The Effect of a Breakfast Rich in Slowly Digestible Starch on Glucose Metabolism: A Statistical Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Experimental Methods
2.1. Selection Criteria for Clinical Studies
2.2. Characteristics of the Relevant Studies
2.3. Included Subjects
2.4. Test Breakfasts
2.5. Experimental Conditions for Metabolic Responses and Glucose Kinetics
2.6. Statistical Analyses
2.7. Binary Categorization and Meta-Analysis
2.8. Modeling the Relationship between the SDS Content of Products and RaE Kinetics
2.9. Association Rules
3. Results
3.1. SDS Content and RaE
3.2. SDS Content and RdE, RaT, and RdT
3.3. SDS and the Glycemic and Insulinemic Responses
3.4. Association Rules between Glycemic and Insulinemic Responses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blaak, E.E.; Antoine, J.M.; Benton, D.; Bjorck, I.; Bozzetto, L.; Brouns, F.; Diamant, M.; Dye, L.; Hulshof, T.; Holst, J.J.; et al. Impact of postprandial glycaemia on health and prevention of disease. Obes. Rev. 2012, 13, 923–984. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [PubMed]
- Brand-Miller, J.; Hayne, S.; Petocz, P.; Colagiuri, S. Low-glycemic index diets in the management of diabetes: A meta-analysis of randomized controlled trials. Diabetes Care 2003, 26, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Leeds, A.; Trew, G.; Margara, R.; Dornhorst, A. Insulin sensitivity in women at risk of coronary heart disease and the effect of a low glycemic diet. Metab. Clin. Exp. 1998, 47, 1245–1251. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Hulshof, T.; Howlett, J. Glycemic response and health—A systematic review and meta-analysis: Relations between dietary glycemic properties and health outcomes. Am. J. Clin. Nutr. 2008, 87, 258S–268S. [Google Scholar] [PubMed]
- Livesey, G.; Taylor, R.; Livesey, H.; Liu, S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am. Clin. Nutr. 2013, 97, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Rizkalla, S.W.; Taghrid, L.; Laromiguiere, M.; Huet, D.E.; Boillot, J.; Rigoir, A.; Elgrably, F.; Slama, G. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: A randomized controlled trial. Diabetes Care 2004, 27, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk—A meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [PubMed]
