Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants
Abstract
:1. Introduction
2. Angiotensin Converting Enzyme and Its Inhibition Mechanism
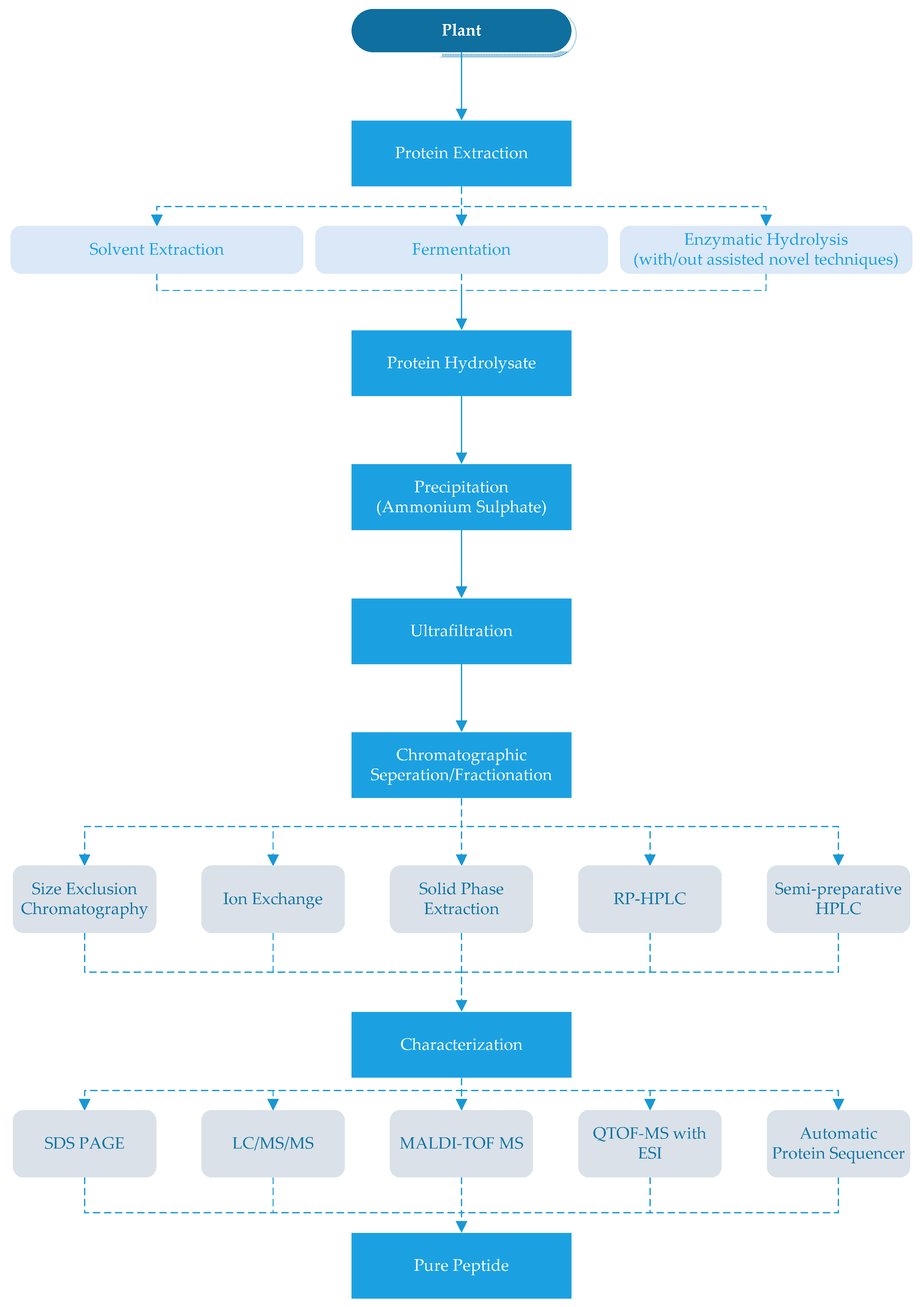
3. Production of ACE-Inhibitory Peptides from Plants
4. Purification of Peptides and Sequencing
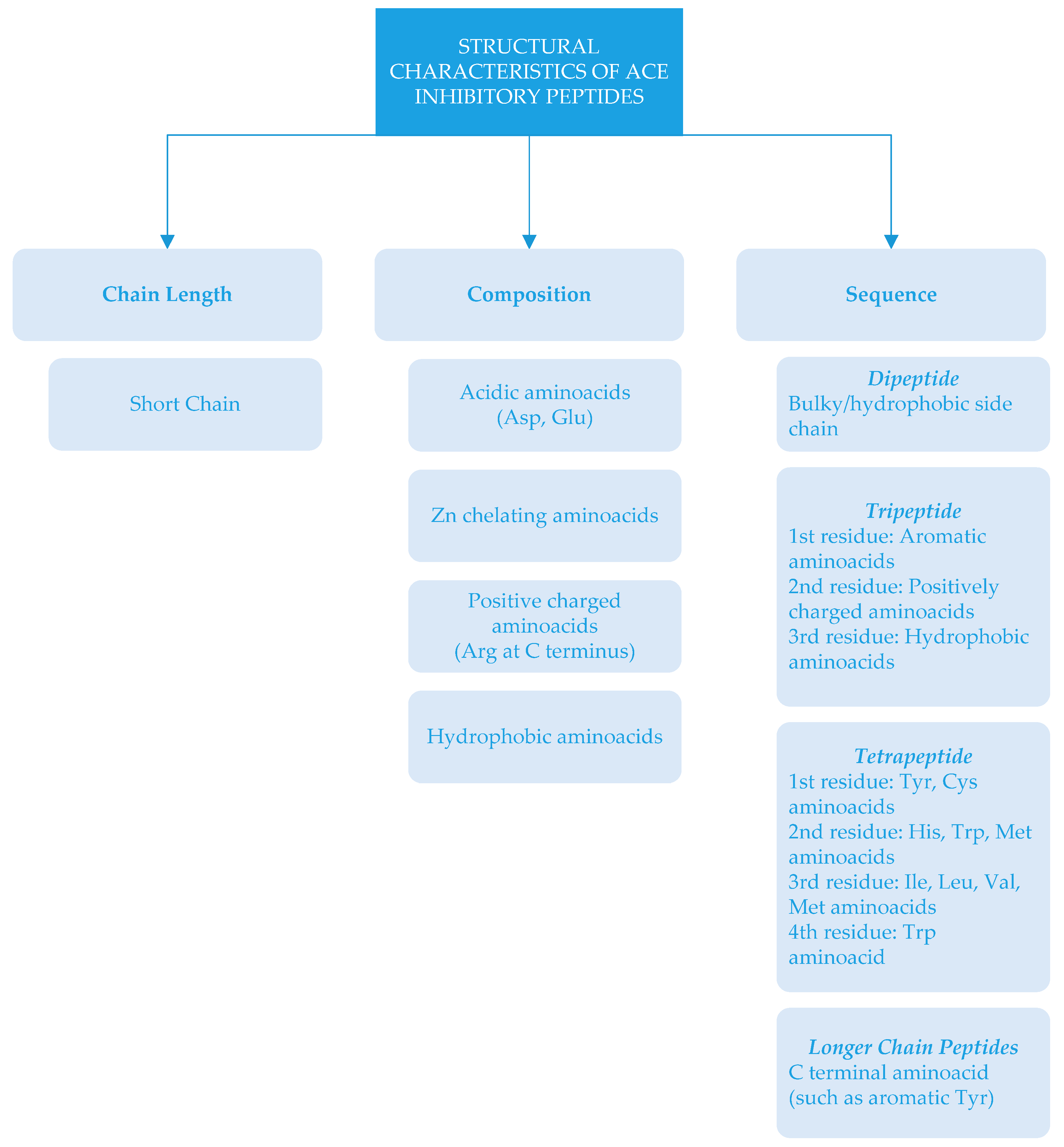
5. Structural Characteristics/Structure Activity Relationship
6. Activity of ACE Inhibitors Derived from Plants
6.1. In Vitro Studies
6.2. In Vivo Studies
7. Bioavailability of ACE Inhibitor Peptides
8. Conclusions and Future Trends
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gouda, K.G.M.; Gowda, L.R.; Rao, A.G.A.; Prakash, V. Angiotensin I-Converting Enzyme Inhibitory Peptide Derived from Glycinin, the 11S Globulin of Soybean (Glycine Max). J. Agric. Food Chem. 2006, 54, 4568–4573. [Google Scholar] [CrossRef] [PubMed]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Food-Originating ACE Inhibitors, Including Antihypertensive Peptides, as Preventive Food Components in Blood Pressure Reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 114–134. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-Inhibitory Activity of Enzymatic Protein Hydrolysates from Lupin and Other Legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [PubMed]
- World Health Organization (WHO). Global Status Report on Noncommunicable Diseases 2014; WHO: Geneve, Switzerland, 2014; p. 176. [Google Scholar]
- Murray, B.A.; FitzGerald, R.J. Angiotensin Converting Enzyme Inhibitory Peptides Derived from Food Proteins: Biochemistry, Bioactivity and Production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [PubMed]
- Jang, J.H.; Jeong, S.C.; Kim, J.H.; Lee, Y.H.; Ju, Y.C.; Lee, J.S. Characterisation of a New Antihypertensive Angiotensin I-Converting Enzyme Inhibitory Peptide from Pleurotus Cornucopiae. Food Chem. 2011, 127, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Coppey, L.J.; Davidson, E.P.; Rinehart, T.W.; Gellett, J.S.; Oltman, C.L.; Lund, D.D.; Yorek, M.A. ACE Inhibitor or Angiotensin II Receptor Antagonist Attenuates Diabetic Neuropathy in Streptozotocin-Induced Diabetic Rats. Diabetes 2006, 55, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H. A Bradykinin-Potentiating Factor (BPF) Present in the Venom of Bothrops Jararaca. Br. J. Pharmacol. Chemother. 1965, 24, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Jimsheena, V.K.; Gowda, L.R. Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides Derived from Arachin by Simulated Gastric Digestion. Food Chem. 2011, 125, 561–569. [Google Scholar] [CrossRef]
- Cheung, I.W.Y.; Nakayama, S.; Hsu, M.N.K.; Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Angiotensim-I Converting Enzyme Inhibitory Activity of Hydrolysates from Oat (Avena Sativa) Proteins by in Silico and in Vitro Analyses. J. Agric. Food Chem. 2009, 57, 9234–9242. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wu, J. LC-MS/MS Coupled with QSAR Modeling in Characterising of Angiotensin I-Converting Enzyme Inhibitory Peptides from Soybean Proteins. Food Chem. 2013, 141, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.; Karak, S.; De, B. Metabolite Profile and Bioactivity of Musa X Paradisiaca L. Flower Extracts. J. Food Biochem. 2016, 40, 724–730. [Google Scholar] [CrossRef]
- Siti, H.N.; Kamisah, Y.; Nur Iliyani, M.I.; Mohamed, S.; Jaarin, K. Citrus Leaf Extract Reduces Blood Pressure and Vascular Damage in Repeatedly Heated Palm Oil Diet-Induced Hypertensive Rats. Biomed. Pharmacother. 2017, 87, 451–460. [Google Scholar] [PubMed]
- Ambigaipalan, P.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant and Angiotensin I Converting Enzyme (ACE) Inhibitory Activities of Date Seed Protein Hydrolysates Prepared Using Alcalase, Flavourzyme and Thermolysin. J. Funct. Foods 2015, 18, 1125–1137. [Google Scholar] [CrossRef]
- Guang, C.; Phillips, R.D. Plant Food-Derived Angiotensin I Converting Enzyme Inhibitory Peptides. J. Agric. Food Chem. 2009, 57, 5113–5120. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ledesma, B.; Del Mar Contreras, M.; Recio, I. Antihypertensive Peptides: Production, Bioavailability and Incorporation into Foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Thewissen, B.G.; Pauly, A.; Celus, I.; Brijs, K.; Delcour, J.A. Inhibition of Angiotensin I-Converting Enzyme by Wheat Gliadin Hydrolysates. Food Chem. 2011, 127, 1653–1658. [Google Scholar]
