Inhibitory Effect of Lychee Seed Saponins on Apoptosis Induced by Aβ25-35 through Regulation of the Apoptotic and NF-κB Pathways in PC12 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Cell Culture
2.3. Collection, Isolation, and Extraction of LSS
2.4. Establishment of an Apoptotic Cell Model of PC12 Cells Induced by Aβ25-35 and Drug Treatment In Vitro
2.5. Apoptotic Analysis of PC12 Cells by Hoechst 33342/PI Fluorescence Double Staining
2.6. Apoptotic Analysis of PC12 Cell by Annexin V/PI Double Staining
2.7. Apoptotic Analysis of PC12 Cells by TUNEL
2.8. Mitochondrial Membrane Potential Examination in PC12 Cells
2.9. Western Blotting Analysis of the Protein Expressions of Bcl-2 and Bax in PC12 Cells
2.10. RNA Isolation and Analysis of NF-κBp65 mRNA Expression in PC12 Cells by RT-PCR
2.11. Nuclear Translocation of NF-κBp65 in PC12 Cells by Immunofluorescence Analysis
2.12. Statistical Analysis
3. Results
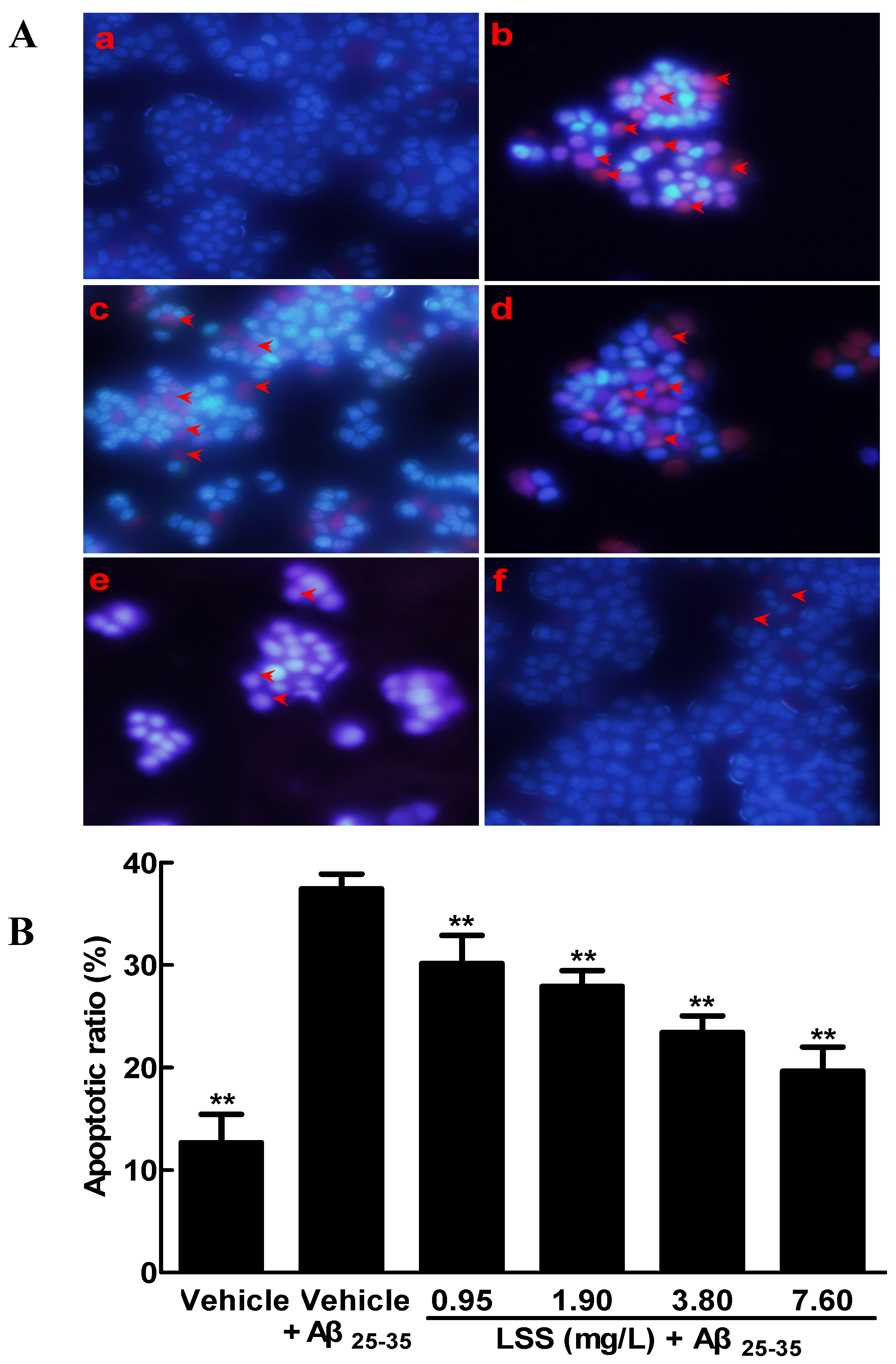
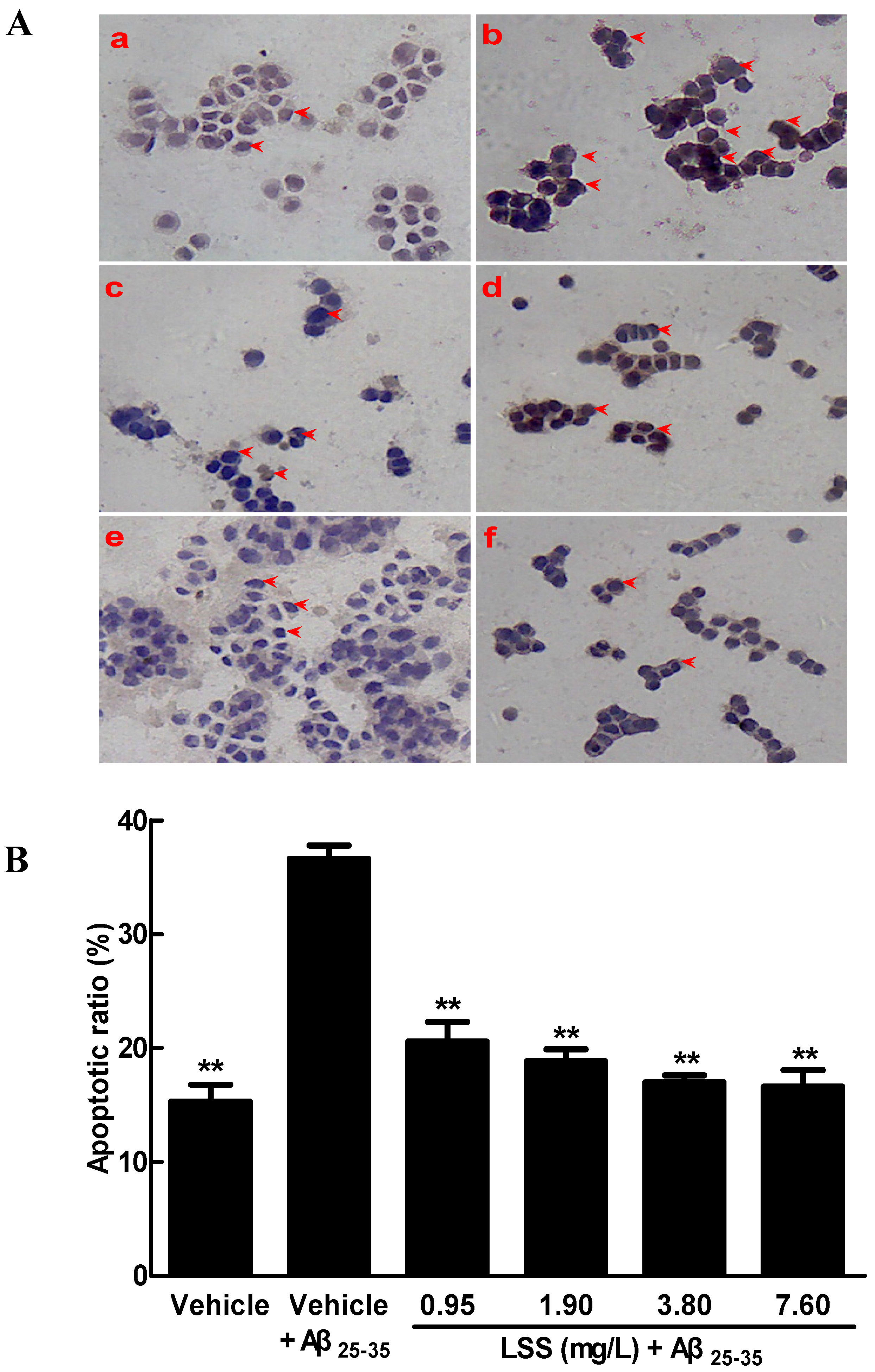
3.1. Effects of LSS on the Aβ25-35-Induced Apoptosis in PC12 Cells by Hoechst 33342/PI Fluorescence Double Staining, Annexin V/PI Double Staining, and TUNEL Analyses
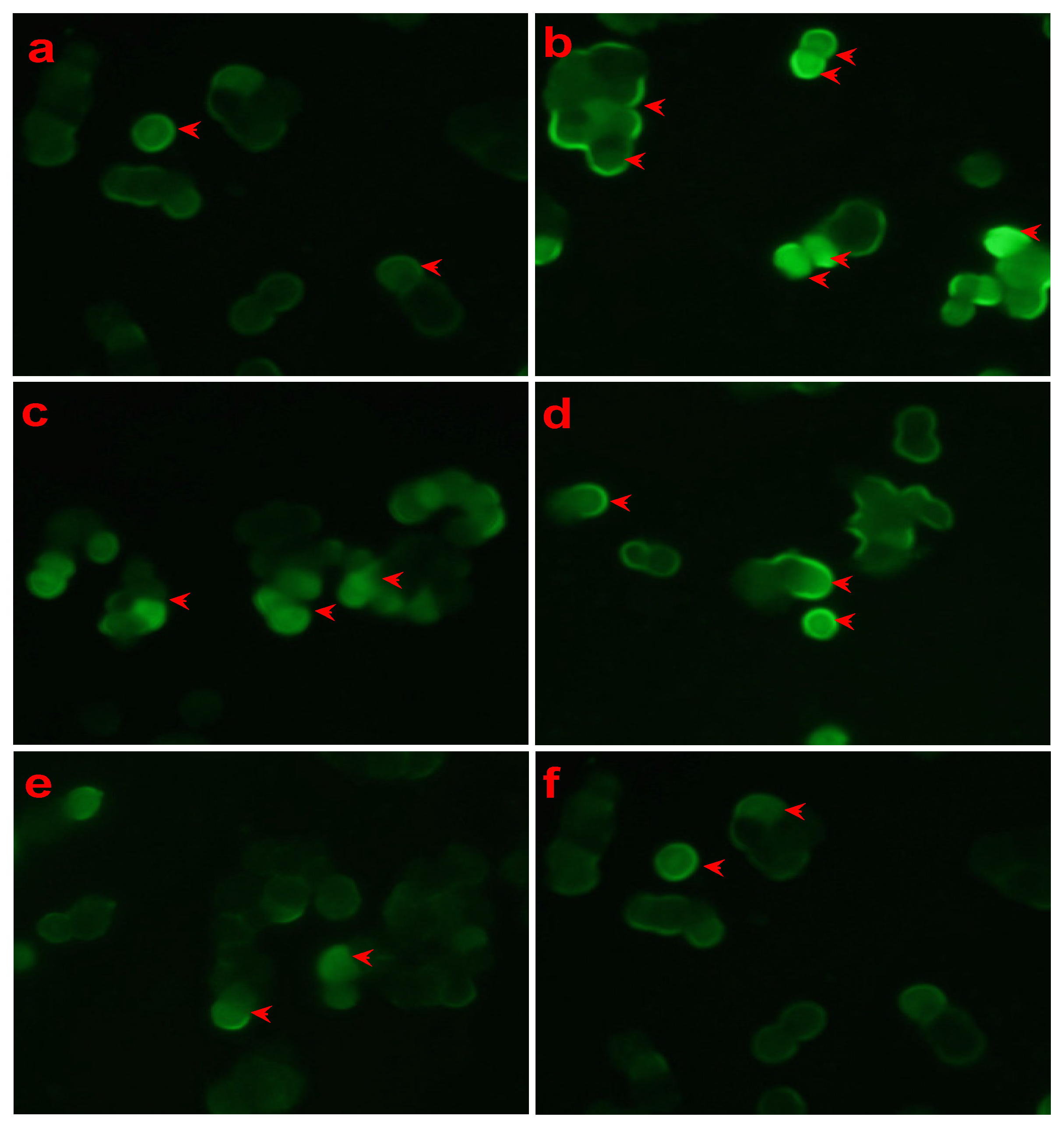
3.2. Effect of LSS on Mitochondrial Membrane Potential in PC12 Cells Exposed to Aβ25-35 with Rhodamine 123 Staining
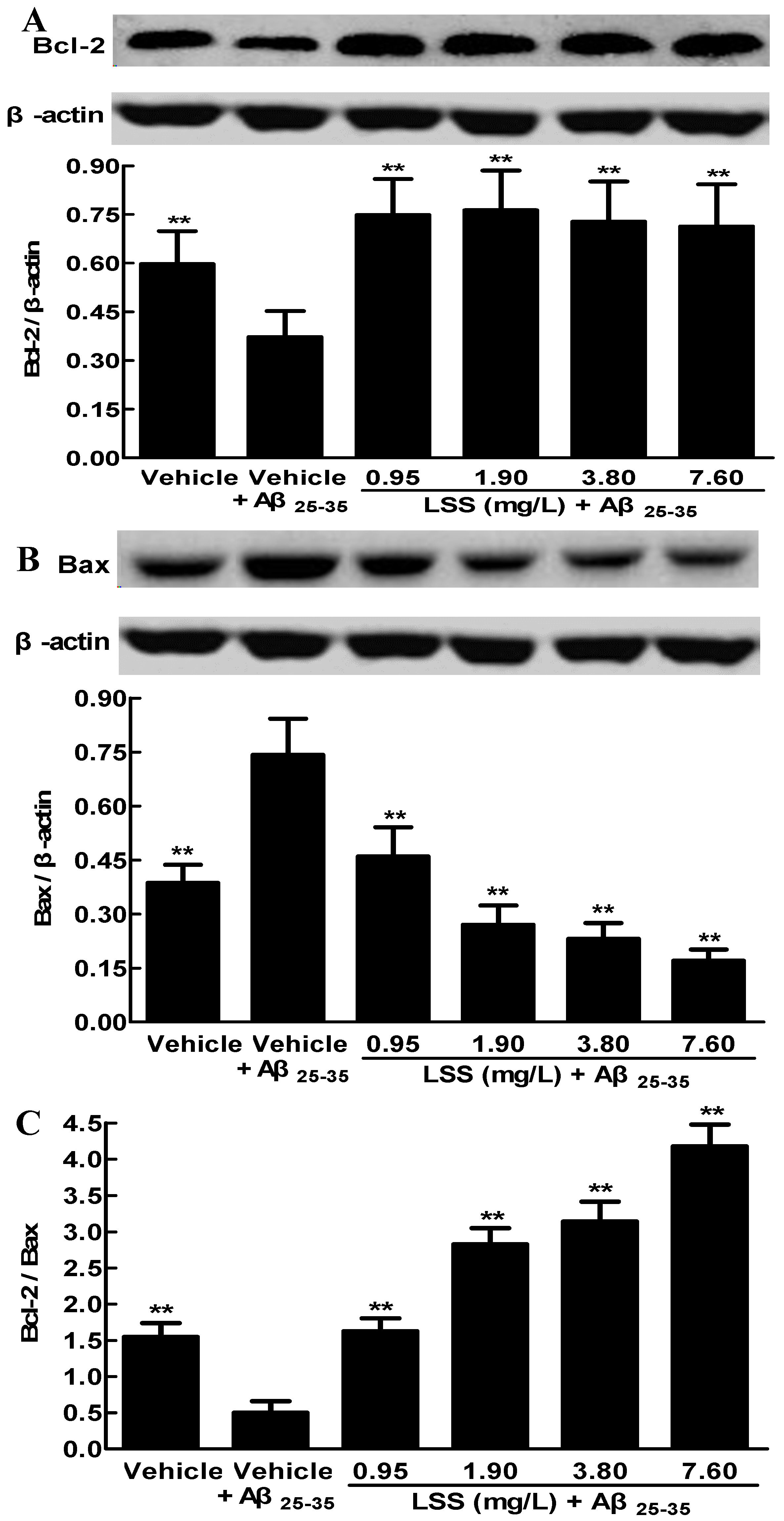
3.3. Effects of LSS on the Protein Expressions of Bcl-2 and Bax in PC12 Cells Induced by Aβ25-35
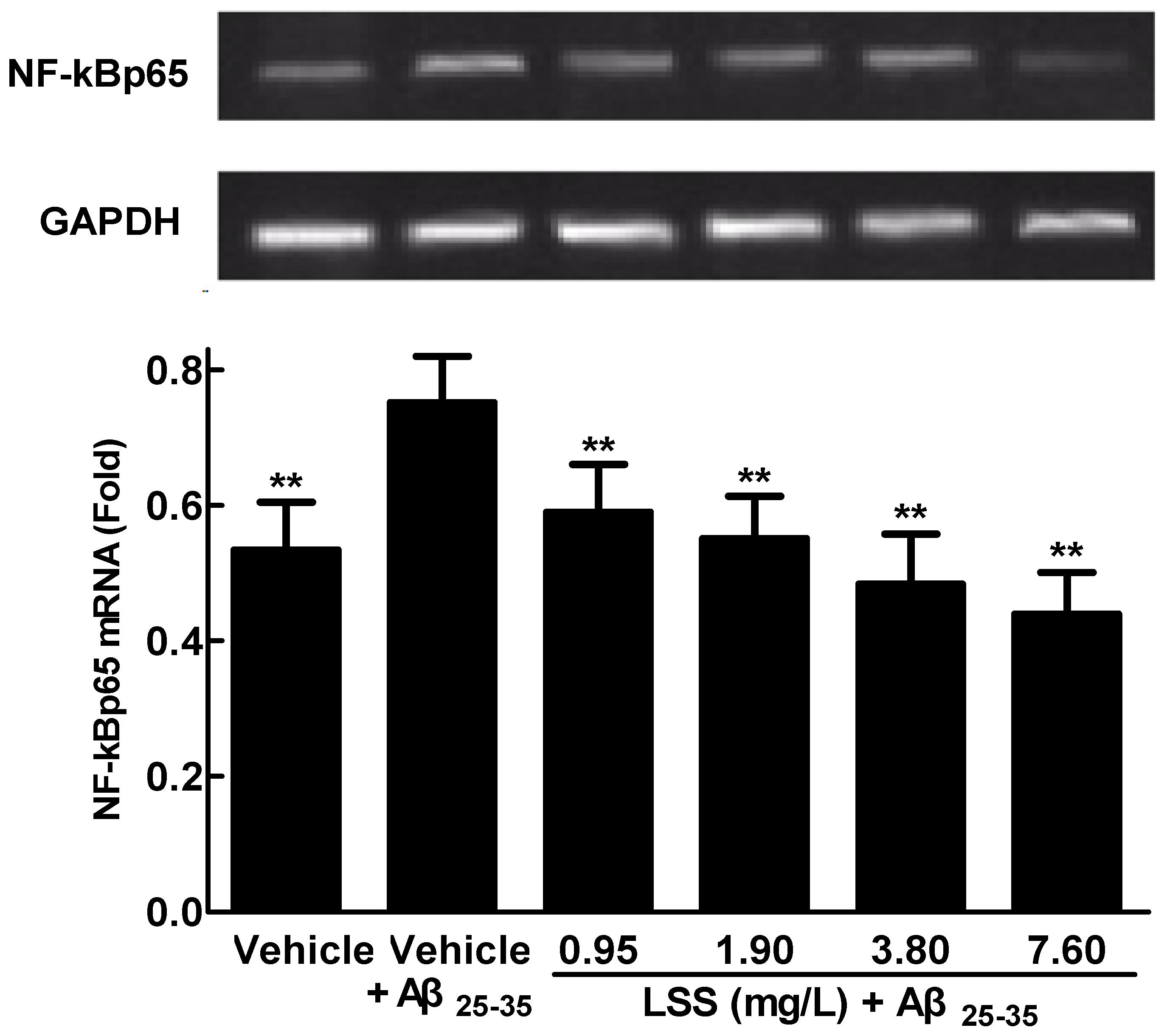
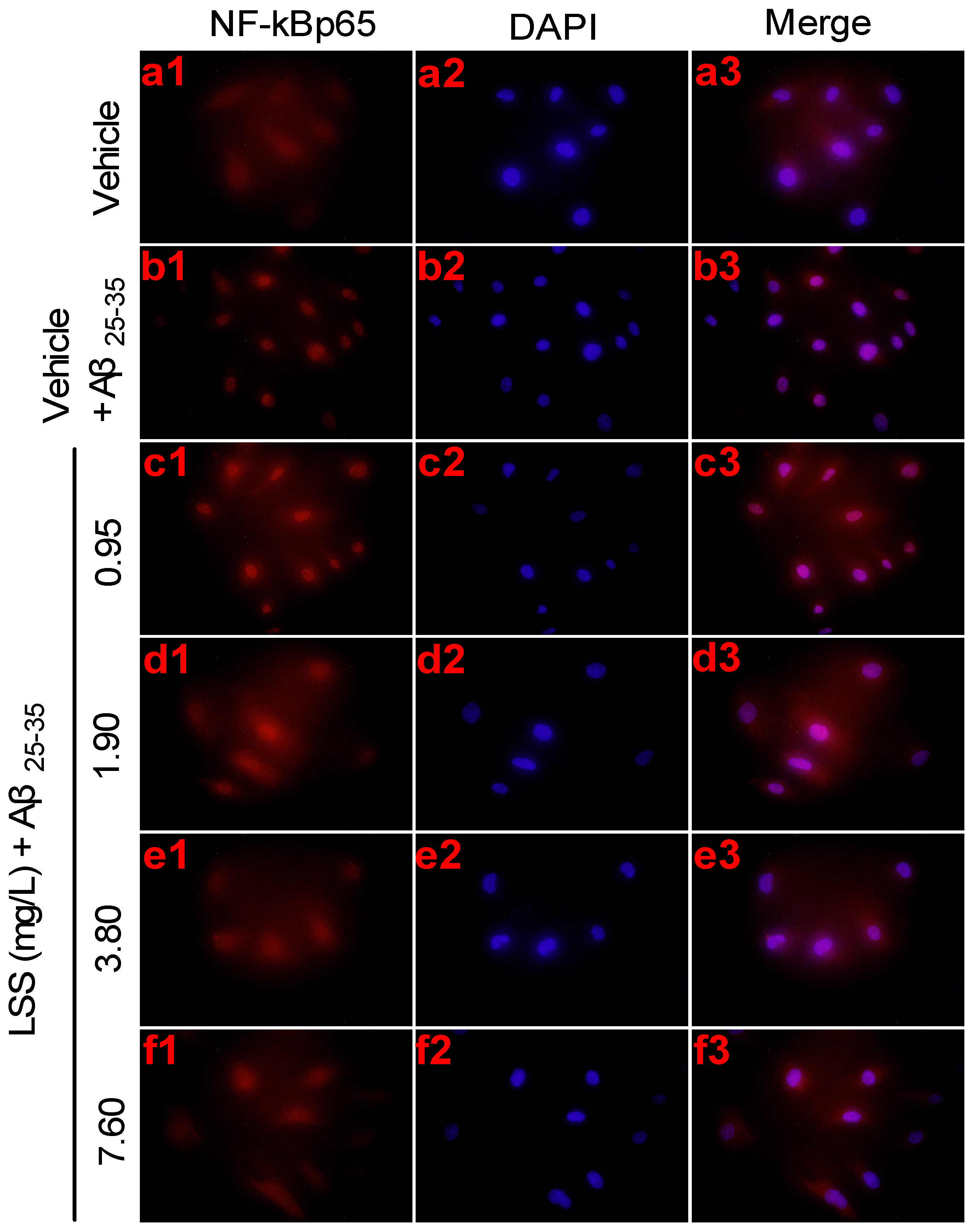
3.4. Effects of LSS on mRNA Expression and Nuclear Translocation of NF-κBp65 in PC12 Cells Treated with Aβ25-35
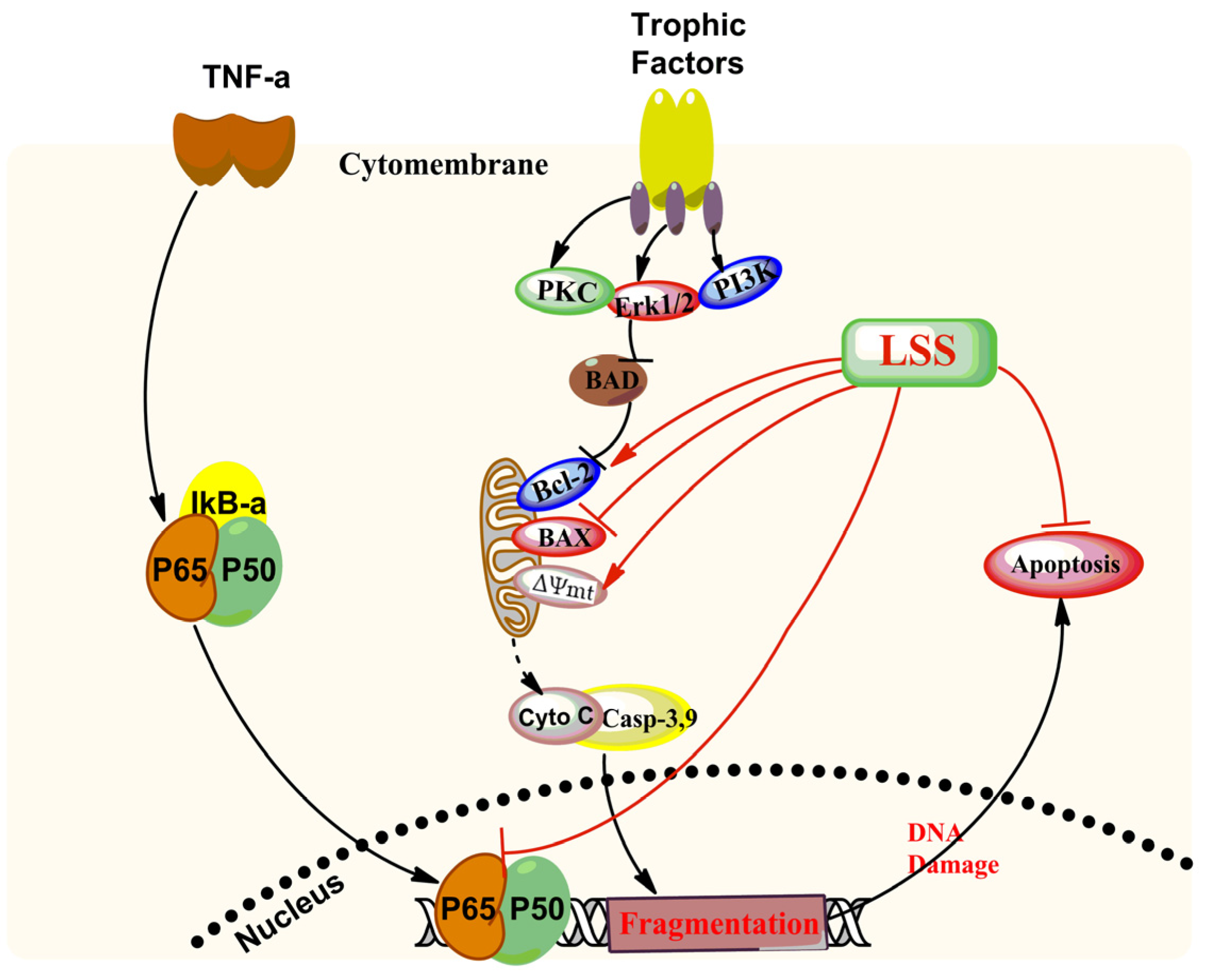
