Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
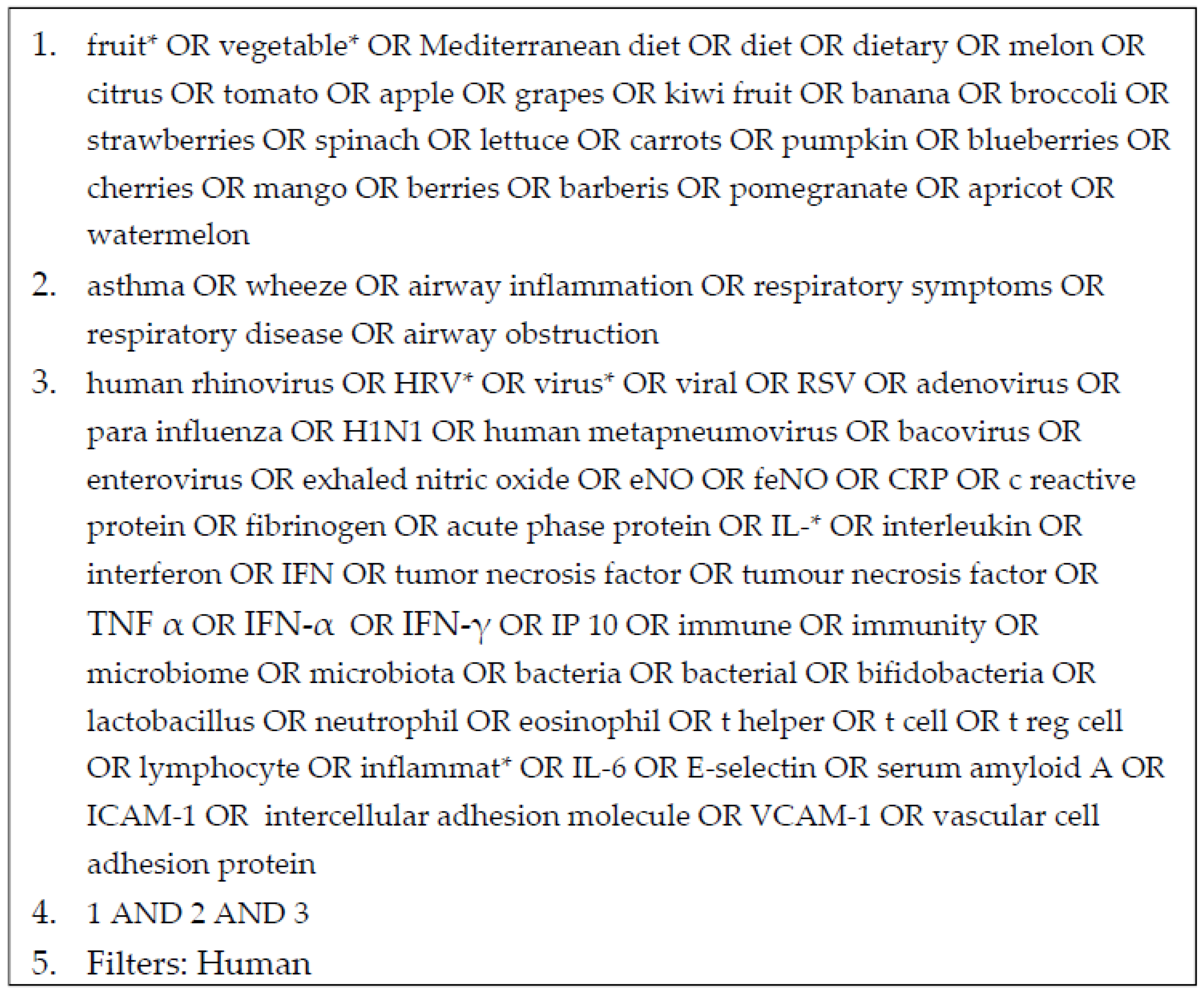
2.1. Search Strategy
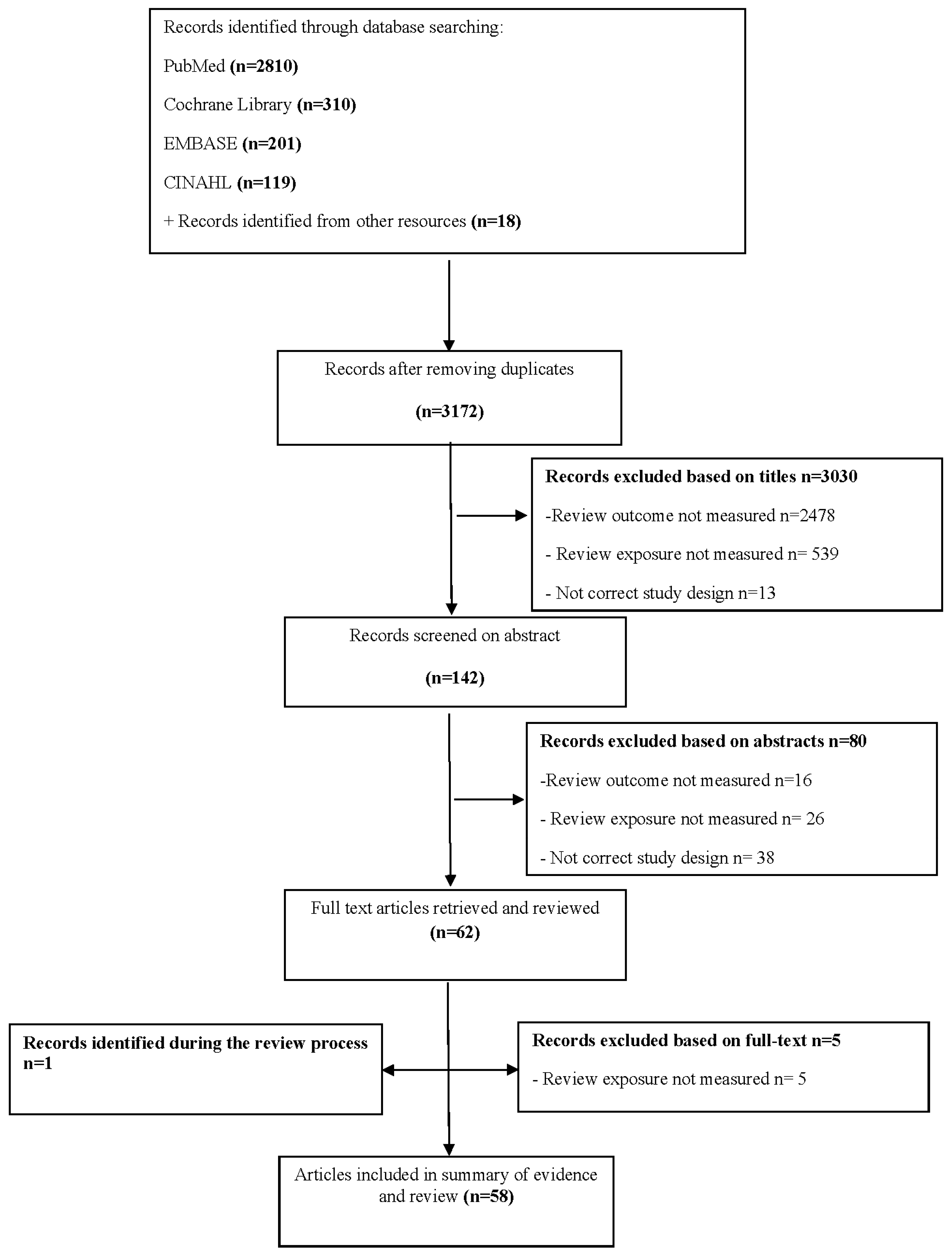
2.2. Study Selection
2.3. Study Quality
2.4. Data Extraction and Study Synthesis
2.5. Statistical Methods
3. Results
3.1. Characteristics of Included Studies
3.2. Studies Conducted in Adults
3.2.1. Cohort Studies
3.2.2. Case-Control Studies
3.2.3. Cross-Sectional Studies
3.2.4. Experimental Trial
3.3. Studies Conducted in Children and Adolescents
3.3.1. Cohort Studies
3.3.2. Case-Control Studies
3.3.3. Cross-Sectional Studies
3.3.4. Experimental Studies
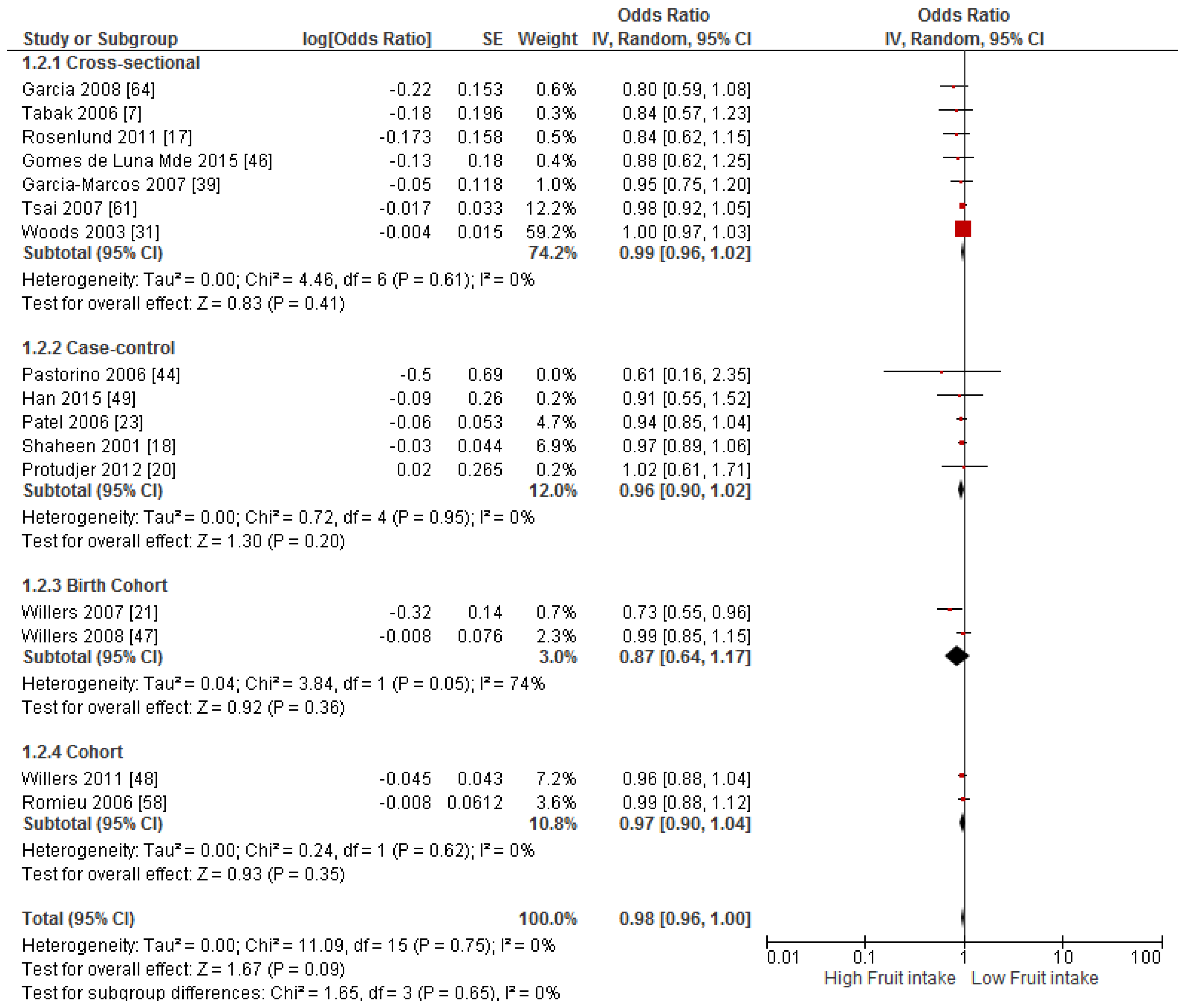
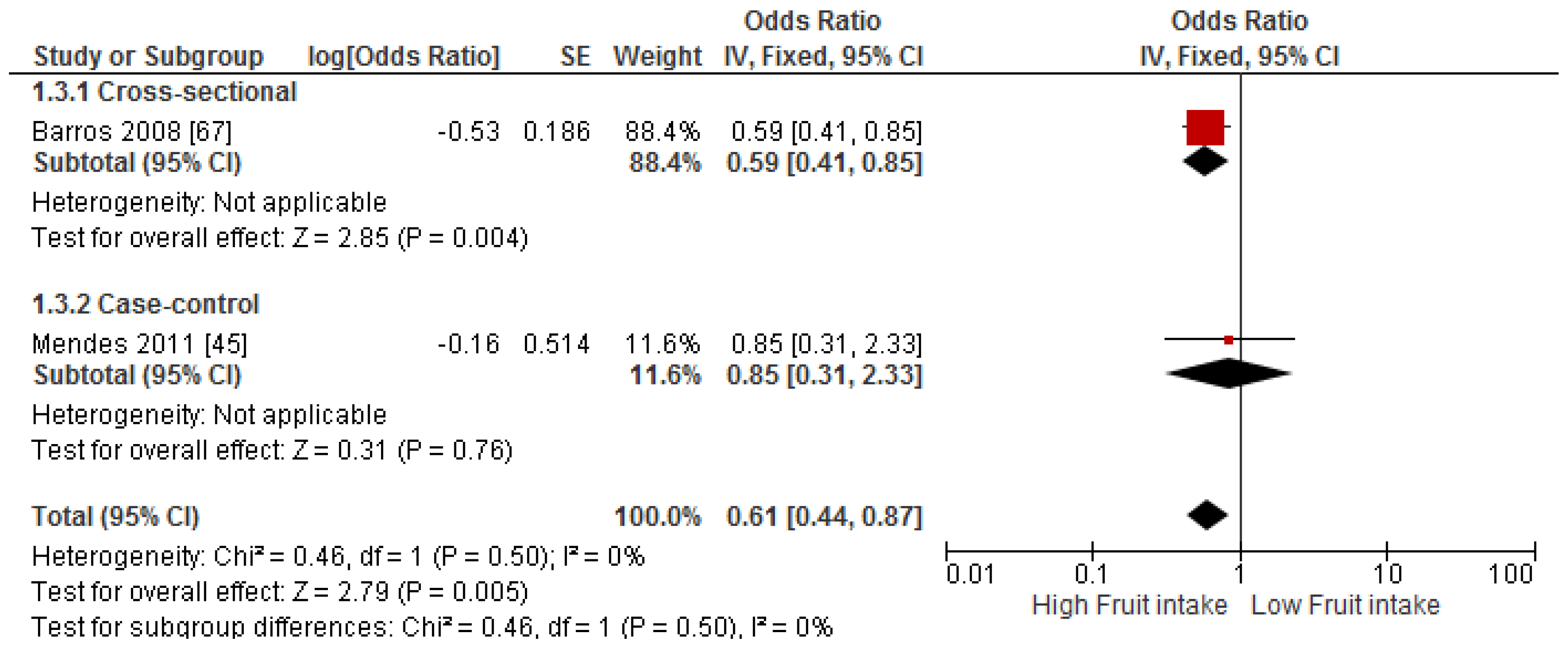
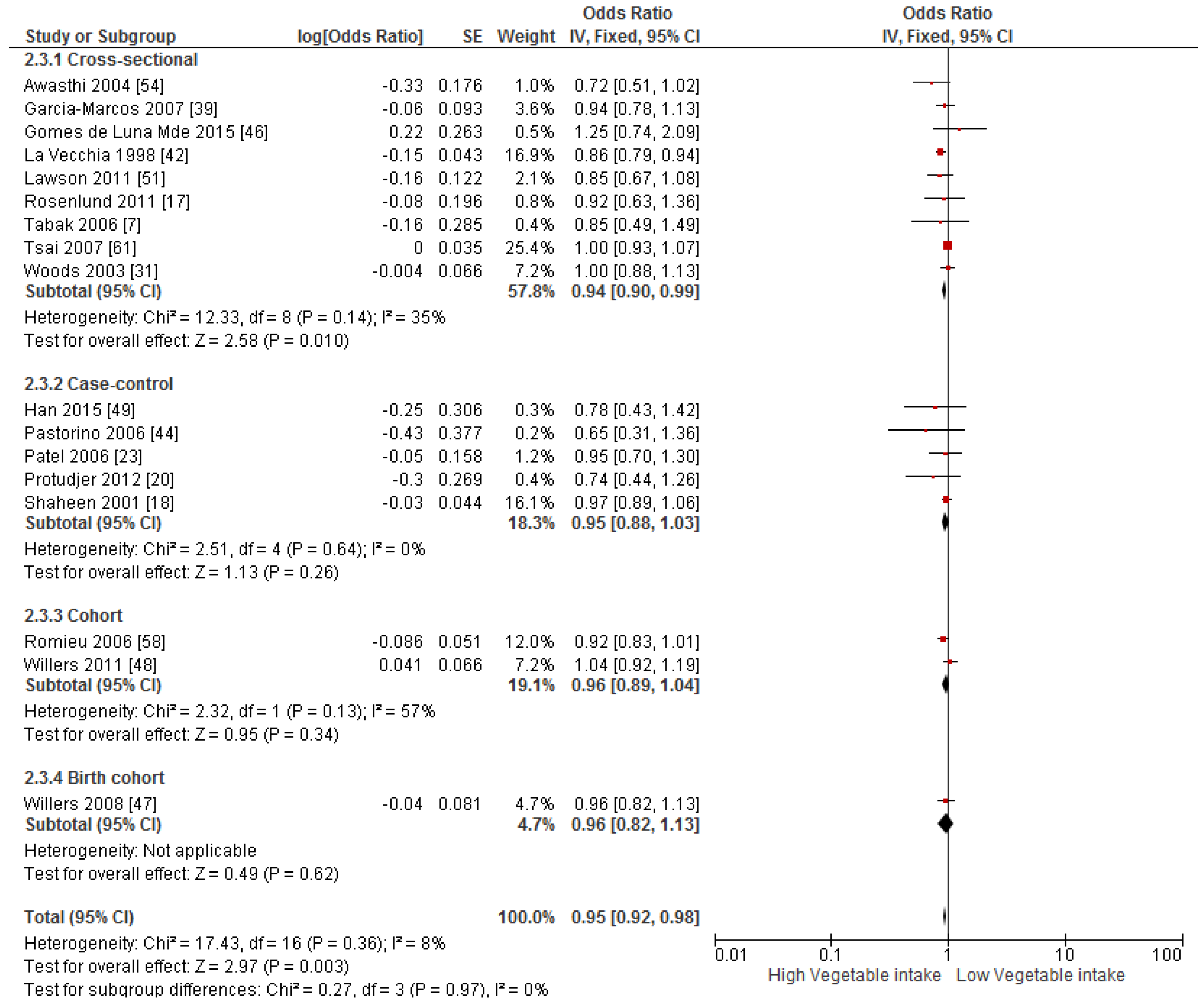
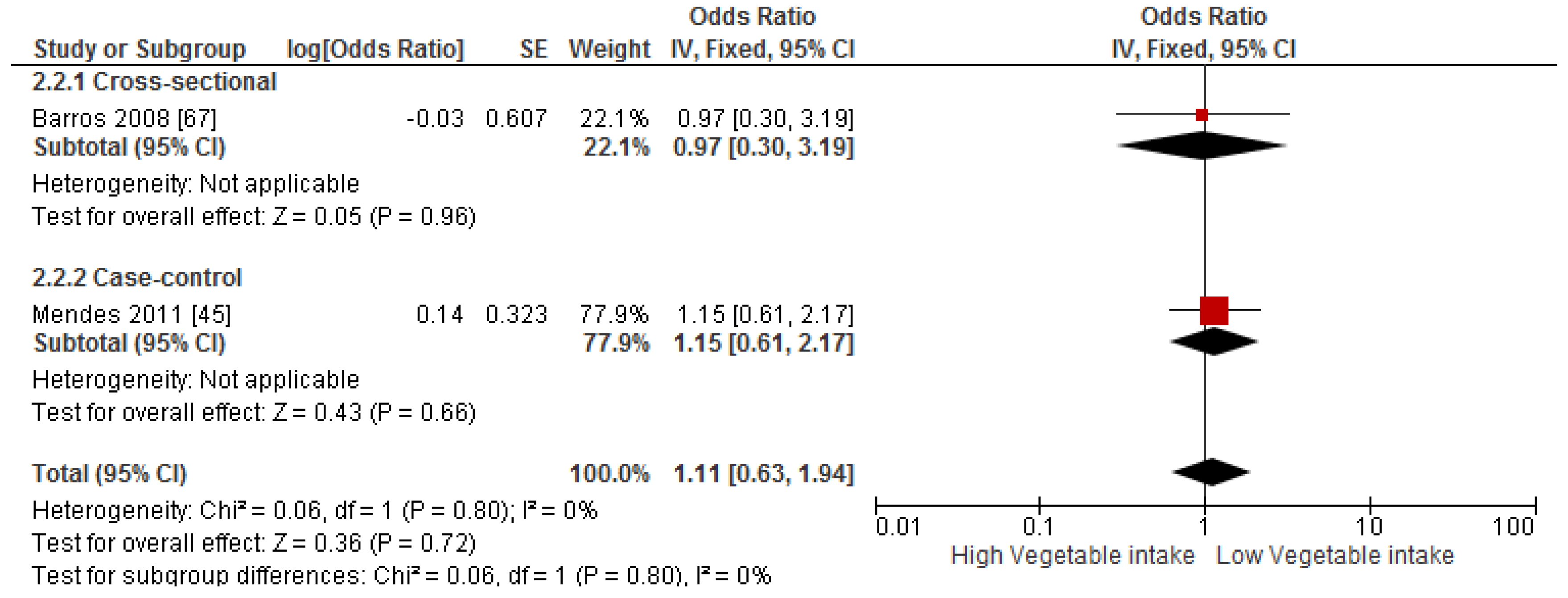
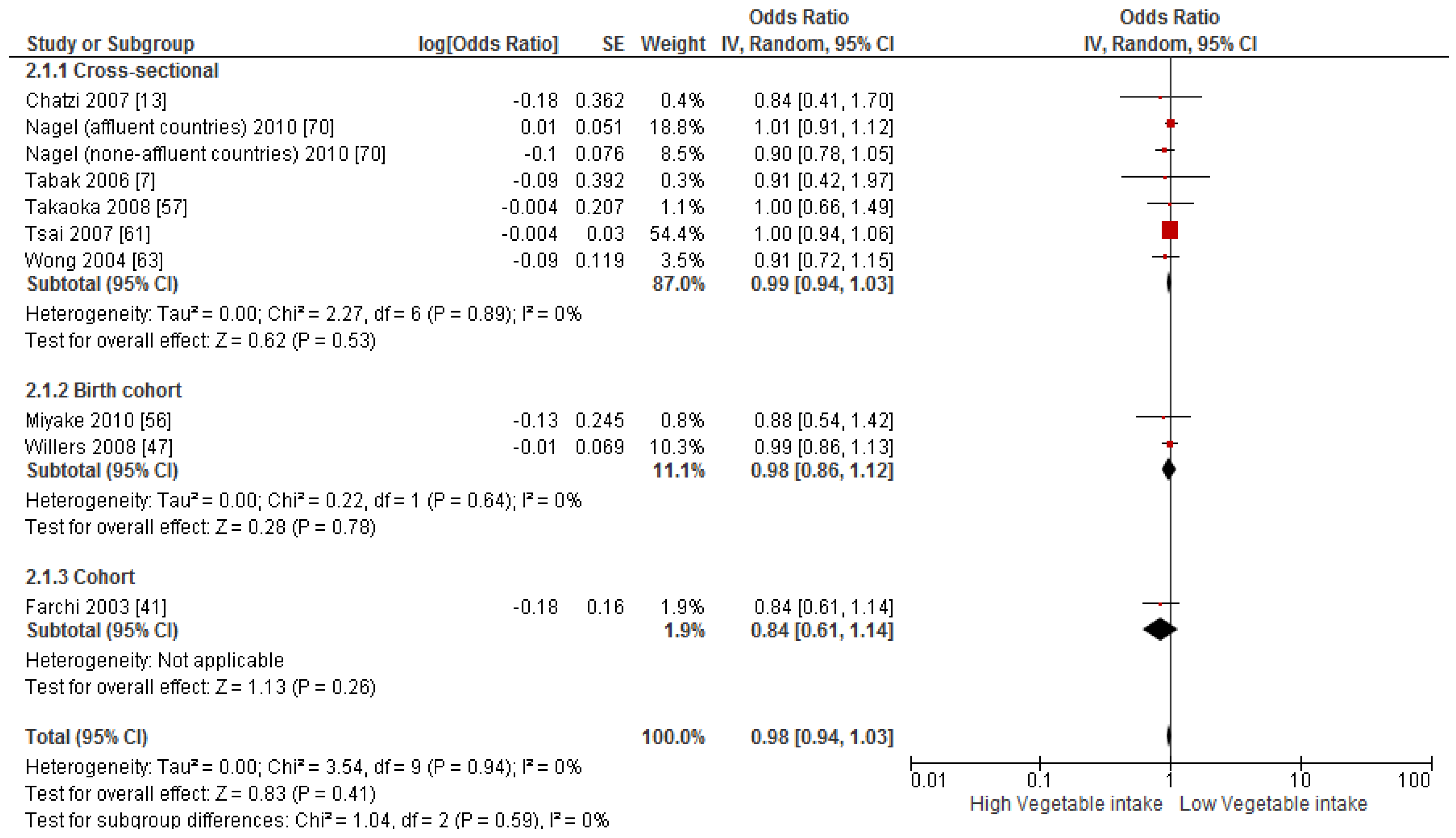
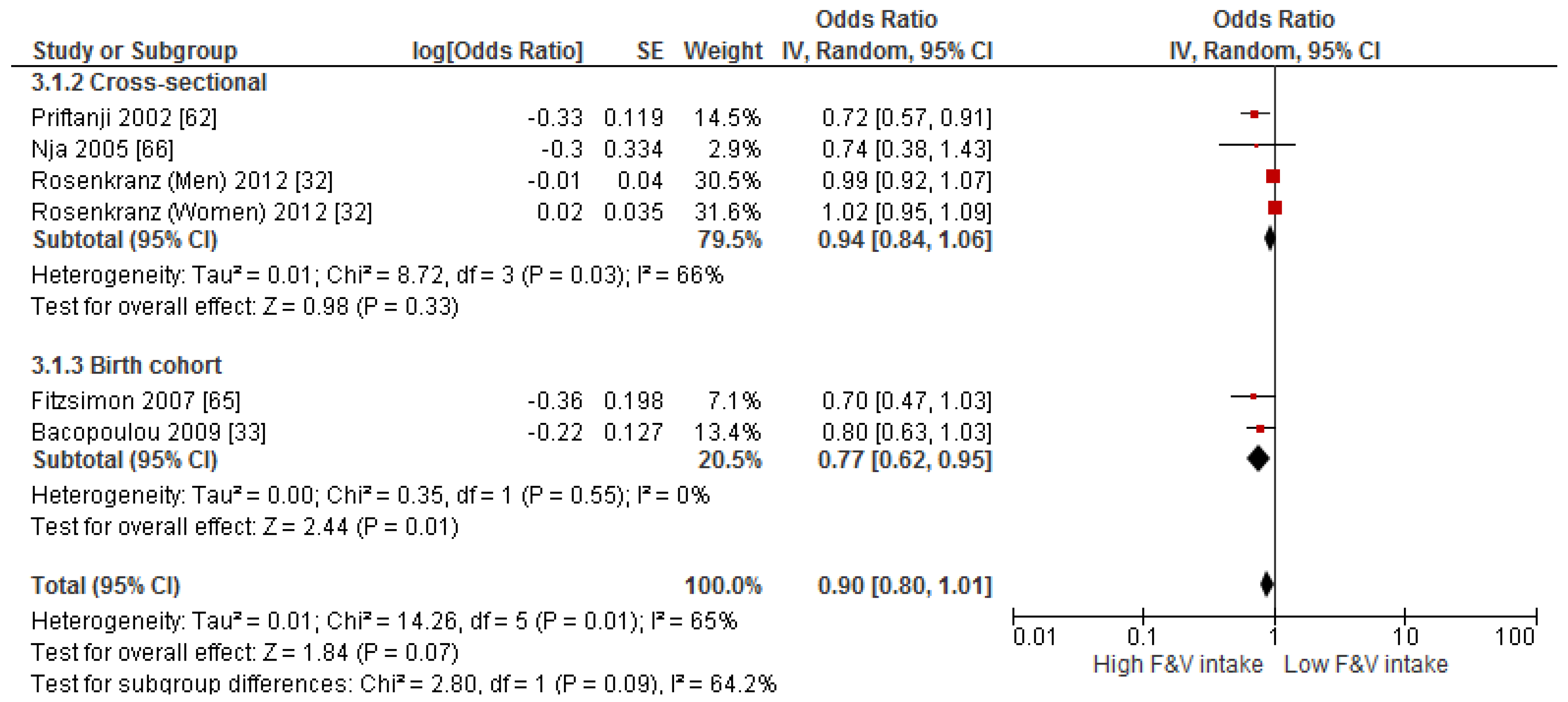
3.4. Findings from Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Okoko, B.J.; Burney, P.G.; Newson, R.B.; Potts, J.F.; Shaheen, S.O. Childhood asthma and fruit consumption. Eur. Respir. J. 2007, 29, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Misso, N.L.; Brooks-Wildhaber, J.; Ray, S.; Vally, H.; Thompson, P.J. Plasma concentrations of dietary and nondietary antioxidants are low in severe asthma. Eur. Respir. J. 2005, 26, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Eder, W.; Ege, M.J.; von Mutius, E. The asthma epidemic. N. Engl. J. Med. 2006, 355, 2226–2235. [Google Scholar] [CrossRef] [PubMed]
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R. The global burden of asthma: Executive summary of the GINA Dissemination Committee Report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Risnes, K.R.; Belanger, K.; Murk, W.; Bracken, M.B. Antibiotic exposure by 6 months and asthma and allergy at 6 years: Findings in a cohort of 1401 US children. Am. J. Epidemiol. 2011, 173, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Berthon, B.S.; Macdonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology 2013, 18, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Tabak, C.; Wijga, A.H.; de Meer, G.; Janssen, N.A.; Brunekreef, B.; Smit, H.A. Diet and asthma in Dutch school children (ISAAC-2). Thorax 2006, 61, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Gibson, P.G. Dietary factors lead to innate immune activation in asthma. Pharmacol. Ther. 2009, 123, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, F.; Tesse, R.; Fucilli, C.; Loffredo, M.S.; Iacoviello, G.; Chinellato, I.; Armenio, L. Correlation between exhaled nitric oxide and dietary consumption of fats and antioxidants in children with asthma. J. Allergy Clin. Immunol. 