- Day, L. Proteins from Land Plants—Potential Resources for Human Nutrition and Food Security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Qu, W.; Ma, H.; Jia, J.; He, R.; Luo, L.; Pan, Z. Enzymolysis Kinetics and Activities of ACE-Inhibitory Peptides from Wheat Germ Protein Prepared with SFP Ultrasound-Assisted Processing. Ultrason. Sonochem. 2012, 19, 1021–1026. [Google Scholar] [CrossRef]
- Zhou, C.; Ma, H.; Yu, X.; Liu, B.; Yagoub, A.E.G.A.; Pan, Z. Pretreatment of Defatted Wheat Germ Proteins (by-Products of Flour Mill Industry) Using Ultrasonic Horn and Bath Reactors: Effect on Structure and Preparation of ACE-Inhibitory Peptides. Ultrason. Sonochem. 2013, 20, 1390–1400. [Google Scholar] [PubMed]
- Jakubczyk, A.; Karas, M.; Baraniak, B.; Pietrzak, M. The Impact of Fermentation and in Vitro Digestion on Formation Angiotensin Converting Enzyme (ACE) Inhibitory Peptides from Pea Proteins. Food Chem. 2013, 141, 3774–3780. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lai, Y.S.; Wu, S.C. Antioxidation, Angiotensin Converting Enzyme Inhibition Activity, Nattokinase, and Antihypertension of Bacillus Subtilis (Natto)-Fermented Pigeon Pea. J. Food Drug Anal. 2015, 23, 750–757. [Google Scholar]
- Rayaprolu, S.; Hettiarachchy, N.; Horax, R.; Satchithanandam, E.; Chen, P.; Mauromoustakos, A. Amino Acid Profiles of 44 Soybean Lines and ACE-I Inhibitory Activities of Peptide Fractions from Selected Lines. J. Am. Oil Chem. Soc. 2015, 92, 1023–1033. [Google Scholar]
- Weng, T.M.; Chen, M.T. Effect of Two-Step Fermentation by Rhizopus Oligosporus and Bacillus Subtilis on Protein of Fermented Soybean. Food Sci. Technol. Res. 2011, 17, 393–400. [Google Scholar] [CrossRef]
- Liu, M.; Du, M.; Zhang, Y.; Xu, W.; Wang, C.; Wang, K.; Zhang, L. Purification and Identification of an ACE-Inhibitory Peptide from Walnut Protein. J. Agric. Food Chem. 2013, 61, 4097–4100. [Google Scholar] [CrossRef] [PubMed]
- Priyanto, A.D.; Doerksen, R.J.; Chang, C.I.; Sung, W.C.; Widjanarko, S.B.; Kusnadi, J.; Lin, Y.C.; Wang, T.C.; Hsu, J.L. Screening, Discovery, and Characterization of Angiotensin-I Converting Enzyme Inhibitory Peptides Derived from Proteolytic Hydrolysate of Bitter Melon Seed Proteins. J. Proteom. 2015, 128, 424–435. [Google Scholar]
- Yang, Y.; Marczak, E.D.; Yokoo, M.; Usui, H.; Yoshikawa, M. Isolation and Antihypertensive Effect of Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Spinach Rubisco. J. Agric. Food Chem. 2003, 51, 4897–4902. [Google Scholar] [CrossRef]
- Maestri, E.; Marmiroli, M.; Marmiroli, N. Bioactive Peptides in Plant-Derived Foodstuffs. J. Proteom. 2015, 147, 140–155. [Google Scholar]
- Vallabha, V.; Tiku, P.K. Antihypertensive Peptides Derived from Soy Protein by Fermentation. Int. J. Pept. Res. Ther. 2014, 20, 161–168. [Google Scholar]
- Wan Mohtar, W.A.A.Q.I.; Hamid, A.A.; Abd-Aziz, S.; Muhamad, S.K.S.; Saari, N. Preparation of Bioactive Peptides with High Angiotensin Converting Enzyme Inhibitory Activity from Winged Bean (Psophocarpus Tetragonolobus (L.) DC.) Seed. J. Food Sci. Technol. 2014, 51, 3658–3668. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A Novel Antioxidant and ACE-Inhibitory Peptide from Rice Bran Protein: Biochemical Characterization and Molecular Docking Study. LWT-Food Sci. Technol. 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Sornwatana, T.; Bangphoomi, K.; Roytrakul, S.; Wetprasit, N.; Choowongkomon, K.; Ratanapo, S. Chebulin: Terminalia Chebula Retz. Fruit-Derived Peptide with Angiotensin-I-Converting Enzyme Inhibitory Activity. Biotechnol. Appl. Biochem. 2015, 62, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Girgih, A.T.; He, R.; Malomo, S.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and Functional Characterization of Hemp Seed (Cannabis Sativa L.) Protein-Derived Antioxidant and Antihypertensive Peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Moayedi, A.; Mora, L.; Aristoy, M.C.; Hashemi, M.; Safari, M.; Toldrá, F. ACE-Inhibitory and Antioxidant Activities of Peptide Fragments Obtained from Tomato Processing By-Products Fermented Using Bacillus Subtilis: Effect of Amino Acid Composition and Peptides Molecular Mass Distribution. Appl. Biochem. Biotechnol. 2016, 181, 48–64. [Google Scholar] [CrossRef]