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Takata, K.; Takada, T.; Ito, A.; Asai, M.; Tawa, M.; Saito, Y.; Ashihara, E.; Tomimoto, H.; Kitamura, Y.; Shimohama, S. Microglial Amyloid-β1-40 phagocytosis dysfunction is caused by high-mobility group box protein-1: Implications for the pathological progression of Alzheimer’s disease. Int. J. Alzheimers Dis. 2012, 2012, 685739. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Yan, L.; Huang, L.Q.; Huang, S.Q.; Zhuang, J.H.; Chen, X.Y.; Lin, Z.; Wu, H.J.; Shao, F.Y.; Zhao, Z.X. Anti-apoptosis effect of astragaloside IV on Alzheimer’s disease rat model via enhancing the expression of Bcl-2 and Bcl-xl. Scand. J. Lab. Anim. Sci. 2010, 37, 75–82. [Google Scholar]
- Yadav, R.S.; Tiwari, N.K. Lipid integration in neurodegeneration: An overview of Alzheimer’s disease. Mol. Neurobiol. 2014, 50, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Xing, G.; Zhou, L.; Dong, M.; Geng, Y.; Li, X.; Li, J.; Wang, G.; Zou, D. Beta-asarone attenuates neuronal apoptosis induced by Beta amyloid in rat hippocampus. Yakugaku Zasshi 2010, 130, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, C.; Quan, R.; Xie, S. The effect of electroacupuncture on neuronal apoptosis and related functions in rats with acute spinal cord injury. Chin. Med. 2014, 5, 199–210. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and oxidative stress in neurodegenerative diseases. J. Alzheimers Dis. 2014, 42, S125–S152. [Google Scholar] [PubMed]
- Kang, D.E.; Roh, S.E.; Woo, J.A.; Liu, T.; Bu, J.H.; Jung, A.R.; Lim, Y. The Interface between cytoskeletal aberrations and mitochondrial dysfunction in Alzheimer's disease and related disorders. Exp. Neurobiol. 2011, 20, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Thakur, V.; Singh, S.N.; Guleria, R. Tumor necrosis factor and Alzheimer’s disease: A cause and consequence relationship. Klin. Psikofarmakol. Bul. 2012, 22, 86–97. [Google Scholar] [CrossRef]
