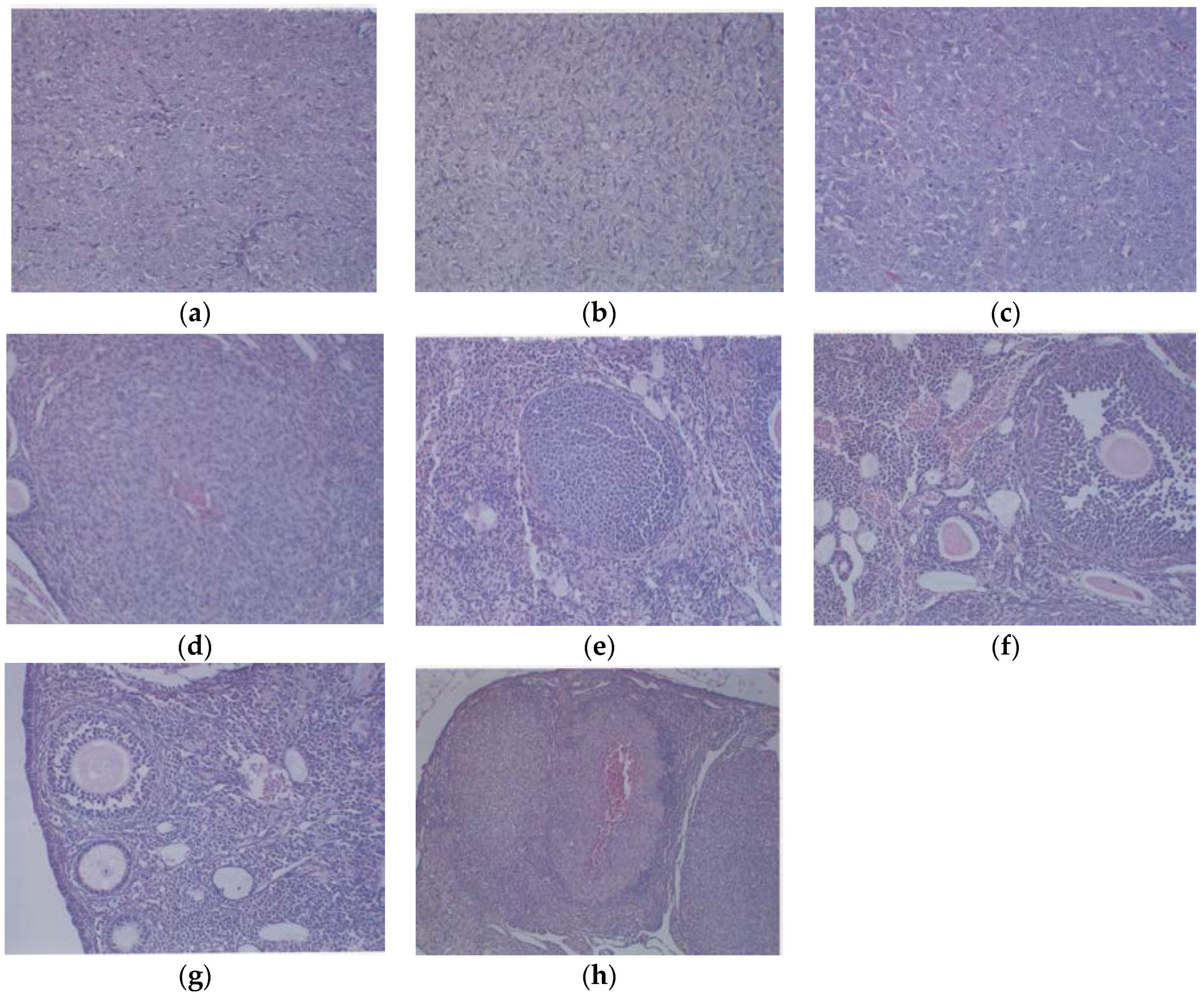
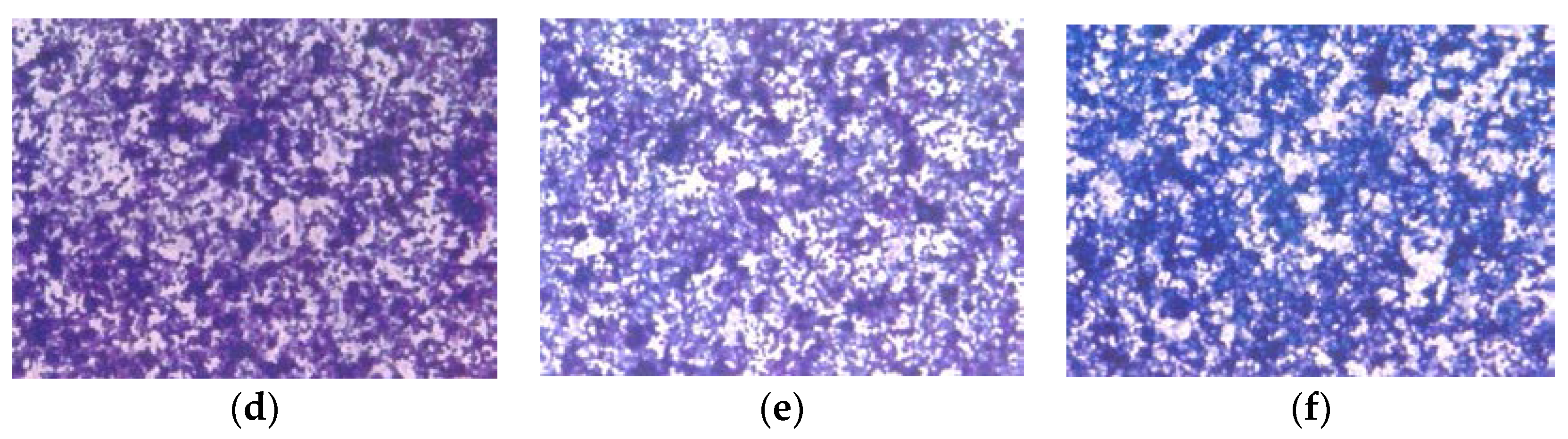
3.1.3. Histology
3.2. In Vitro
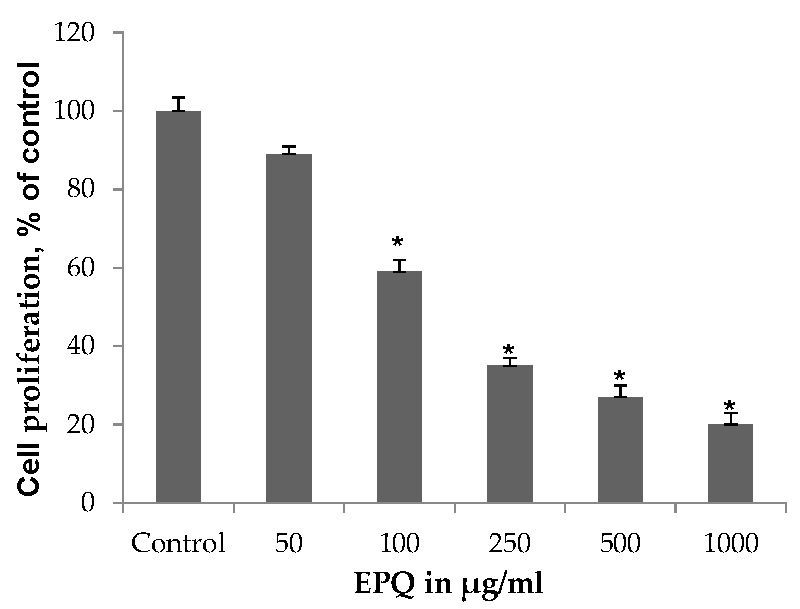
3.2.1. Cell Proliferation