- Augustin, L.S.; Franceschi, S.; Jenkins, D.J.A.; Kendall, C.W.C.; La Vecchia, C. Glycemic index in chronic disease: A review. Eur. J. Clin. Nutr. 2002, 56, 1049–1071. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266S–273S. [Google Scholar] [PubMed]
- Salmeron, J.; Ascherio, A.; Rimm, E.B.; Colditz, G.A.; Spiegelman, D.; Jenkins, D.J.; Stampfer, M.J.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997, 20, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, J.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997, 277, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Normand, S.; Khalfallah, Y.; Louche-Pelissier, C.; Pachiaudi, C.; Antoine, J.M.; Blanc, S.; Desage, M.; Riou, J.P.; Laville, M. Influence of dietary fat on postprandial glucose metabolism (exogenous and endogenous) using intrinsically (13)C-enriched durum wheat. Br. J. Nutr. 2001, 86, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Ghafari, H.; Wolever, T.M.; Taylor, R.H.; Jenkins, A.L.; Barker, H.M.; Fielden, H.; Bowling, A.C. Relationship between rate of digestion of foods and post-prandial glycaemia. Diabetologia 1982, 22, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Schenk, S.; Davidson, C.J.; Zderic, T.W.; Byerley, L.O.; Coyle, E.F. Different glycemic indexes of breakfast cereals are not due to glucose entry into blood but to glucose removal by tissue. Am. J. Clin. Nutr. 2003, 78, 742–748. [Google Scholar] [PubMed]
- Vinoy, S.; Lesdéma, A.; Cesbron-Lavau, G.; Goux, A.; Meynier, A. Creating Food Products with a Lower Glycemic Index; CRC Pres: Boca Raton, FL, USA, 2016; Chapter 13; pp. 285–318. [Google Scholar]
- Meynier, A.; Goux, A.L.; Atkinson, F.; Brack, O.; Vinoy, S. Postprandial glycaemic response: How is it influenced by characteristics of cereal products? Br. J. Nutr. 2015, 113, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Korach-André, M.; Roth, H.; Barnoud, D.; Péan, M.; Péronnet, F.; Leverve, X. Glucose appearance in the peripheral circulation and liver glucose output in men after a large 13C starch meal. Am. J. Clin. Nutr. 2004, 80, 881–886. [Google Scholar] [PubMed]