- García, M.C.; Endermann, J.; González-García, E.; Marina, M.L. HPLC-Q-TOF-MS Identification of Antioxidant and Antihypertensive Peptides Recovered from Cherry (Prunus Cerasus L.) Subproducts. J. Agric. Food Chem. 2015, 63, 1514–1520. [Google Scholar] [PubMed]
- Makinen, S.; Johannson, T.; Vegarud Gerd, E.; Pihlava, J.M.; Pihlanto, A. Angiotensin I-Converting Enzyme Inhibitory and Antioxidant Properties of Rapeseed Hydrolysates. J. Funct. Foods 2012, 4, 575–583. [Google Scholar]
- Toopcham, T.; Mes, J.J.; Wichers, H.J.; Roytrakul, S.; Yongsawatdigul, J. Bioavailability of Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides Derived from Virgibacillus Halodenitrificans SK1-3-7 Proteinases Hydrolyzed Tilapia Muscle Proteins. Food Chem. 2017, 220, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Liu, H.; Liu, L.; Hu, H.; Wang, Q.; Adhikari, B. Isolation, Purification and Molecular Mechanism of a Peanut Protein-Derived ACE-Inhibitory Peptide. PLoS ONE 2014, 9, e111188. [Google Scholar]
- Lee, D.H.; Kim, J.H.; Park, J.S.; Choi, Y.J.; Lee, J.S. Isolation and Characterization of a Novel Angiotensin I-Converting Enzyme Inhibitory Peptide Derived from the Edible Mushroom Tricholoma Giganteum. Peptides 2004, 25, 621–627. [Google Scholar]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-Inhibitory and Antioxidant Properties of Potato (Solanum Tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [PubMed]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Gomez, R.; Martinez-Villaluenga, C. High-Pressure Improves Enzymatic Proteolysis and the Release of Peptides with Angiotensin I Converting Enzyme Inhibitory and Antioxidant Activities from Lentil Proteins. Food Chem. 2015, 171, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Qiu, N.; Yi, J. Production and Characterization of Angiotensin Converting Enzyme (ACE) Inhibitory Peptides from Apricot (Prunus Armeniaca L.) Kernel Protein Hydrolysate. Eur. Food Res. Technol. 2010, 231, 13–19. [Google Scholar] [CrossRef]
- White, B.L.; Sanders, T.H.; Davis, J.P. Potential ACE-Inhibitory Activity and nanoLC-MS/MS Sequencing of Peptides Derived from Aflatoxin Contaminated Peanut Meal. LWT-Food Sci. Technol. 2014, 56, 537–542. [Google Scholar]
- Malomo, S.A.; Onuh, J.O.; Girgih, A.T.; Aluko, R.E. Structural and Antihypertensive Properties of Enzymatic Hemp Seed Protein Hydrolysates. Nutrients 2015, 7, 7616–7632. [Google Scholar]
- Deddish, P.A.; Wang, J.; Michel, B.; Morris, P.W.; Davidson, N.O.; Skidgel, R.A.; Erdös, E.G. Naturally Occurring Active N-Domain of Human Angiotensin I-Converting Enzyme. Proc. Natl. Acad. Sci. USA 1994, 91, 7807–7811. [Google Scholar] [CrossRef] [PubMed]
- Aluko, R.E. Structure and Function of Plant Protein-Derived Antihypertensive Peptides. Curr. Opin. Food Sci. 2015, 4, 44–50. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric Assay and Properties of the Angiotensin-Converting Enzyme of Rabbit Lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [PubMed]
- Rawlings, N.D.; Barrett, A.J. Evolutionary Families of Metallopeptidases. Biochem. J. 1993, 290, 205–218. [Google Scholar] [PubMed]
- Ondetti, M.A.; Rubin, B.; Cushman, D.W. Design of Specific Inhibitors of Angiotensin-Converting Enzyme: New Class of Orally Active Antihypertensive Agents. Science 1977, 196, 441–444. [Google Scholar] [CrossRef]
- Johnston, C.I.; Risvanis, J. Preclinical Pharmacology of Angiotensin II Receptor Antagonists. Am. J. Hypertens. 1997, 10, 306–310. [Google Scholar] [CrossRef]
- Chiang, W.-D.; Tsou, M.-J.; Tsai, Z.-Y.; Tsai, T.-C. Angiotensin I-Converting Enzyme Inhibitor Derived from Soy Protein Hydrolysate and Produced by Using Membrane Reactor. Food Chem. 2006, 98, 725–732. [Google Scholar] [CrossRef]
- He, H.L.; Liu, D.; Ma, C.B. Review on the Angiotensin-I-Converting Enzyme (ACE) Inhibitor Peptides from Marine Proteins. Appl. Biochem. Biotechnol. 2013, 169, 738–749. [Google Scholar] [CrossRef]
- Van der Ven, C.; Gruppen, H.; de Bont, D.B.A.; Voragen, A.G.J. Optimisation of the Angiotensin Converting Enzyme Inhibition by Whey Protein Hydrolysates Using Response Surface Methodology. Int. Dairy J. 2002, 12, 813–820. [Google Scholar]
- De Gobba, C.; Tompa, G.; Otte, J. Bioactive Peptides from Caseins Released by Cold Active Proteolytic Enzymes from Arsukibacterium Ikkense. Food Chem. 2014, 165, 205–215. [Google Scholar] [PubMed]