- Mei, J.M.; Niu, C.S. Effects of CDNF on 6-OHDA-induced apoptosis in PC12 cells via modulation of Bcl-2/Bax and caspase-3 activation. Neurol. Sci. 2014, 35, 1275–1280. [Google Scholar] [PubMed]
- Zhang, H.; Wu, S.; Xing, D. Inhibition of Aβ25-35-induced cell apoptosis by Low-power-laser-irradiation (LPLI) through promoting Akt-dependent YAP cytoplasmic translocation. Cell Signal. 2012, 24, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kim, M.O. Melatonin ameliorates amyloid beta-induced memory deficits, tau hyperphosphorylation and neurodegeneration via PI3/Akt/GSk3β pathway in the mouse hippocampus. J. Pineal. Res. 2015, 59, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Fujishiro, N.; Imanaga, I.; Sakamoto, Y. Role of ATP decrease in secretion induced by mitochondrial dysfunction in guinea-pig adrenal chromaffin cells. J. Physiol. 2002, 539, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Marsillach, J.; Ferré, N.; Camps, J.; Riu, F.; Rull, A.; Joven, J. Moderately high folic acid supplementation exacerbates experimentally induced liver fibrosis in rats. Exp. Biol. Med. 2008, 233, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Obulesu, M.; Lakshmi, M.J. Apoptosis in Alzheimer’s disease: An understanding of the physiology, pathology and therapeutic avenues. Neurochem. Res. 2014, 39, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, C. Research progress on the antineoplastic pharmacological effects and mechanisms of litchi seeds. Chin. Med. 2015, 6, 20–26. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, B.; Yang, S.; Chen, Y.; Zhao, M.; Ashraf, M.; Jiang, Y. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi ( Litchi sinensis Sonn.) seeds. Food Chem. 2009, 116, 1–7. [Google Scholar] [CrossRef]