- Livesey, G.; Wilson, P.D.G.; Dainty, J.R.; Brown, J.C.; Faulks, R.M.; Roe, M.A.; Newman, T.A.; Eagles, J.; Mellon, F.A.; Greenwood, R.H. Simultaneous time-varying systemic appearance of oral and hepatic glucose in adults monitored with stable isotopes. Am. J. Physiol. 1998, E717–E728. [Google Scholar] [PubMed]
- Wachters-Hagedoorn, R.E.; Priebe, M.G.; Heimweg, J.A.J.; Heiner, A.M.; Englyst, K.N.; Holst, J.J.; Stellaard, F.; Vonk, R.J. The rate of intestinal glucose absorption is correlated with plasma glucose-dependent insulinotropic polypeptide concentrations in healthy men. J. Nutr. 2006, 136, 1511–1516. [Google Scholar] [PubMed]
- Robertson, M.D.; Livesey, G.; Mathers, J.C. Quantitative kinetics of glucose appearance and disposal following a 13C-labelled starch-rich meal: Comparison of male and female subjects. Br. J. Nutr. 2002, 87, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, Y.; Hagander, B.; Bjorck, I. Metabolic responses to starch in oat and wheat products. On the importance of food structure, incomplete gelatinization or presence of viscous dietary fibre. Eur. J. Clin. Nutr. 1995, 49, 189–199. [Google Scholar] [PubMed]
- Lehmann, U.; Robin, F. Slowly digestible starch—Its structure and health implications: A review. Trends Food Sci. Technol. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Liljeberg, H.; Granfeldt, Y.; Bjorck, I. Metabolic responses to starch in bread containing intact kernels versus milled flour. Eur. J. Clin. Nutr. 1992, 46, S61–S75. [Google Scholar]
- Holm, J.; Bjoerck, I. Bioavailability of starch in various wheat-based bread products: Evaluation of metabolic responses in healthy subjects and rate and extent of in vitro starch digestion. Am. J. Clin. Nutr. 1992, 55, 420–429. [Google Scholar] [PubMed]
- Heaton, K.W.; Marcus, S.N.; Emmett, P.M.; Bolton, C.H. Particle size of wheat, maize, and oat test meals: Effects on plasma glucose and insulin responses and on the rate of starch digestion in vitro. Am. J. Clin. Nutr. 1988, 47, 675–682. [Google Scholar] [PubMed]