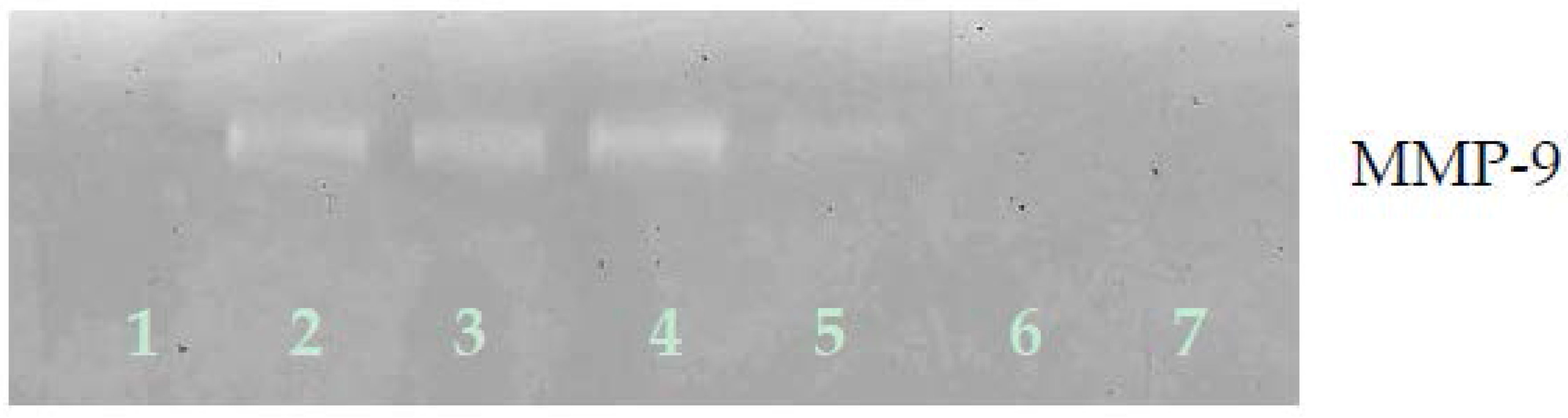
3.2.2. Gelatinase Zymography
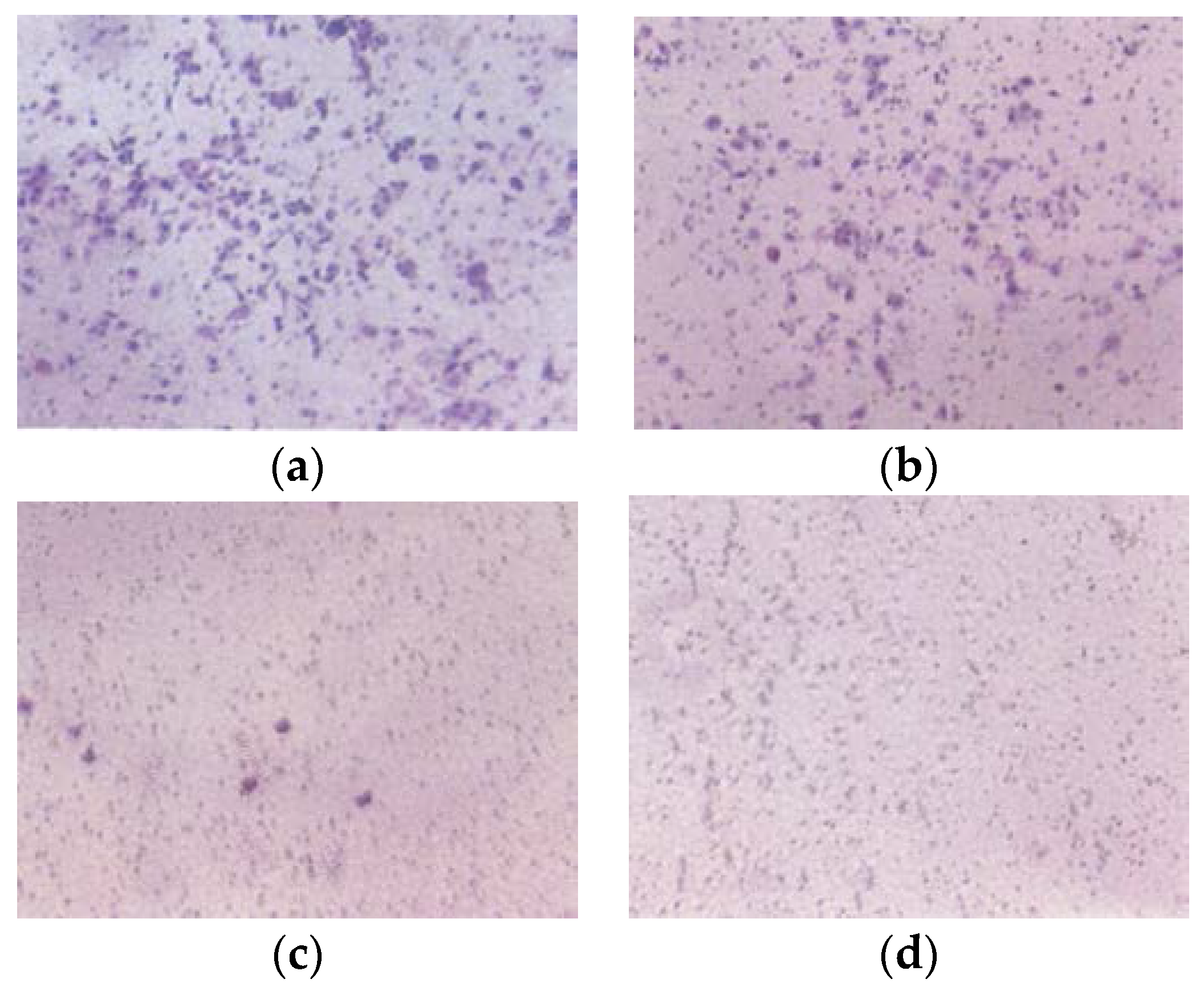
3.2.3. Matrigel Invasion
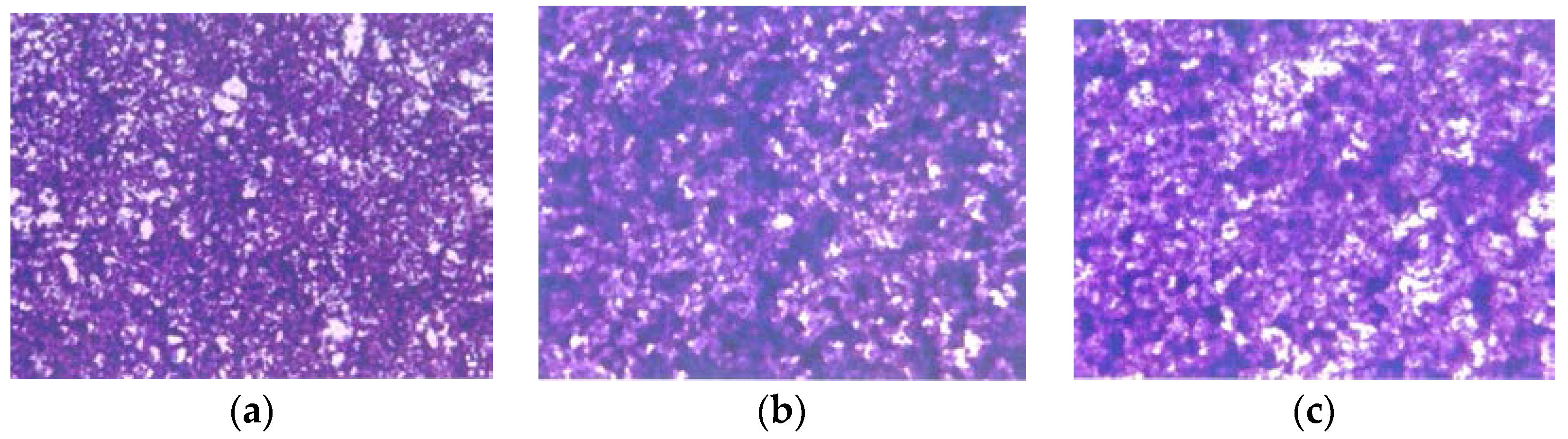
3.2.4. Morphology: H & E Staining
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Cancer Society: What Are the Key Statistics about Ovarian Cancer? Available online: http://www.cancer.org/cancer/ovariancancer/detailedguide/ovarian-cancer-key-statistics (accessed on 28 April 2016).
- Ovarian Cancer National Alliance: Ovarian Cancer: Statistics. Available online: http://www.ovariancancer.org/about/statistics/ (accessed on 28 April 2016).