- Xu, X.; Xie, H.; Wang, Y.; Wei, X. A-type proanthocyanidins from lychee seeds and their antioxidant and antiviral activities. J. Agric. Food Chem. 2010, 58, 11667–11672. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.I.; Zhu, Y.T.; Huang, Z.Y.; He, J.J.; Pei, J. Experimental studies on anti-fluvirus effect of litchi seed in vivo. Chin. J. Ethnomed. Ethnopharm. 2011, 9, 34–36. [Google Scholar]
- Xiao, L.; Pan, J.; Rao, W. The research of protective effect of litchi seed of experimental liver injury in mice. China J. Tradit. Chin. Med. Pharm. 2005, 20, 42–43. [Google Scholar]
- Deng, Z.J.; Guo, J.W.; Pan, J.Q. Pharmacologic and pharmacodynamic effects of effective element of litchi and litchi seed. Pharma. Today 2009, 19, 7–9. [Google Scholar]
- Xiao, Z.; Guo, J.; Feng, X.U.; Pharmacy, D.O.; Hospital, F.C. Effect of litchi saponin and litchi flavones on insulin resistance in HepG2 cells. J. Pharma. Pract. 2015, 33, 316–318. [Google Scholar]
- Yaidikar, L.; Thakur, S. Arjunolic acid, a pentacyclic triterpenoidal saponin of Terminalia Arjuna bark protects neurons from oxidative stress associated damage in focal cerebral ischemia and reperfusion. Pharmacol. Rep. 2015, 67, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Yan, X.D.; Qi, L.S.; Li, L.; Hu, G.Y.; Li, P.; Zhao, G. Ginsenoside Rd attenuates Abeta25-35-induced oxidative stress and apoptosis in primary cultured hippocampal neurons. Chem. Biol. Interact. 2015, 239, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q. The intervention of Lychee seed saponins on rats with Alzheimer’s disease. Luzhou Med. Coll. 2010, 33, 1–3. [Google Scholar]