- Englyst, K.N.; Vinoy, S.; Englyst, H.N.; Lang, V. Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Br. J. Nutr. 2003, 89, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Veenstra, J.; Hudson, G.J. Measurement of rapidly available glucose (RAG) in plant foods: A potential in vitro predictor of the glycaemic response. Br. J. Nutr. 1996, 75, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Nazare, J.A.; De Rougemont, A.; Normand, S.; Sauvinet, V.; Sothier, M.; Vinoy, S.; Désage, M.; Laville, M. Effect of postprandial modulation of glucose availability: Short- and long-term analysis. Br. J. Nutr. 2010, 103, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Vinoy, S.; Normand, S.; Meynier, A.; Sothier, M.; Louche-Pelissier, C.; Peyrat, J.; Maitrepierre, C.; Nazare, J.A.; Brand-Miller, J.; Laville, M. Cereal processing influences postprandial glucose metabolism as well as the GI effect. J. Am. Col. Nutr. 2013, 32, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Péronnet, F.; Meynier, A.; Sauvinet, V.; Normand, S.; Bourdon, E.; Mignault, D.; St-Pierre, D.H.; Laville, M.; Rabasa-Lhoret, R.; Vinoy, S. Plasma glucose kinetics and response of insulin and GIP following a cereal breakfast in female subjects: Effect of starch digestibility. Eur. J. Clin. Nutr. 2015, 69, 740–745. [Google Scholar] [CrossRef] [PubMed]
- PubMed. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 1 January 2005).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097–e1000097. [Google Scholar] [CrossRef] [PubMed]
- Tissot, S.; Normand, S.; Guilluy, R.; Pachiaudi, C.; Beylot, M.; Laville, M.; Cohen, R.; Mornex, R.; Riou, J.P. Use of a new gas chromatograph isotope ratio mass spectrometer to trace exogenous 13C labelled glucose at a very low level of enrichment in man. Diabetologia 1990, 33, 449–456. [Google Scholar] [CrossRef] [PubMed]
- LaMorte, W.W. Case-Control Studies; Retrieved 2 September 2013; Boston University School of Public Health: Boston, MA, USA, 2013. [Google Scholar]