- Espejo-Carpio, F.J.; De Gobba, C.; Guadix, A.; Guadix, E.M.; Otte, J. Angiotensin I-Converting Enzyme Inhibitory Activity of Enzymatic Hydrolysates of Goat Milk Protein Fractions. Int. Dairy J. 2013, 32, 175–183. [Google Scholar] [CrossRef]
- Qu, W.; Ma, H.; Zhao, W.; Pan, Z. ACE-Inhibitory Peptides Production from Defatted Wheat Germ Protein by Continuous Coupling of Enzymatic Hydrolysis and Membrane Separation: Modeling and Experimental Studies. Chem. Eng. J. 2013, 226, 139–145. [Google Scholar] [CrossRef]
- Bao, C.; Chen, H.; Chen, L.; Cao, J.; Meng, J. Comparison of ACE Inhibitory Activity in Skimmed Goat and Cow Milk Hydrolyzed by Alcalase, Flavourzyme, Neutral Protease and Proteinase K. Acta Univ. Cibiniensis Ser. E Food Technol. 2016, 20, 77–84. [Google Scholar] [CrossRef]
- Mohan, A.; Julian, D.; Udenigwe, C.C. Encapsulation of Bioactive Whey Peptides in Soy Lecithin-Derived Nanoliposomes: Influence of Peptide Molecular Weight. Food Chem. 2016, 213, 143–148. [Google Scholar] [PubMed]
- Lourenço da Costa, E.; Antonio da Rocha Gontijo, J.; Netto, F.M. Effect of Heat and Enzymatic Treatment on the Antihypertensive Activity of Whey Protein Hydrolysates. Int. Dairy J. 2007, 17, 632–640. [Google Scholar]
- Matsubara, H. Observations on the Specificity of Thermolysin with Synthetic Peptides. Biochem. Biophys. Res. Commun. 1966, 24, 427–430. [Google Scholar] [CrossRef]
- Arnold, U.; Rucknagel, K.P.; Schierhorn, A.; Ulbrich-Hofmann, R. Thermal Unfolding and Proteolytic Susceptibility of Ribonuclease A. Eur. J. Biochem. 1996, 237, 862–869. [Google Scholar] [PubMed]
- García-Tejedor, A.; Sánchez-Rivera, L.; Castelló-Ruiz, M.; Recio, I.; Salom, J.B.; Manzanares, P. Novel Antihypertensive Lactoferrin-Derived Peptides Produced by Kluyveromyces Marxianus: Gastrointestinal Stability Profile and in Vivo Angiotensin I-Converting Enzyme (ACE) Inhibition. J. Agric. Food Chem. 2014, 62, 1609–1616. [Google Scholar]
- Jung, W.K.; Park, P.J.; Byun, H.G.; Moon, S.H.; Kim, S.K. Preparation of Hoki (Johnius Belengerii) Bone Oligophosphopeptide with a High Affinity to Calcium by Carnivorous Intestine Crude Proteinase. Food Chem. 2005, 91, 333–340. [Google Scholar] [CrossRef]
- Jung, W.K.; Mendis, E.; Je, J.Y.; Park, P.J.; Byeng, W.S.; Hyoung, C.K.; Yang, K.C.; Kim, S.K. Angiotensin I-Converting Enzyme Inhibitory Peptide from Yellowfin Sole (Limanda Aspera) Frame Protein and Its Antihypertensive Effect in Spontaneously Hypertensive Rats. Food Chem. 2006, 94, 26–32. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yoon, S.; Yu, D.Y.; Lönnerdal, B.; Chung, B.H. Novel Angiotensin-I-Converting Enzyme Inhibitory Peptides Derived from Recombinant Human α(s1)-Casein Expressed in Escherichia Coli. J. Dairy Res. 1999, 66, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Wu, J. A New Approach for Identification of Novel Antihypertensive Peptides from Egg Proteins by QSAR and Bioinformatics. Food Res. Int. 2010, 43, 1371–1378. [Google Scholar]
- Joshi, S.; Satyanarayana, T. Biotechnology of Cold-Active Proteases. Biology 2013, 2, 755–783. [Google Scholar]
- Kasana, R.C. Proteases from Psychrotrophs: An Overview. Crit. Rev. Microbiol. 2010, 36, 134–145. [Google Scholar] [PubMed]
- Aghajari, N.; Van Petegem, F.; Villeret, V.; Chessa, J.P.; Gerday, C.; Haser, R.; Van Beeumen, J. Crystal Structures of a Psychrophilic Metalloprotease Reveal New Insights into Catalysis by Cold-Adapted Proteases. Proteins Struct. Funct. Genet. 2003, 50, 636–647. [Google Scholar] [PubMed]
- Kuddus, M.; Ramteke, P.W. Recent Developments in Production and Biotechnological Applications of Cold Active Microbial Proteases. Crit. Rev. Microbiol. 2012, 38, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Cao, L. Immobilised Enzymes: Science or Art? Curr. Opin. Chem. Biol. 2005, 9, 217–226. [Google Scholar]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of Enzyme Activity, Stability and Selectivity via Immobilization Techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Mendes, A.A.; Freitas, L.; de Carvalho, A.K.F.; de Oliveira, P.C.; de Castro, H.F. Immobilization of a Commercial Lipase from Penicillium Camembertii (Lipase G) by Different Strategies. Enzyme Res. 2011, 2011, 967239. [Google Scholar] [CrossRef] [PubMed]