- Qian, X.; Cao, H.; Ma, Q.; Wang, Q.; He, W.; Qin, P.; Ji, B.; Yuan, K.; Yang, F.; Liu, X. Allopregnanolone attenuates Aβ25-35-induced neurotoxicity in PC12 cells by reducing oxidative stress. Int. J. Clin. Exp. Med. 2015, 8, 13610–13615. [Google Scholar] [PubMed]
- Hu, Z.; Guan, W.; Wang, W.; Huang, L.; Xing, H.; Zhu, Z. Synthesis of b-alanine C60 derivative and its protective effect on hydrogen peroxide-induced apoptosis in rat pheochromocytoma cells. Cell Biol. Int. 2007, 31, 798–804. [Google Scholar] [PubMed]
- Schutte, B.; Nuydens, R.; Geerts, H.; Ramaekers, F. Annexin V binding assay as a tool to measure apoptosis indifferentiated neuronal cells. J. Neurosci. Meth. 1998, 86, 63–69. [Google Scholar] [CrossRef]
- Jang, J.H.; Aruoma, O.I.; Jen, L.S.; Chung, H.Y.; Surh, Y.J. Ergothioneine rescues PC12 cells from β-amyloid-induced apoptotic death. Free Radic. Biol. Med. 2004, 36, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Myhre, O.; Utkilen, H. Metal dyshomeostasis and inflammation in Alzheimer’s and Parkinson’s diseases: Possible impact of environmental exposures. Oxid. Med. Cell. Longev. 2013, 2013, 726954. [Google Scholar] [PubMed]
- Yagami, T.; Ueda, K.; Sakaeda, T.; Itoh, N.; Sakaguchi, G.; Okamura, N.; Hori, Y.; Fujimoto, M. Protective effects of a selective l-type voltage-sensitive calcium channel blocker, S-312-d, on neuronal cell death. Biochem. Pharmacol. 