- Borgelt, C. Frequent item set mining. WIREs Data Min. Knowl. Discov. 2012, 2, 437–456. [Google Scholar] [CrossRef]
- Tufféry Stéphane. Data Mining et Statistique Décisionnelle—Intelligence Des Données; Technip: Paris, France, 2012. [Google Scholar]
- Eelderink, C.; Moerdijk-Poortvliet, T.C.W.; Wang, H.; Schepers, M.; Preston, T.; Boer, T.; Vonk, R.J.; Schierbeek, H.; Priebe, M.G. The glycemic response does not reflect the in vivo starch digestibility of fiber-rich wheat products in healthy men. J. Nutr. 2012, 142, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Eelderink, C.; Schepers, M.; Preston, T.; Vonk, R.J.; Oudhuis, L.; Priebe, M.G. Slowly and rapidly digestible starchy foods can elicit a similar glycemic response because of differential tissue glucose uptake in healthy men. Am. J. Clin. Nutr. 2012, 96, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Priebe, M.G.; Wachters-Hagedoorn, R.E.; Heimweg, J.A.J.; Small, A.; Preston, T.; Elzinga, H.; Stellaard, F.; Vonk, R.J. An explorative study of in vivo digestive starch characteristics and postprandial glucose kinetics of wholemeal wheat bread. Eur. J. Nutr. 2008, 47, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Woolnough, J.W.; Monro, J.A.; Brennan, C.S.; Bird, A.R. Simulating human carbohydrate digestion in vitro: A review of methods and the need for standardisation. Int. J. Food Sci. Technol. 2008, 43, 2245–2256. [Google Scholar] [CrossRef]
- Aller, E.E.J.G.; Abete, I.; Astrup, A.; Alfredo-Martinez, J.; Baak, M.A. Starches, sugars and obesity. Nutrients 2011, 3, 341–369. [Google Scholar] [CrossRef] [PubMed]
- Vinoy, S.; Laville, M.; Feskens, E.J.M. Slow-Release Carbohydrates: Growing Evidence on Metabolic Responses and Public Health Interest. Food Nutr. Res. 2016, 60, 31662. [Google Scholar] [CrossRef] [PubMed]
- Harbis, A.; Perdreau, S.; Vincent-Baudry, S.; Charbonnier, M.; Bernard, M.C.; Raccah, D.; Senft, M.; Lorec, A.M.; Defoort, C.; Portugal, H.; et al. Glycemic and insulinemic meal responses modulate postprandial hepatic and intestinal lipoprotein accumulation in obese, insulin-resistant subjects. Am. J. Clin. Nutr. 2004, 80, 896–902. [Google Scholar] [PubMed]