- Otağ, F.B.; Hayta, M. Efeects of Ultrasound, Microwave, Fermentation and Heat Treatments on Angiotensin-I Converting Enzyme Activity of Chickpea Bioactive Peptides. J. Food 2016, 41, 9–14. [Google Scholar]
- Jia, J.; Ma, H.; Zhao, W.; Wang, Z.; Tian, W.; Luo, L.; He, R. The Use of Ultrasound for Enzymatic Preparation of ACE-Inhibitory Peptides from Wheat Germ Protein. Food Chem. 2010, 119, 336–342. [Google Scholar]
- Hayes, M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Putting Microbes to Work: Diary Fermentation, Cell Factories and Bioactive Peptides. Part II: Bioactive Peptide Functions. Biotechnol. J. 2007, 2, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Synthesis of Angiotensin I-Converting Enzyme (ACE)-Inhibitory Peptides and gamma-Aminobutyric Acid (GABA) during Sourdough Fermentation by Selected Lactic Acid Bacteria. J. Agric. Food Chem. 2008, 56, 6936–6943. [Google Scholar] [CrossRef]
- Nejati, F.; Rizzello, C.G.; Di Cagno, R.; Sheikh-Zeinoddin, M.; Diviccaro, A.; Minervini, F.; Gobbetti, M. Manufacture of a Functional Fermented Milk Enriched of Angiotensin-I Converting Enzyme (ACE)-Inhibitory Peptides and γ-Amino Butyric Acid (GABA). LWT-Food Sci. Technol. 2013, 51, 183–189. [Google Scholar] [CrossRef]
- Shu, G.; Yang, H.; Chen, H.; Zhang, Q.; Tian, Y. Effect of Incubation Time, Inoculum Size, Temperature, Pasteurization Time, Goat Milk Powder and Whey Powder on Ace Inhibitory Activity in Fermented Milk by L. Plantarum LP69. Acta Sci. Pol. Technol. Aliment. 2015, 14, 107–116. [Google Scholar] [PubMed]
- Fernández, M.; Benito, M.J.; Martín, A.; Casquete, R.; Córdoba, J.J.; Córdoba, M.G. Influence of Starter Culture and a Protease on the Generation of ACE-Inhibitory and Antioxidant Bioactive Nitrogen Compounds in Iberian Dry-Fermented Sausage “salchichon”. Heliyon 2016. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Van Camp, J.; Decroos, K.; Van Wijmelbeke, L.; Verstraete, W. The Impact of Fermentation and In Vitro Digestion on the Formation of Angiotensin-I-Converting Enzyme Inhibitory Activity from Pea and Whey Protein. J. Dairy Sci. 2003, 86, 429–438. [Google Scholar] [CrossRef]
- García-Tejedor, A.; Sánchez-Rivera, L.; Recio, I.; Salom, J.B.; Manzanares, P. Dairy Debaryomyces Hansenii Strains Produce the Antihypertensive Casein-Derived Peptides LHLPLP and HLPLP. LWT-Food Sci. Technol. 2015, 61, 550–556. [Google Scholar]
- Hang, M.; Zhao, X.-H. Fermentation Time and Ethanol/water-Based Solvent System Impacted in Vitro ACE-Inhibitory Activity of the Extract of Mao-Tofu Fermented by Mucor Spp. CyTA-J. Food 2012, 10, 137–143. [Google Scholar]
- Wang, D.; Wang, L.J.; Zhu, F.X.; Zhu, J.Y.; Chen, X.D.; Zou, L.; Saito, M.; Li, L.T. In Vitro and in Vivo Studies on the Antioxidant Activities of the Aqueous Extracts of Douchi (A Traditional Chinese Salt-Fermented Soybean Food). Food Chem. 2008, 107, 1421–1428. [Google Scholar] [CrossRef]
- Chaves-López, C.; Serio, A.; Paparella, A.; Martuscelli, M.; Corsetti, A.; Tofalo, R.; Suzzi, G. Impact of Microbial Cultures on Proteolysis and Release of Bioactive Peptides in Fermented Milk. Food Microbiol. 2014, 42, 117–121. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive Peptides from Marine Processing Waste and Shellfish: A Review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar]
- Jimsheena, V.K.; Gowda, L.R. Arachin Derived Peptides as Selective Angiotensin I-Converting Enzyme (ACE) Inhibitors: Structure-Activity Relationship. Peptides 2010, 31, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Yust, M.M.; Pedroche, J.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Production of ACE-Inhibitory Peptides by Digestion of Chickpea Legumin with Alcalase. Food Chem. 2003, 81, 363–369. [Google Scholar]
- Natesh, R.; Schwager, S.L.U.; Sturrock, E.D.; Acharya, K.R. Crystal Structure of the Human Enzyme—Lisinopril Complex. Nature 2003, 421, 1427–1429. [Google Scholar] [CrossRef] [PubMed]
- López-Fandiño, R.; Otte, J.; van Camp, J. Physiological, Chemical and Technological Aspects of Milk-Protein-Derived Peptides with Antihypertensive and ACE-Inhibitory Activity. Int. Dairy J. 2006, 16, 1277–1293. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; Meisel, H. Milk Protein-Derived Peptide Inhibitors of Angiotensin-I-Converting Enzyme. Br. J. Nutr. 2000, 84, S33–S37. [Google Scholar]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure-Activity Relationship Study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [PubMed]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure-Activity Relationship Modeling of Peptides Containing 4–10 Amino Acid Residues. QSAR Comb. Sci. 2006, 25, 873–880. [Google Scholar]