2007, 119, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.C.; Xiang, X.; Qiu, D.; Ng, T.P.; Lam, S.F.; Hegele, R.G. Epidemiology of respiratory viruses in patients hospitalized with near-fatal asthma, acute exacerbations of asthma, or chronic obstructive pulmonary disease. Am. J. Med. 2003, 115, 272–277. [Google Scholar] [CrossRef]
- Bowler, R.P. Oxidative stress in the pathogenesis of asthma. Cur. Allergy Asthma Rep. 2004, 4, 116–122. [Google Scholar] [CrossRef]
- Greene, L.S. Asthma, oxidant stress, and diet. Nutrition 1999, 15, 899–907. [Google Scholar] [CrossRef]
- Chatzi, L.; Apostolaki, G.; Bibakis, I.; Skypala, I.; Bibaki-Liakou, V.; Tzanakis, N.; Kogevinas, M.; Cullinan, P. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax 2007, 62, 677–683. [Google Scholar] [PubMed]
- Tay, H.; Wark, P.A.; Bartlett, N.W. Advances in the treatment of virus-induced asthma. Expert Rev. Respir. Med. 2016, 10, 629–641. [Google Scholar]
- Andreeva-Gateva, P.A.; Stamenova, E.; Gatev, T. The place of inhaled corticosteroids in the treatment of chronic obstructive pulmonary disease: A narrative review. Postgrad. Med. 2016, 128, 474–484. [Google Scholar] [PubMed]
- Academy of Nutrition and Dietetics. Quality Criteria Checklist: Primary Research in Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process. Available online: http://andevidencelibrary.com/files/Docs/2012_Jan_EA_Manual.pdf (accessed on 13 July 2016).
- Rosenlund, H.; Kull, I.; Pershagen, G.; Wolk, A.; Wickman, M.; Bergstrom, A. Fruit and vegetable consumption in relation to allergy: Disease-related modification of consumption? J. Allergy Clin. Immunol. 2011, 127, 1219–1225. [Google Scholar] [PubMed]
- Shaheen, S.; Sterne, J.C.; Thompson, R.; Songhurst, C.; Margetts, B.; Burney, P.J. Dietary Antioxidants and Asthma in Adults. Am. J. Respir. Crit. Care Med. 2001, 164, 1823–1828. [Google Scholar] [PubMed]