- Garsetti, M.; Vinoy, S.; Lang, V.; Holt, S.; Loyer, S.; Brand-Miller, J.C. The glycemic and insulinemic index of plain sweet biscuits: Relationships to in vitro starch digestibility. J. Am. Coll. Nutr. 2005, 24, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Colagiuri, S.; Gerich, J.; Tuomilehto, J. Guideline for management of postmeal glucose in diabetes. Diabetes Res. Clin. Pract. 2014, 103, 256–268. [Google Scholar]
- NaAN, E.P. Guidance on the scientific requirements for health claims related to appetite ratings, weight management, and blood glucose concentrations. EFSA J. 2012, 10, 2604–2604. [Google Scholar]
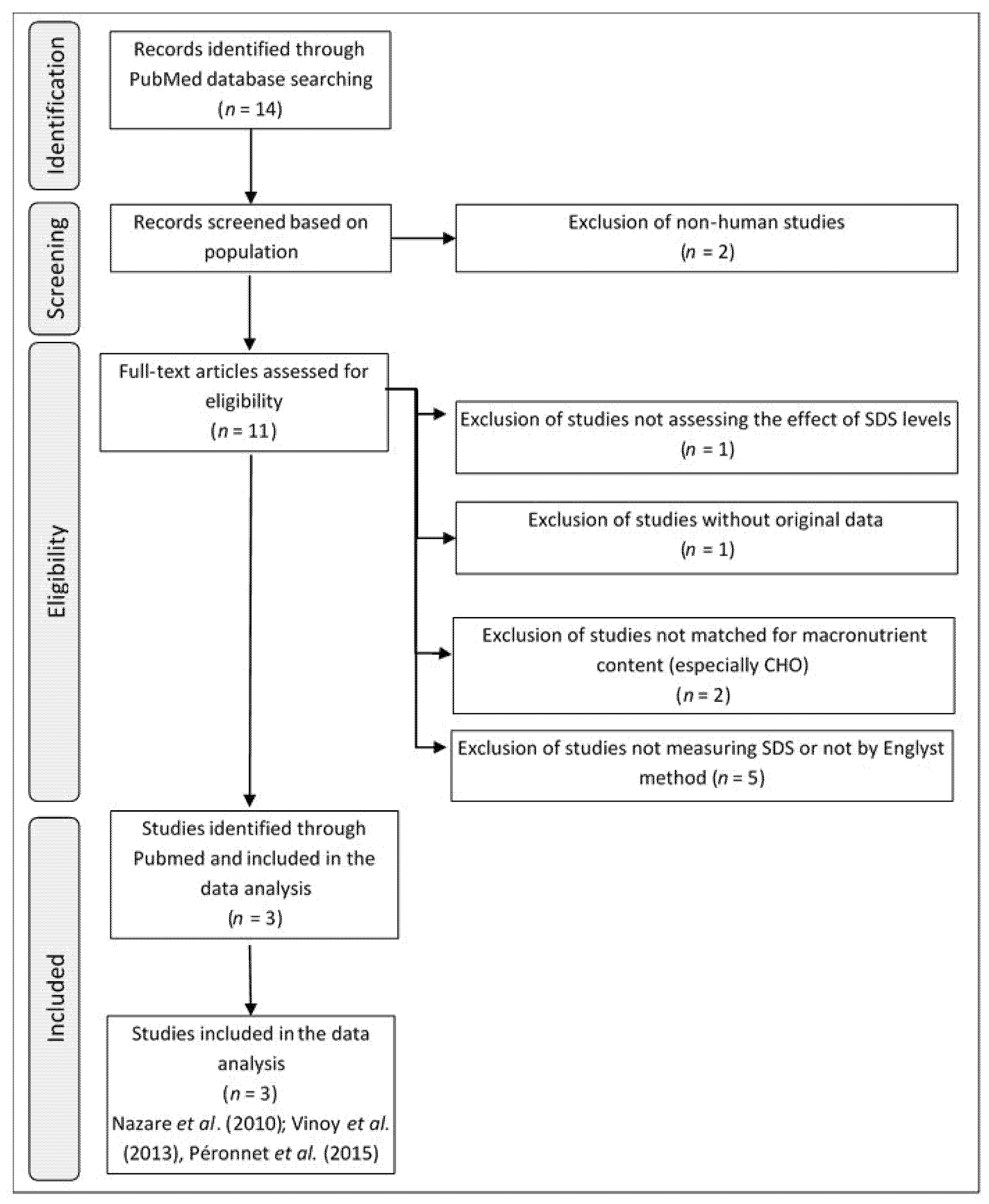
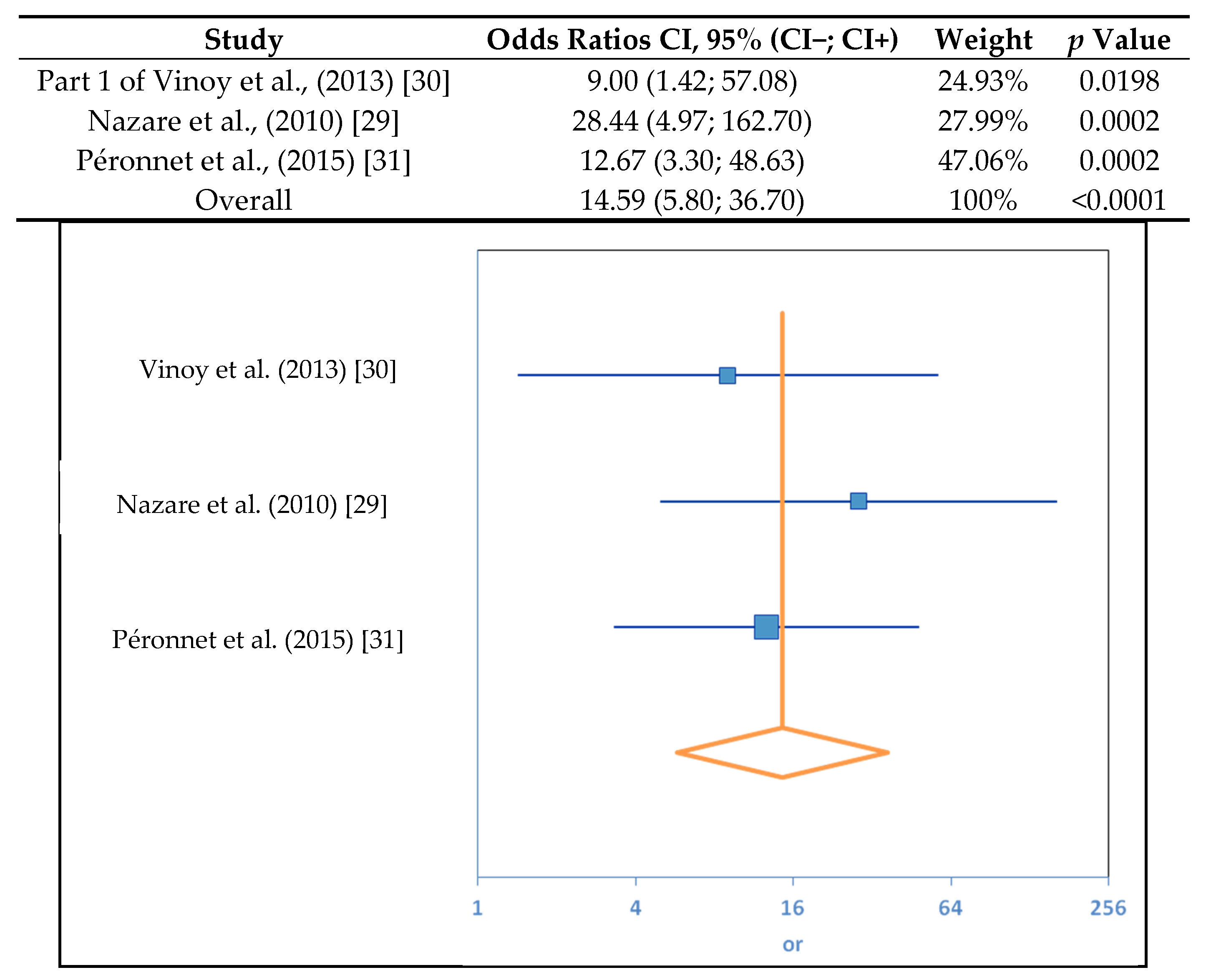
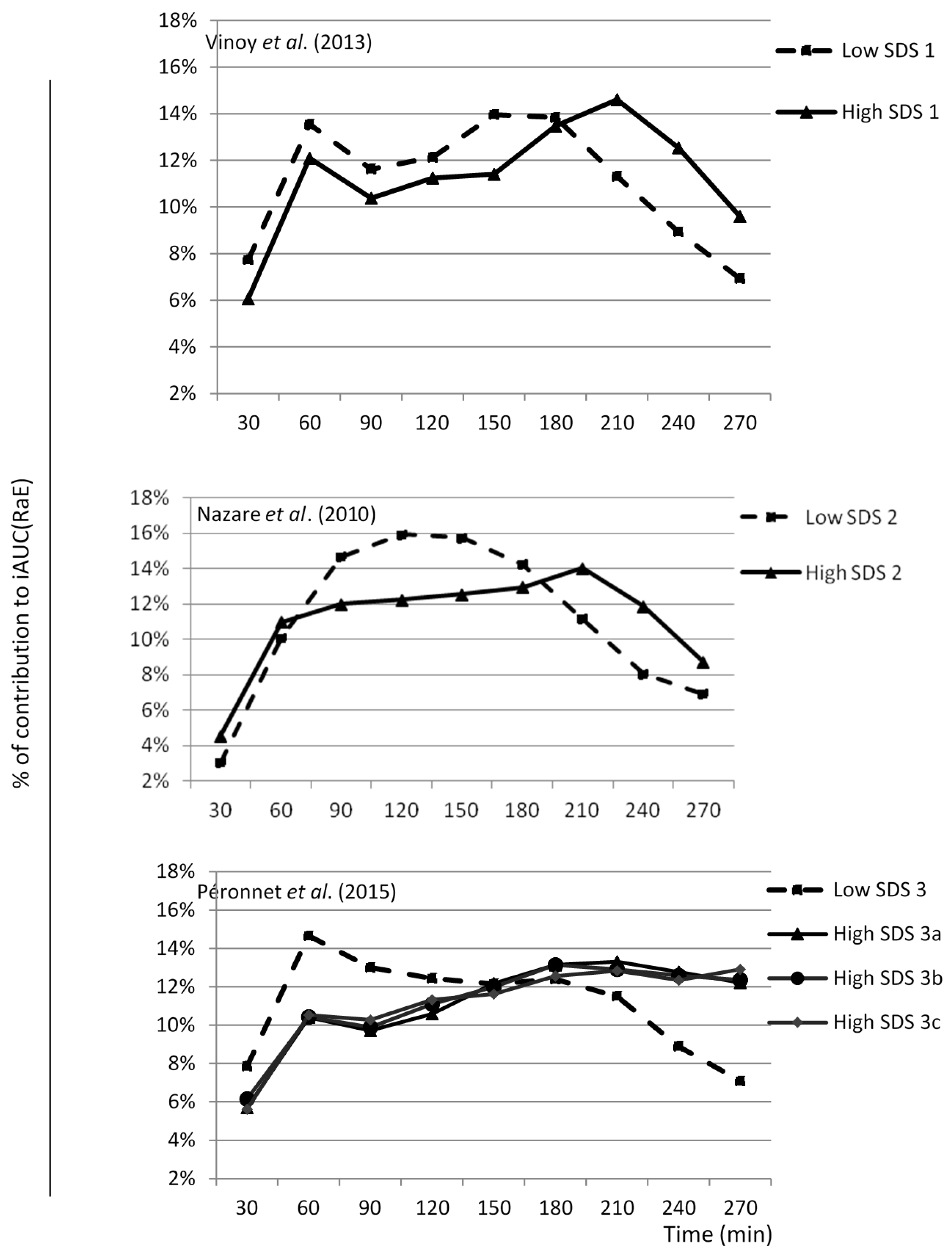
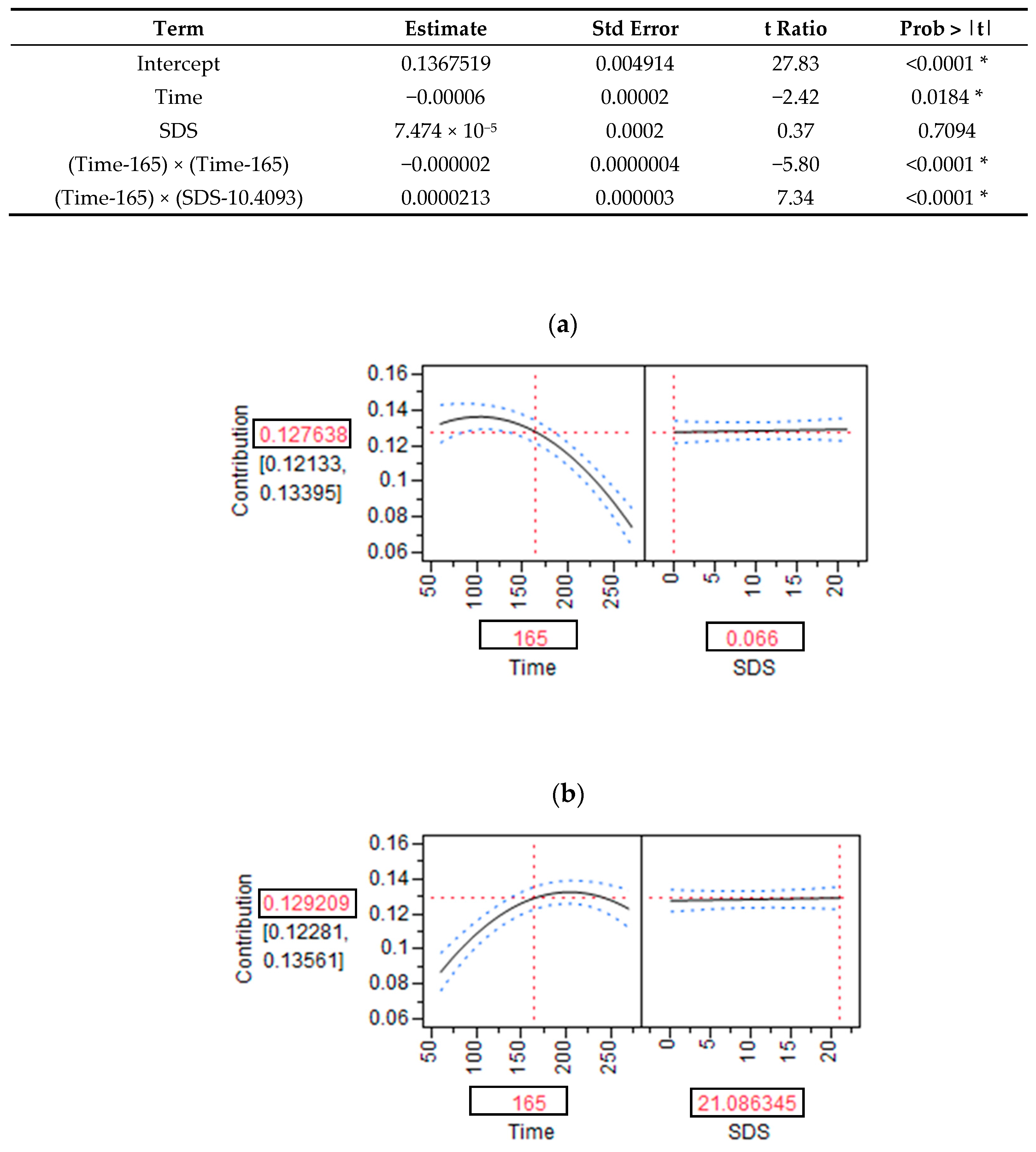
| (a) | ||||
| Publication Reference | Study Design | Population | Adaptation Period | Number of Subjects |
| Nazare et al., 2010. [29] | Randomized, parallel | Non-diabetic overweight men and pre-menopausal women (age: 20–60 years, mean 38.3; BMI: 25–30 kg/m2, mean 27.3 SD 1.5) | None (only day one without adaptation period) | 19 (11 men and 8 women) |
| 19 (9 men and 10 women) | ||||
| Vinoy et al., 2013. [30] | Randomized, open, cross-over (includes two parts) | Healthy subjects (age: 18–40 years, mean 24.0; BMI: 20–25 kg/m2, mean 22.6 SD 1.8) | 3 week adaptation to tested products * | 12 men (part one—isotope measurements and metabolic measurements) 13 men (part 2—metabolic measurements only) |
| Péronnet et al., 2015. [31] | Randomized, Open-label, cross-over | Young, lean, healthy subjects (age: 19–26 years, mean 22.0; BMI: 20.2–24.4 kg/m2, mean 22.0 SD 1.5) | None | 16 women |
| (b) | ||||
| Pooled Experiments | Number of Subjects | Age (Years) Mean ± SD (Min – Max) − Median | BMI (kg/m2) Mean ± SD (Min – Max) − Median | Weight (kg) Mean ± SD (Min – Max) − Median |
| Glucose kinetics measure by isotopic method | N = 66 * (34 women and 32 men) | 32.0 ± 10.4 (19–57) − 29 | 25.1 ± 3.0(20.2–30.7) − 25.9 | 71.6 ± 10.8 (52.6–91.2) − 70.0 |