2004, 67, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.; Ferreiro, E.; Pereira, C.; Oliveira, C.R. ER stress is involved in Aβ-induced GSK-3β activation and tau phosphorylation. J. Neurosci. Res. 2008, 86, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.Y.; Lei, Y.; Tang, X.C.; Zhang, H.Y. Donepezil attenuates Aβ-associated mitochondrial dysfunction and reduces mitochondrial Aβ accumulation in vivo and in vitro. Neuropharmacology 2015, 95, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Teng, J. β-asarone prevents Aβ25-35-induced inflammatory responses and autophagy in SH-SY5Y cells: Down expression Beclin-1, LC3B and up expression Bcl-2. Int. J. Clin. Exp. Med. 2015, 8, 20658–20663. [Google Scholar] [PubMed]
- Xu, J.; Zhang, R.; Zuo, P.; Yang, N.; Ji, C.; Liu, W.; Wang, Y.; Wang, H.; Wu, A.; Yue, Y. Aggravation effect of isoflurane on Aβ(25-35)-induced apoptosis and tau hyperphosphorylation in PC12 cells. Cell Mol. Neurobiol. 2012, 32, 1343–1351. [Google Scholar] [PubMed]
- Tian, X.Q.; Zhang, L.D.; Wang, J.M.; Dai, J.G.; Shen, S.S.; Yang, L.; Huang, P.L. The protective effect of hyperbaric oxygen and Ginkgo biloba extract on Aβ25-35-induced oxidative stress and neuronal apoptosis in rats. Behav. Brain Res. 2013, 242, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jazvinšćak, J.M.; Hof, P.R.; Šimić, G. Ceramides in Alzheimer’s disease: Key Mediators of Neuronal Apoptosis Induced by Oxidative Stress and Aβ Accumulation. Oxid. Med. Cell. Longev. 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cavallucci, V.; Ferraina, C.; D’Amelio, M. Key role of mitochondria in Alzheimer’s disease synaptic dysfunction. Curr. Pharm. Des. 2013, 19, 6440–6450. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, L.; Sun, M.; Zhou, T.; Zhong, K.; Wang, H.; Liu, Y.; Liu, X.; Xiao, R.; Ge, J. Xylocoside G reduces amyloid-β induced neurotoxicity by inhibiting NF-κB signaling pathway in neuronal cells. J. Alzheimers Dis. 2012, 30, 263–275. [Google Scholar] [PubMed]