- Romieu, I.; Barraza-Villarreal, A.; Escamilla-Nunez, C.; Texcalac-Sangrador, J.L.; Hernandez-Cadena, L.; Diaz-Sanchez, D.; de Batlle, J.; Del Rio-Navarro, B.E. Dietary intake, lung function and airway inflammation in Mexico City school children exposed to air pollutants. Respir. Res. 2009, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Protudjer, J.L.; Sevenhuysen, G.P.; Ramsey, C.D.; Kozyrskyj, A.L.; Becker, A.B. Low vegetable intake is associated with allergic asthma and moderate-to-severe airway hyperresponsiveness. Pediatr. Pulmonol. 2012, 47, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Willers, S.M.; Devereux, G.; Craig, L.C.; McNeill, G.; Wijga, A.H.; Abou El-Magd, W.; Turner, S.W.; Helms, P.J.; Seaton, A. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax 2007, 62, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Butland, B.K.; Strachan, D.P.; Anderson, H.R. Fresh fruit intake and asthma symptoms in young British adults: Confounding or effect modification by smoking? Eur. Respir. J. 1999, 13, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.D.; Welch, A.A.; Bingham, S.A.; Luben, R.N.; Day, N.E.; Khaw, K.T.; Lomas, D.A.; Wareham, N.J. Dietary antioxidants and asthma in adults. Thorax 2006, 61, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, A.W.; Antoniak, M.; Venn, A.J.; Davies, L.; Goodwin, A.; Salfield, N.; Britton, J.R.; Lewis, S.A. A natural experiment on the impact of fruit supplementation on asthma symptoms in children. Eur. Respir. J. 2009, 33, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Larsen, V.; Bush, A.; Boyle, R.J.; Shaheen, S.O.; Warner, J.O.; Athersuch, T.; Mudway, I.; Burney, P.G.J. O06-The Chelsea, asthma and fresh fruit intake in children (CHAFFINCH) trial-pilot study. Clin. Transl. Allergy 2014, 4, O6. [Google Scholar] [CrossRef]
- Cook, D.G.; Carey, I.M.; Whincup, P.H.; Papacosta, O.; Chirico, S.; Bruckdorfer, K.R.; Walker, M. Effect of fresh fruit consumption on lung function and wheeze in children. Thorax 1997, 52, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.A.; Antoniak, M.; Venn, A.J.; Davies, L.; Goodwin, A.; Salfield, N.; Britton, J.; Fogarty, A.W. Secondhand smoke, dietary fruit intake, road traffic exposures, and the prevalence of asthma: A cross-sectional study in young children. Am. J. Epidemiol. 2005, 161, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Powell, H.; Gibson, P.G. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: Proof of concept. Free Radic. Res. 2008, 42, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Baines, K.J.; Wood, L.G.; Gibson, P.G. The nutrigenomics of asthma: Molecular mechanisms of airway neutrophilia following dietary antioxidant withdrawal. OMICS 2009, 13, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Garg, M.L.; Smart, J.M.; Scott, H.A.; Barker, D.; Gibson, P.G. Manipulating antioxidant intake in asthma: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Woods, R.K.; Walters, E.H.; Raven, J.M.; Wolfe, R.; Ireland, P.D.; Thien, F.C.; Abramson, M.J. Food and nutrient intakes and asthma risk in young adults. Am. J. Clin. Nutr. 2003, 78, 414–421. [Google Scholar] [PubMed]
- Rosenkranz, R.R.; Rosenkranz, S.K.; Neessen, K.J. Dietary factors associated with lifetime asthma or hayfever diagnosis in Australian middle-aged and older adults: A cross-sectional study. Nutr. J. 2012, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Bacopoulou, F.; Veltsista, A.; Vassi, I.; Gika, A.; Lekea, V.; Priftis, K.; Bakoula, C. Can we be optimistic about asthma in childhood? A Greek cohort study. J. Asthma 2009, 46, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, F.; Priftis, K.N.; Papadimitriou, A.; Papadopoulos, M.; Roma, E.; Kapsokefalou, M.; Anthracopoulos, M.B.; Panagiotakos, D.B. Adherence to the Mediterranean type of diet is associated with lower prevalence of asthma symptoms, among 10–12 years old children: The PANACEA study. Pediatr. Allergy Immunol. 2011, 22, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Alphantonogeorgos, G.; Panagiotakos, D.B.; Grigoropoulou, D.; Yfanti, K.; Papoutsakis, C.; Papadimitriou, A.; Anthracopoulos, M.B.; Bakoula, C.; Priftis, K.N. Investigating the associations between Mediterranean diet, physical activity and living environment with childhood asthma using path analysis. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Panagiotakos, D.B.; Hatziagorou, E.; Antonogeorgos, G.; Matziou, V.N.; Tsanakas, J.N.; Gratziou, C.; Tsabouri, S.; Priftis, K.N. Antioxidant foods consumption and childhood asthma and other allergic diseases: The Greek cohorts of the ISAAC II survey. Allergol. Immunopathol. 2015, 43, 353–360. [Google Scholar]