- Duffy, M.J. The role of proteolytic enzymes in cancer invasion and metastasis. Clin. Exp. Metastasis. 1992, 10, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Goldberg, I.; Gotlieb, W.H.; Kopoiovic, J.; Ben-Baruch, G.; Nesland, J.M.; Berner, A.; Byrne, M.; Reich, R. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin. Exp. Metastasis 1999, 17, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Goldberg, I.; Gotlieb, W.H.; Kopoiovic, J.; Ben-Baruch, G.; Nesland, J.M.; Reich, R. The prognostic value of metalloproteinases and angiogenic factors in ovarian carcinoma. Mol. Cell Endocrinol. 2002, 187, 39–45. [Google Scholar] [CrossRef]
- Wu, X.; Li, H.; Kang, L.; Li, L.; Wang, W.; Shan, B. Activated matrix metalloproteinase-2 a potential maker of prognosis for epithelial ovarian cancer. Gynecol. Oncol. 2002, 84, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Lopata, A.; Agresta, F.; Quinn, M.A.; Smith, C.; Ostor, A.G.; Salamonsen, L.A. Detection of endometrial cancer by determination of matrix metalloproteinases in the uterine cavity. Gynecol. Oncol. 2003, 90, 318–324. [Google Scholar] [CrossRef]
- Torng, P.L.; Mao, T.L.; Chan, W.Y.; Huang, S.C.; Lin, C.T. Prognostic significance of stromal metalloproteinase-2 in ovarian adenocarcinoma and in relation to carcinoma progression. Gynecol. Oncol. 2004, 92, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Pauling, L. Plasmin-induced proteolysis and the role of apoprotein(a), lysine and synthetic analogs. Orthomol. Med. 1992, 7, 17–23. [Google Scholar]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Micronutrient synergy—A new tool in effective control of metastasis and other key mechanisms of cancer. Cancer Metastasis Rev. 2010, 29, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. A nutrient mixture modulates ovarian ES-2 cancer progression by inhibiting xenograft tumor growth and cellular MMP secretion, migration and invasion. Int. J. Clin. Exp. Med. 2016, 9, 814–822. [Google Scholar]
- Shaw, T.J.; Senterman, M.K.; Dawson, K.; Crane, C.A.; Vanderhyden, B.C. Characterization of intraperitoneal, orthotopic and metastatic xenograft models of human ovarian cancer. Mol. Therapy 2004, 10, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Aldercreutz, H. Western diet and Western disease: Some hormonal and biochemical mechanisms and associations. Scand. J. Clin. Lab. Investig. 1990, 50 (Suppl. 201), 3–23. [Google Scholar] [CrossRef]
- Miller, A.B. Diet and Cancer: A review. Acta Oncol. 1990, 29, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chen, Y.H.; Wang, P.; Zhang, J.; Gurewich, V.; Zhang, P.; Liu, J.N. The blockage of high-affinity lysine binding sites of plasminogen by EACA significantly inhibits prourokinase-induced plasminogen activation. Biochem. Biophys. Acta 2002, 1596, 182–192. [Google Scholar] [CrossRef]
- Kemberling, J.K.; Hampton, J.A.; Keck, R.W.; Gomez, M.A.; Selman, S.H. Inhibition of bladder tumor growth by the green tea derivative epigallocatechin-3-gallate. J. Urol. 2003, 170, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.; Matsushima, M. Preventive effects of urinary bladder tumors induced by N-butyl-N-(4-hydroxybutyl)-nitrosamine in rat by green tea leaves. Int. J. Urol. 2003, 10, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Valcic, S.; Timmermann, B.N.; Alberts, D.S.; Wachter, G.A.; Krutzsch, M.; Wymer, J.; Guillen, J.M. Inhibitory effect of six green tea catechins and caffeine on the growth of four selected human tumor cell lines. Anticancer Drugs 1996, 7, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, H.; Ahmed, N. Tea polyphenols: Prevention of cancer and optimizing health. Am. J. Clin. Nutr. 2000, 71, 1698s–1702s. [Google Scholar] [PubMed]
- Yang, G.Y.; Liao, J.; Kim, K.; Yurtow, E.J.; Yang, C.S. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 1998, 19, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Fujiki, H.; Kobayashi, H.; Go, H.; Miyado, K.; Sadano, H.; Shimikawa, R. Effect of (-) epigallocatechin gallate, the main constituent of green tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett. 1992, 65, 51–54. [Google Scholar] [CrossRef]