- Schmitt, K.; Brown, S.; Frank, S.; Eckert, A. Mitochondrial dynamics in Alzheimer’s disease. Drug Aging 2010, 27, 181–192. [Google Scholar] [CrossRef]
- Morais, V.A.; De, S.B. Mitochondria dysfunction and neurodegenerative disorders: Cause or consequence. J. Alzheimers Dis. 2010, 20, S255–S263. [Google Scholar] [PubMed]
- Kadenbach, B.; Arnold, S.; Lee, I.; Hüttemann, M. The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim. Biophys. Acta 2004, 1655, 400–408. [Google Scholar] [PubMed]
- Bartley, M.G.; Marquardt, K.; Kirchhof, D.; Wilkins, H.M.; Patterson, D.; Linseman, D.A. Overexpression of amyloid-β protein precursor induces mitochondrial oxidative stress and activates the intrinsic apoptotic cascade. J. Alzheimers Dis. 2012, 28, 855–868. [Google Scholar] [PubMed]
- Yu, W.; Mechawar, N.; Krantic, S.; Quirion, R. Evidence for the involvement of apoptosis-inducing factor–mediated caspase-independent neuronal death in Alzheimer disease. Am. J. Pathol. 2010, 176, 2209–2218. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Inagaki, Y.; Liu, Y. Research progress on flavonoids isolated from traditional Chinese medicine in treatment of Alzheimer’s disease. Intract. Rare Dis. Res. 2013, 2, 3–10. [Google Scholar]
- Gong, Q.H.; Shi, X.R.; Hong, Z.Y.; Pan, L.L.; Liu, X.H.; Zhu, Y.Z. A new hope for neurodegeneration: Possible role of hydrogen sulfide. J. Alzheimers Dis. 2011, 24, 173–182. [Google Scholar] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Puangmalai, N.; Somani, A.; Thangnipon, W.; Ballard, C.; Broadstock, M. A genetically immortalized human stem cell line: A promising new tool for Alzheimer’s disease therapy. EXCLI J. 2015, 14, 1135–1144. [Google Scholar] [PubMed]