- Chatzi, L.; Torrent, M.; Romieu, I.; Garcia-Esteban, R.; Ferrer, C.; Vioque, J.; Kogevinas, M.; Sunyer, J. Mediterranean diet in pregnancy is protective for wheeze and atopy in childhood. Thorax 2008, 63, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Calatayud-Saez, F.M.; Calatayud Moscoso Del Prado, B.; Gallego Fernandez-Pacheco, J.G.; Gonzalez-Martin, C.; Alguacil Merino, L.F. Mediterranean diet and childhood asthma. Allergol. Immunopathol. 2016, 44, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marcos, L.; Canflanca, I.M.; Garrido, J.B.; Varela, A.L.; Garcia-Hernandez, G.; Guillen Grima, F.; Gonzalez-Diaz, C.; Carvajal-Uruena, I.; Arnedo-Pena, A.; Busquets-Monge, R.M.; et al. Relationship of asthma and rhinoconjunctivitis with obesity, exercise and Mediterranean diet in Spanish schoolchildren. Thorax 2007, 62, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Garcia-Marcos, L.; Alfonseda Rojas, J.D.; Valverde-Molina, J.; Sanchez-Solis, M. Mediterranean diet as a protective factor for wheezing in preschool children. J. Pediatr. 2008, 152, 823–828. [Google Scholar] [PubMed]
- Farchi, S.; Forastiere, F.; Agabiti, N.; Corbo, G.; Pistelli, R.; Fortes, C.; Dell’Orco, V.; Perucci, C.A. Dietary factors associated with wheezing and allergic rhinitis in children. Eur. Respir. J. 2003, 22, 772–780. [Google Scholar] [CrossRef] [PubMed]
- La Vecchia, C.; Decarli, A.; Pagano, R. Vegetable consumption and risk of chronic disease. Epidemiology 1998, 9, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Forastiere, F.; Pistelli, R.; Sestini, P.; Fortes, C.; Renzoni, E.; Rusconi, F.; Dell’Orco, V.; Ciccone, G.; Bisanti, L. Consumption of fresh fruit rich in vitamin C and wheezing symptoms in children. SIDRIA Collaborative Group, Italy (Italian Studies on Respiratory Disorders in Children and the Environment). Thorax 2000, 55, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, A.C.; Rimazza, R.D.; Leone, C.; Castro, A.P.; Sole, D.; Jacob, C.M. Risk factors for asthma in adolescents in a large urban region of Brazil. J. Asthma 2006, 43, 695–700. [Google Scholar] [CrossRef]
- Mendes, A.P.; Zhang, L.; Prietsch, S.O.; Franco, O.S.; Gonzales, K.P.; Fabris, A.G.; Catharino, A. Factors associated with asthma severity in children: A case-control study. J. Asthma 2011, 48, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Luna Mde, F.; Gomes de Luna, J.R.; Fisher, G.B.; de Almeida, P.C.; Chiesa, D.; Carlos da Silva, M.G. Factors associated with asthma in adolescents in the city of Fortaleza, Brazil. J. Asthma 2015, 52, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Willers, S.M.; Wijga, A.H.; Brunekreef, B.; Kerkhof, M.; Gerritsen, J.; Hoekstra, M.O.; de Jongste, J.C.; Smit, H.A. Maternal food consumption during pregnancy and the longitudinal development of childhood asthma. Am. J. Respir. Crit. Care Med. 2008, 178, 124–131. [Google Scholar] [PubMed]