- Pripp, A.H.; Ardö, Y. Modelling Relationship between Angiotensin-(I)-Converting Enzyme Inhibition and the Bitter Taste of Peptides. Food Chem. 2007, 102, 880–888. [Google Scholar] [CrossRef]
- Wu, S.; Qi, W.; Su, R.; Li, T.; Lu, D.; He, Z. CoMFA and CoMSIA Analysis of ACE-Inhibitory, Antimicrobial and Bitter-Tasting Peptides. Eur. J. Med. Chem. 2014, 84, 100–106. [Google Scholar] [CrossRef]
- Li, G.H.; Liu, H.; Shi, Y.H.; Le, G.W. Direct Spectrophotometric Measurement of Angiotensin I-Converting Enzyme Inhibitory Activity for Screening Bioactive Peptides. J. Pharm. Biomed. Anal. 2005, 37, 219–224. [Google Scholar]
- Lam, L.H.; Shimamura, T.; Sakaguchi, K.; Noguchi, K.; Ishiyama, M.; Fujimura, Y.; Ukeda, H. Assay of Angiotensin I-Converting Enzyme-Inhibiting Activity Based on the Detection of 3-Hydroxybutyric Acid. Anal. Biochem. 2007, 364, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.; Castillo, J.; Quinones, M.; Garcia-Vallve, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of Angiotensin-Converting Enzyme Activity by Flavonoids: Structure-Activity Relationship Studies. PLoS ONE 2012, 7, e49493. [Google Scholar]
- Wu, J.; Aluko, R.E.; Muir, A.D. Improved Method for Direct High-Performance Liquid Chromatography Assay of Angiotensin-Converting Enzyme-Catalyzed Reactions. J. Chromatogr. A 2002, 950, 125–130. [Google Scholar] [PubMed]
- Sagardia, I.; Roa-Ureta, R.H.; Bald, C. A New QSAR Model, for Angiotensin I-Converting Enzyme Inhibitory Oligopeptides. Food Chem. 2013, 136, 1370–1376. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Augustijns, P.; Morel, N.; Van Camp, J.; Opsomer, A.; Verstraete, W. In Vitro Intestinal Transport and Antihypertensive Activity of ACE Inhibitory Pea and Whey Digests. Int. J. Food Sci. Nutr. 2005, 56, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Alabaster, V. The Fall and Rise of in Vivo Pharmacology. Trends Pharmacol. Sci. 2002, 23, 13–18. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Mcclements, D.J.; Zou, L.; Zhang, R.; Salvia-Trujillo, L.; Kumosani, T.; Xiao, H. Enhancing Nutraceutical Performance Using Excipient Foods: Designing Food Structures and Compositions to Increase Bioavailability. Compr. Rev. Food Sci. Food Saf. 2015, 14, 824–847. [Google Scholar]
- Jao, C.-L.; Huang, S.-L.; Hsu, K.-C. Angiotensin I-Converting Enzyme Inhibitory Peptides: Inhibition Mode, Bioavailability, and Antihypertensive Effects. Biomedicine 2012, 2, 130–136. [Google Scholar]
- Vermeirssen, V.; Van Camp, J.; Verstraete, W. Review Article Bioavailability of Angiotensin I Converting Enzyme Inhibitory Peptides. Br. J. Nutr. 2004, 92, 357–366. [Google Scholar]
- Ding, L.; Wang, L.; Yu, Z.; Zhang, T.; Liu, J. Digestion and Absorption of an Egg White ACE-Inhibitory Peptide in Human Intestinal Caco-2 Cell Monolayers. Int. J. Food Sci. Nutr. 2016, 67, 111–116. [Google Scholar] [CrossRef]
- Fernández-Musoles, R.; Salom, J.B.; Castelló-Ruiz, M.; Contreras, M.; del, M.; Recio, I.; Manzanares, P. Bioavailability of Antihypertensive Lactoferricin B-Derived Peptides: Transepithelial Transport and Resistance to Intestinal and Plasma Peptidases. Int. Dairy J. 2013, 32, 69–174. [Google Scholar]
- Gallego, M.; Grootaert, C.; Mora, L.; Aristoy, M.C.; Van Camp, J.; Toldrá, F. Transepithelial Transport of Dry-Cured Ham Peptides with ACE Inhibitory Activity through a Caco-Cell Monolayer. J. Funct. Foods 2016, 21, 388–395. [Google Scholar]
- Cinq-Mars, C.D.; Hu, C.; Kitts, D.D.; Li-Chan, E.C. Investigations into Inhibitor Type and Mode, Simulated Gastrointestinal Digestion, and Cell Transport of the Angiotensin I-Converting Enzyme—Inhibitory Peptides in Pacific Hake (Merluccius Productus) Fillet Hydrolysate. J. Agric. Food Chem. 2008, 56, 410–419. [Google Scholar]
- Satake, M.; Enjoh, M.; Nakamura, Y.; Takano, T.; Kawamura, Y.; Arai, S.; Shimizu, M. Transepithelial Transport of the Bioactive Tripeptide, Val-Pro-Pro, in Human Intestinal Caco-2 Cell Monolayers. Biosci. Biotechnol. Biochem. 2002, 66, 378–384. [Google Scholar] [CrossRef]