- Li, N.; Liu, B.; Dluzen, D.E.; Jin, Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J. Ethnopharmacol. 2007, 111, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Zhang, H.; Liu, J.; Zhang, Z.; Wang, L.; Yin, H. Cytotoxicity of Lychee seed saponins on normal PC12 cell and determination of their effective concentration to inhibit cytotoxicity of Aβ. J. Luzhou Med. Coll. 2011, 34, 594–598. [Google Scholar]
- Wang, X.; Wu, J.; Yu, C.; Tang, Y.; Liu, J.; Chen, H.; Jin, B.; Mei, Q.; Cao, S.; Qin, D. Lychee seed saponins improve cognitive function and prevent neuronal injury via inhibiting neuronal apoptosis in a rat model of Alzheimer’s disease. Nutrients 2017, 9, 105. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, H.; Liu, J.; Chen, R.; Tang, Y.; Chen, H.; Gu, L.; Li, M.; Cao, S.; Qin, D.; et al. Inhibitory Effect of Lychee Seed Saponins on Apoptosis Induced by Aβ25-35 through Regulation of the Apoptotic and NF-κB Pathways in PC12 Cells. Nutrients 2017, 9, 337. https://doi.org/10.3390/nu9040337
Wang X, Zhang H, Liu J, Chen R, Tang Y, Chen H, Gu L, Li M, Cao S, Qin D, et al. Inhibitory Effect of Lychee Seed Saponins on Apoptosis Induced by Aβ25-35 through Regulation of the Apoptotic and NF-κB Pathways in PC12 Cells. Nutrients. 2017; 9(4):337. https://doi.org/10.3390/nu9040337
Chicago/Turabian StyleWang, Xiuling, Hong Zhang, Jian Liu, Rong Chen, Yong Tang, Haixia Chen, Li Gu, Mao Li, Shousong Cao, Dalian Qin, and et al. 2017. "Inhibitory Effect of Lychee Seed Saponins on Apoptosis Induced by Aβ25-35 through Regulation of the Apoptotic and NF-κB Pathways in PC12 Cells" Nutrients 9, no. 4: 337. https://doi.org/10.3390/nu9040337