- Willers, S.M.; Wijga, A.H.; Brunekreef, B.; Scholtens, S.; Postma, D.S.; Kerkhof, M.; de Jongste, J.C.; Smit, H.A. Childhood diet and asthma and atopy at 8 years of age: The PIAMA birth cohort study. Eur. Respir. J. 2011, 37, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Y.; Forno, E.; Brehm, J.M.; Acosta-Perez, E.; Alvarez, M.; Colon-Semidey, A.; Rivera-Soto, W.; Campos, H.; Litonjua, A.A.; Alcorn, J.F.; et al. Diet, interleukin-17, and childhood asthma in Puerto Ricans. Ann. Allergy Asthma Immunol. 2015, 115, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, F.D.; Berhane, K.T.; Li, Y.F.; Gauderman, W.J.; McConnell, R.; Peters, J. Children’s lung function and antioxidant vitamin, fruit, juice, and vegetable intake. Am. J. Epidemiol. 2003, 158, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.A.; Janssen, I.; Bruner, M.W.; Madani, K.; Pickett, W. Urban-rural differences in asthma prevalence among young people in Canada: The roles of health behaviors and obesity. Ann. Allergy Asthma Immunol. 2011, 107, 220–228. [Google Scholar] [PubMed]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [PubMed]
- Nwaru, B.I.; Erkkola, M.; Ahonen, S.; Kaila, M.; Kronberg-Kippila, C.; Ilonen, J.; Simell, O.; Knip, M.; Veijola, R.; Virtanen, S.M. Intake of antioxidants during pregnancy and the risk of allergies and asthma in the offspring. Eur. J. Clin. Nutr. 2011, 65, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Kalra, E.; Roy, S.; Awasthi, S. Prevalence and risk factors of asthma and wheeze in school-going children in Lucknow, North India. Indian Pediatr. 2004, 41, 1205–1210. [Google Scholar] [PubMed]
- Agrawal, S.; Pearce, N.; Ebrahim, S. Prevalence and risk factors for self-reported asthma in an adult Indian population: A cross-sectional survey. Int. J. Tuberc. Lung Dis. 2013, 17, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy 2010, 65, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, M.; Norback, D. Diet among Japanese female university students and asthmatic symptoms, infections, pollen and furry pet allergy. Respir. Med. 2008, 102, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Romieu, I.; Varraso, R.; Avenel, V.; Leynaert, B.; Kauffmann, F.; Clavel-Chapelon, F. Fruit and vegetable intakes and asthma in the E3N study. Thorax 2006, 61, 209–215. [Google Scholar] [PubMed]