| Metabolic responses | N = 79 ** (34 women and 45 men) | 30.6 ± 10.1 (19–57) – 26.0 | 24.8 ± 2.9 (20.2–30.7) – 25.0 | 72.7 ± 10.1 (52.6–98.5) − 71.9 |
| Publication Reference | Type of Cereal Products | Product Portion Size | Quantity of SDS Per Portion of Product | Foods Included in the Breakfasts Other Than Cereal Products | Composition of the Whole Breakfast | ||||
|---|---|---|---|---|---|---|---|---|---|
| Energy (kcal) | CHO g (% E) | Proteins g (% E) | Fat g (% E) | ||||||
| Nazare et al., 2010 [29] | High-SDS product (biscuit) | 80 g | 21 g | Semi-skimmed milk | 432 | 68 (62) | 13 (12) | 13 (26) | |
| Low-SDS product (extruded) | 80 g | 0.4 g | 429 | 72 (62) | 13 (12) | 11 (26) | |||
| Vinoy et al., 2013 [30] | High-SDS product (biscuit) | 70 g | 12 g | Semi-skimmed milk | Tea or coffee without sugar or with intense sweetener | 435 | 62 (57) | 12 (11) | 15 (32) |
| Low-SDS product (extruded) | 70 g | 0.9 g | Whole milk | 450 | 68 (61) | 12 (11) | 14 (28) | ||
| Péronnet et al., 2015 [31] | High-SDS product 1 (biscuit) | 72 g | 15 g | Skimmed milk | Tea or coffee without sugar or with intense sweetener | 380 | 58 (61) | 10 (10) | 12 (29) |
| High-SDS product 2 (biscuit) | 72 g | 17 g | 378 | 58 (62) | 10 (10) | 12 (28) | |||
| High-SDS product 3 (biscuit) | 72 g | 16 g | 379 | 58 (62) | 10 (10) | 12 (28) | |||
| Low-SDS product (extruded) | 66 g | 0.1 g | Skimmed milk with blend of added fats | 381 | 58 (61) | 10 (10) | 12 (29) | ||
| (a) | ||||
| Study | OR | CI | Weight | p |
| Part one of Vinoy et al., 2013 [30] | 9.00 | (1.42; 57.12) | 25.22% | 0.0198 |
| Nazare et al., 2010 [29] | 72.25 | (9.10; 573.74) | 20.06% | 0.00005 |
| Péronnet et al., 2015 [31] | 7.20 | (2.05; 25.24) | 54.73% | 0.0020 |
| 12.09 | (4.78; 30.59) | 100% | 0.0001 | |
| (b) | ||||
| Study | OR | CI | Weight | p |
| Part one of Vinoy et al., 2013 [30] | 1.96 | (0.39; 9.93) | 22.13% | 0.4164 |
| Nazare et al., 2010 [29] | 2.94 | (0.79; 10.99) | 33.54% | 0.1088 |
| Péronnet et al., 2015 [31] | 1.89 | (0.60; 5.95) | 44.33% | 0.2766 |
| 2.21 | [1.03; 4.74] | 100% | 0.0418 | |
| (c) | ||||
| Study | OR | CI | Weight | p |