- Megías, C.; Pedroche, J.; Yust, M.D.M.; Alaiz, M.; Girón-Calle, J.; Millán, F.; Vioque, J. Stability of Sunflower Protein Hydrolysates in Simulated Gastric and Intestinal Fluids and Caco-2 Cell Extracts. LWT-Food Sci. Technol. 2009, 42, 1496–1500. [Google Scholar]
- Picariello, G.; Iacomino, G.; Mamone, G.; Ferranti, P.; Fierro, O.; Gianfrani, C.; Di Luccia, A.; Addeo, F. Transport across Caco-2 Monolayers of Peptides Arising from in Vitro Digestion of Bovine Milk Proteins. Food Chem. 2013, 139, 203–212. [Google Scholar]
- Miguel, M.; Dávalos, A.; Manso, M.A.; De La Peña, G.; Lasunción, M.A.; López-Fandiño, R. Transepithelial Transport across Caco-2 Cell Monolayers of Antihypertensive Egg-Derived Peptides. PepT1-Mediated Flux of Tyr-Pro-Ile. Mol. Nutr. Food Res. 2008, 52, 1507–1513. [Google Scholar] [PubMed]
- Yea Chay, S.; Kiat Tan, W.; Saari, N. LER Preparation and Characterisation of Nanoliposomes Containing Winged Bean Seeds Bioactive Peptides—Prof Nereide Mandou. J. Microencapsul. 2015, 32, 488–495. [Google Scholar]
- Davarci, F.; Turan, D.; Ozcelik, B.; Poncelet, D. The Influence of Solution Viscosities and Surface Tension on Calcium-Alginate Microbead Formation Using Dripping Technique. Food Hydrocoll. 2017, 62, 119–127. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Karadag, A.; Duman, Ş.; Özkal, B.; Özçelik, B. Fortification of Dark Chocolate with Spray Dried Black Mulberry (Morus Nigra) Waste Extract Encapsulated in Chitosan-Coated Liposomes and Bioaccessability Studies. Food Chem. 2016, 201, 205–212. [Google Scholar]
- Ruiz Ruiz, J.C.; Rubí, M.; Campos, S.; Abram, D.; Ancona, B.; Antonio, L.; Guerrero, C.; Mx, C. Encapsulation of Phaseolus Lunatus Protein Hydrolysate with Angiotensin-Converting Enzyme Inhibitory Activity. ISRN Biotechnol. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Lin, F.; Chen, L.; Liang, R.; Zhang, Z.; Wang, J.; Cai, M.; Li, Y. Pilot-Scale Production of Low Molecular Weight Peptides from Corn Wet Milling Byproducts and the Antihypertensive Effects in Vivo and in Vitro. Food Chem. 2011, 124, 801–807. [Google Scholar]
- White, B.L.; Oakes, A.J.; Shi, X.; Price, K.M.; Lamb, M.C.; Sobolev, V.S.; Sanders, T.H.; Davis, J.P. Development of a Pilot-Scale Process to Sequester Aflatoxin and Release Bioactive Peptides from Highly Contaminated Peanut Meal. LWT-Food Sci. Technol. 2013, 51, 492–499. [Google Scholar] [CrossRef]
- Firdaous, L.; Dhulster, P.; Amiot, J.; Doyen, A.; Lutin, F.; Vézina, L.P.; Bazinet, L. Investigation of the Large-Scale Bioseparation of an Antihypertensive Peptide from Alfalfa White Protein Hydrolysate by an Electromembrane Process. J. Memb. Sci. 2010, 355, 175–181. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y. Bioactive Peptides and Protein Hydrolysates: Research Trends and Challenges for Application as Nutraceuticals and Functional Food Ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37. [Google Scholar]
- Cheung, L.K.Y.; Aluko, R.E.; Cliff, M.A.; Li-Chan, E.C.Y. Effects of Exopeptidase Treatment on Antihypertensive Activity and Taste Attributes of Enzymatic Whey Protein Hydrolysates. J. Funct. Foods 2015, 13, 262–275. [Google Scholar] [CrossRef]
| Substrate | Production Method | Purification Method | IC50 Value | Sequencing and Molecular Mass Determination | Peptide Sequence and Molecular Weight | Reference |
|---|---|---|---|---|---|---|
| Mushroom (Tricholoma giganteum) | Solvent extraction and enzymatic hydrolysis | Ultrafiltration (UF), size exclusion chromatography (SEC) with Sephadex G-25 column chromatography, and reverse-phase high performance liquid chromatography (RP-HPLC) | Water extract: 310 µg/mL | Protein sequencer | Gly-Gln-Pro 301 Da | [39] |
| UF: 280 µg/mL | ||||||
| SEC: 240 µg/mL | ||||||
| RP-HPLC: 40 µg/mL | ||||||
| Mushroom (Pleurotus cornucopiae) | Water and methanol extraction | UF, SEC with Sephadex G-25 column, solid phase extraction (SPE), strong cation exchange (SCX) solid phase extraction, RP-HPLC | Water extract: 6000 µg/mL | Liquid chromatography tandem mass spectrometry (LC-MS/MS) | Fr 1: Arg-Leu-Pro-Ser-Glu-Phe-Asp-Leu-Ser-Ala-Phe-Leu-Arg-Ala (1622.85 Da); Fr 2: Arg-Leu-Ser-Gly-Gln-Thr-Ile-Glu-Val-Thr-Ser-Glu-Tyr-Leu- Phe-Arg-His (2037.26 Da) | [6] |
| UF: 5300 µg/mL | ||||||