- Uddenfeldt, M.; Janson, C.; Lampa, E.; Leander, M.; Norbäck, D.; Larsson, L.; Rask-Andersen, A. High BMI is related to higher incidence of asthma, while a fish and fruit diet is related to a lower: Results from a long-term follow-up study of three age groups in Sweden. Respir. Med. 2010, 104, 972–980. [Google Scholar] [PubMed]
- Lee, S.C.; Yang, Y.H.; Chuang, S.Y.; Huang, S.Y.; Pan, W.H. Reduced medication use and improved pulmonary function with supplements containing vegetable and fruit concentrate, fish oil and probiotics in asthmatic school children: A randomised controlled trial. Br. J. Nutr. 2013, 110, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.J.; Tsai, A.C. The association of diet with respiratory symptoms and asthma in schoolchildren in Taipei, Taiwan. J. Asthma 2007, 44, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Priftanji, A.V.; Qirko, E.; Burr, M.L.; Layzell, J.C.; Williams, K.L. Factors associated with asthma in Albania. Allergy 2002, 57, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.W.; Ko, F.W.; Hui, D.S.; Fok, T.F.; Carr, D.; von Mutius, E.; Zhong, N.S.; Chen, Y.Z.; Lai, C.K. Factors associated with difference in prevalence of asthma in children from three cities in China: Multicentre epidemiological survey. BMJ 2004, 329, 486. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Aristizabal, G.; Vasquez, C.; Rodriguez-Martinez, C.E.; Sarmiento, O.L.; Satizabal, C.L. Prevalence of and factors associated with current asthma symptoms in school children aged 6–7 and 13–14 yr old in Bogota, Colombia. Pediatr. Allergy Immunol. 2008, 19, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimon, N.; Fallon, U.; O'Mahony, D.; Loftus, B.G.; Bury, G.; Murphy, A.W.; Kelleher, C.C. Mothers’ dietary patterns during pregnancy and risk of asthma symptoms in children at 3 years. Ir. Med. J. 2007, 100, 27–32. [Google Scholar]