| Part one of Vinoy et al., 2013 [30] | 4.00 | (0.73; 21.83) | 22.24% | 0.1094 |
| Nazare et al., 2010 [29] | 4.69 | (1.19; 18.42) | 34.23% | 0.0268 |
| Péronnet et al., 2015 [31] | 3.88 | (1.15; 13.05) | 43.53% | 0.0285 |
| 4.17 | (1.87; 9.28) | 100.00% | 0.0005 | |
| Study | OR | CI | Weight | p |
|---|---|---|---|---|
| Part one of Vinoy et al., 2013 [30] | 2.00 | (0.38; 10.41) | 21.66% | 0.4102 |
| Nazare et al., 2010 [29] | 6.43 | (1.52; 27.24) | 28.26% | 0.0116 |
| Part two of Vinoy et al., 2013 [30] | 7.50 | (1.31; 43.03) | 19.31% | 0.0237 |
| Péronnet et al., 2015 [31] | 6.39 | (1.60; 25.48) | 30.77% | 0.0086 |
| 5.13 | (2.38; 11.06) | 100% | 0.0001 |
| Association Rule | Support (%) | Confidence (%) | Lift |
|---|---|---|---|
| {Low glycemic iAUC}→low insulinemic iAUC | 39.4 | 73.4 | 1.352 |
| {High glycemic iAUC}→high insulinemic iAUC | 31.4 | 67.9 | 1.485 |
| {Low insulinemic iAUC}→low glycemic iAUC | 39.4 | 72.6 | 1.352 |
| {High insulinemic iAUC}→high glycemic iAUC | 31.4 | 68.8 | 1.485 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinoy, S.; Meynier, A.; Goux, A.; Jourdan-Salloum, N.; Normand, S.; Rabasa-Lhoret, R.; Brack, O.; Nazare, J.-A.; Péronnet, F.; Laville, M. The Effect of a Breakfast Rich in Slowly Digestible Starch on Glucose Metabolism: A Statistical Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 318. https://doi.org/10.3390/nu9040318
Vinoy S, Meynier A, Goux A, Jourdan-Salloum N, Normand S, Rabasa-Lhoret R, Brack O, Nazare J-A, Péronnet F, Laville M. The Effect of a Breakfast Rich in Slowly Digestible Starch on Glucose Metabolism: A Statistical Meta-Analysis of Randomized Controlled Trials. Nutrients. 2017; 9(4):318. https://doi.org/10.3390/nu9040318
Chicago/Turabian StyleVinoy, Sophie, Alexandra Meynier, Aurélie Goux, Nathalie Jourdan-Salloum, Sylvie Normand, Rémi Rabasa-Lhoret, Olivier Brack, Julie-Anne Nazare, François Péronnet, and Martine Laville. 2017. "The Effect of a Breakfast Rich in Slowly Digestible Starch on Glucose Metabolism: A Statistical Meta-Analysis of Randomized Controlled Trials" Nutrients 9, no. 4: 318. https://doi.org/10.3390/nu9040318