| SEC: 3860 µg/mL | ||||||
| SCX: 1500 µg/mL | ||||||
| RP-HPLC: | ||||||
| Fr 1: 460 µg/mL | ||||||
| Fr 2: 1140 µg/mL | ||||||
| Potato | Enzymatic hydrolysis with alcalase, neutrase and esperase | UF (3, 5 and 10 kDa cut off), SPE, HPLC | 18–86 µg/mL | Matrix-assisted laser desorption ionization (MALDI)-time of flight (TOF) mass spectrometry (MS) | 704–850 Da | [40] |
| Wheat | Solvent extraction and enzymatic hydrolysis | Immobilized metal-affinity chromatography and semi-preparative RP-HPLC | 20 µg/mL | - | - | [17] |
| Soybean | Lactobacillus casei spp. pseudoplantarum fermentation | Semi-preparative HPLC | 17.2 µg/mL2 | Protein sequencer | N-terminal of the peptide: Leu-Ile-Val-Thr-Gln | [29] |
| Enzymatic hydrolysis with thermolysin, pepsin and trypsin | RP-UPLC | Predicted by QSAR modelling based on peptide sequences: 3.4–470.7 µM | Reverse-phase ultra performance liquid chromatography tandem mass spectrometry (RP-UPLC-MS/MS) | 12 dipeptide, 10 tripeptide, 7 tetrapeptide, 4 pentapeptie, 1 hexapeptide (200–600 Da) | [11] | |
| Terminalia chebula Tree | Enzymatic hydrolysis with pepsin | Filtration (3–kDa cut off), RP-HPLC, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS- PAGE) and nano-LC-MS/MS | 100 µM | Nano-liquid chromatography tandem mass spectrometry (Nano-LC-MS/MS) | Asp-Glu-Asn-Ser-Lys-Phe 738.5 Da | [32] |
| Lentil | HP assisted proteolysis with different proteolytic enzymes | UF (3–kDa cut off), SPE | - | MALDI TOF/TOF MS/MS | 13 different peptides (1105–2614 Da) | [41] |
| Walnut | Enzymatic hydrolysis with proteinase | UF (3–kDa cut off), SEC with Sephadex G-15 and anion exchange chromatography, and HPLC | 25.67 μg/mL | MALDI TOF MS | Trp-Pro-Glu-Arg-Pro-Pro-Gln-Ile-Pro 1033.42 Da | [25] |
| Tomato waste | Bacillus subtilis fermentation | 8200 µg/mL2 | MALDI TOF MS | 500–800 Da | [34] | |
| Rice bran | Enzymatic hydrolysis with trypsin | UF (different cut off; <4 kDa, 4–6 kDa, >6 kDa), SEC with Sephadex G-25, RP-HPLC | 76 µM | Quardrupole time-of-flight mass spectrometer (Q-TOF-MS) with an electro-spray ionization (ESI) (Q-TOF-MS with ESI) | Tyr-Ser-Lys 395 Da | [31] |
| Apricot kernel | Enzymatic hydrolysis with different proteolytic enzymes | UF (1 and 5 kDa MWCO) | Enzymatic hydrolysate: 378 µg/mL | - | - | [42] |
| UF (<5 kDa molecular weight cut off (MWCO): 849 µg/mL | ||||||
| UF (1–5 kDa MWCO): 601 µg/mL | ||||||
| UF (<1 kDa MWCO): 150 µg/mL | ||||||
| Date seed flour | Enzymatic hydrolysis with alcalase, flavourzyme, thermolysin and their mixture | - | 530 µg/mL2 (alcalase and thermolysin enzyme mixture) | Quadrupole orthogonal time-of-flight (QqTOF)-MS/MS hybrid tandem mass spectrometer (QqTOF-MS/MS) | 2.06–116.8 kDa | [14] |
| Peanut | Enzymatic hydrolysis with alcalase | UF (10kDa cut off), SEC | 44.4 μg/mL2 | Nano-LC-MS/MS | 271 unique peptides 295–782 Da | [43] |
| Bitter melon seed | Enzymatic hydrolysis with thermolysin | UF (3 kDa cut off), HPLC | 8.64 µM | LC-MS/MS | Val-Ser-Gly-Ala-Gly-Arg-Tyr 708 Da | [26] |
| Pea | Lactobacillus plantarum fermentation | SEC (Sephadex G-10), HPLC | 64.04 µM | LC-MS/MS | Lys-Glu-Asp-Asp-Glu-Glu-Glu-Glu-Gln-Glu-Glu-Glu 1593.58 Da | [21] |
| Spinach | Enzymatic hydrolysis with pepsin-pancreatin | RP-HPLC | Fr 1: 4.2 µM | Protein sequencer | Fr 1: Ile-Ala-Tyr-Lys-Pro-Ala-Gly | [27] |
| Fr 2: 2.1 µM | Fr 2: Met-Arg-Trp-Arg-Asp | |||||
| Fr 3: 0.6 µM | Fr 3: Met-Arg-Trp | |||||
| Fr 4: 0.38 µM | Fr 4: Leu-Arg-Ile-Pro-Val-Ala | |||||
| Cherry subproduct | Enzymatic hydrolysis with alcalase, flavourzyme and thermolysin | UF (3 and 5 kDa cut-off) | 310 µg/mL2 (thermolysin hydrolyzate) | RP-HPLC-Q-TOF-MS | 21 different peptides | [35] |
| Hemp seed | Enzymatic hydrolysis with alcalase, pepsin, papain and pepsin-pancreatin | SEC | 16–228 µg/mL | 300–9560 Da | [44] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. https://doi.org/10.3390/nu9040316
Daskaya-Dikmen C, Yucetepe A, Karbancioglu-Guler F, Daskaya H, Ozcelik B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients. 2017; 9(4):316. https://doi.org/10.3390/nu9040316
Chicago/Turabian StyleDaskaya-Dikmen, Ceren, Aysun Yucetepe, Funda Karbancioglu-Guler, Hayrettin Daskaya, and Beraat Ozcelik. 2017. "Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants" Nutrients 9, no. 4: 316. https://doi.org/10.3390/nu9040316