- Hara, Y. Green tea: Health Benefits and Applications; Marcel Dekker: New York, NY, USA; Basel, Switzerland, 2001. [Google Scholar]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Blasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.I.; Cossarizza, A. Quercetin and cancer chemoprevention. Evid.-Based Complement. Altern. Med. 2011, 591356. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Roomi, M.W.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int. J. Oncol. 2013, 42, 55–64. [Google Scholar] [PubMed]
- Naidu, K.A.; Karl, R.C.; Coppola, D. Antiproliferative and proapoptotic effect of ascorbyl stearate in human pancreatic cancer cells: Association with decreased expression of insulin-like growth factor 1 receptor. Dig. Dis. Sci. 2003, 48, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Anthony, H.M.; Schorah, C.J. Severe hypovitaminosis C in lung-cancer patients: The utilization of vitamin C in surgical repair and lymphocyte-related host resistance. Br. J. Cancer 1982, 46, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Maramag, C.; Menon, M.; Balaji, K.C.; Reddy, P.G.; Laxmanan, S. Effect of vitamin C on prostate cancer cells in vitro: Effect on cell number, viability and DNA synthesis. Prostate 1997, 32, 188–195. [Google Scholar] [CrossRef]
- Koh, W.S.; Lee, S.J.; Lee, H.; Park, C.; Park, M.H.; Kim, W.S.; Yoon, S.S.; Park, K.; Hong, S.I.; Chung, M.H.; Park, C.H. Differential effects and transport kinetics of ascorbate derivatives in leukemic cell lines. Anticancer Res. 1998, 8, 2487–2493. [Google Scholar]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. PNAS 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed]
- Nunez, C.; Ortiz de Apodaca, Y.; Ruiz, A. Ascorbic acid in the plasma and blood cells of women with breast cancer. The effect of consumption of food with an elevated content of this vitamin. Nutr. Hosp. 1995, 10, 68–372. [Google Scholar]
- Kurbacher, C.M.; Wagner, U.; Kolster, B.; Andreotti, P.E.; Krebs, D.; Bruckner, H.W. Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett. 1996, 103, 183–189. [Google Scholar] [CrossRef]
- Kawakami, S.; Kageyama, Y.; Fujii, Y.; Kihara, K.; Oshima, H. Inhibitory effects of N-acetyl cysteine on invasion and MMP 9 production of T24 human bladder cancer cells. Anticancer Res. 2001, 21, 213–219. [Google Scholar] [PubMed]
- Morini, M.; Cai, T.; Aluigi, M.G.; Noonan, D.M.; Masiello, L.; De Floro, S.; D’Agostinin, F.; Albini, A.; Fassima, G. The role of the thiol N-acetyl cysteine in the prevention of tumor invasion and angiogenesis. Int. J. Biol. Markers 1999, 14, 268–271. [Google Scholar] [PubMed]
- Yoon, S.O.; Kim, M.M.; Chung, A.S. Inhibitory effects of selenite on invasion of HT 1080 tumor cells. J. Biol. Chem. 2001, 276, 20085–20092. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P.; Dzau, V.J. Nitric oxide synthase: Role in the genesis of vascular disease. Annu. Rev. Med. 1997, 48, 489–509. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roomi, M.W.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. A Specific Mixture of Nutrients Suppresses Ovarian Cancer A-2780 Tumor Incidence, Growth, and Metastasis to Lungs. Nutrients 2017, 9, 303. https://doi.org/10.3390/nu9030303
Roomi MW, Kalinovsky T, Rath M, Niedzwiecki A. A Specific Mixture of Nutrients Suppresses Ovarian Cancer A-2780 Tumor Incidence, Growth, and Metastasis to Lungs. Nutrients. 2017; 9(3):303. https://doi.org/10.3390/nu9030303
Chicago/Turabian StyleRoomi, Mohd Waheed, Tatiana Kalinovsky, Matthias Rath, and Aleksandra Niedzwiecki. 2017. "A Specific Mixture of Nutrients Suppresses Ovarian Cancer A-2780 Tumor Incidence, Growth, and Metastasis to Lungs" Nutrients 9, no. 3: 303. https://doi.org/10.3390/nu9030303