- Nja, F.; Nystad, W.; Lodrup Carlsen, K.C.; Hetlevik, O.; Carlsen, K.H. Effects of early intake of fruit or vegetables in relation to later asthma and allergic sensitization in school-age children. Acta Paediatr. 2005, 94, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Moreira, A.; Fonseca, J.; de Oliveira, J.F.; Delgado, L.; Castel-Branco, M.G.; Haahtela, T.; Lopes, C.; Moreira, P. Adherence to the Mediterranean diet and fresh fruit intake are associated with improved asthma control. Allergy 2008, 63, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, N.; Abalkhail, B.; Seaton, A. Diet and childhood asthma in a society in transition: A study in urban and rural Saudi Arabia. Thorax 2000, 55, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Niti, M.; Yap, K.B.; Tan, W.C. Dietary and supplemental antioxidant and anti-inflammatory nutrient intakes and pulmonary function. Public Health Nutr. 2014, 17, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Weinmayr, G.; Kleiner, A.; Garcia-Marcos, L.; Strachan, D.P. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax 2010, 65, 516–522. [Google Scholar] [PubMed]
- Garcia-Larsen, V.; Arthur, R.; Kato, B.; Potts, J.F.; Burney, P.G. Fruit and vegetable intake and its association with asthma in adults across Europe: Evidence from the GA2LEN follow-up survey. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 58. [Google Scholar]
- Leonardi, S.; Pecoraro, R.; Filippelli, M.; Miraglia del Giudice, M.; Marseglia, G.; Salpietro, C.; Arrigo, T.; Stringari, G.; Rico, S.; La Rosa, M.; et al. Allergic reactions to foods by inhalation in children. Allergy Asthma Proc. 2014, 35, 51–56. [Google Scholar]
- Wood, L.G.; Garg, M.L.; Simpson, J.L.; Mori, T.A.; Croft, K.D.; Wark, P.A.; Gibson, P.G. Induced sputum 8-isoprostane concentrations in inflammatory airway diseases. Am. J. Respir. Crit. Care Med. 2005, 171, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Gibson, P.G.; Garg, M.L. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur. Respir. J. 2003, 21, 177–186. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 16 July 2016).
- Wood, L.G.; Garg, M.L.; Blake, R.J.; Garcia-Caraballo, S.; Gibson, P.G. Airway and Circulating Levels of Carotenoids in Asthma and Healthy Controls. J. Am. Coll. Nutr. 2005, 24, 448–455. [Google Scholar] [PubMed]
- Mastrorilli, C.; Posa, D.; Cipriani, F.; Caffarelli, C. Asthma and allergic rhinitis in childhood: What’s new. Pediatr. Allergy Immunol. 2016, 27, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, P.; Asher, M.I.; Bjorksten, B.; Burr, M.; Pearce, N.; Robertson, C.F. Diet and asthma, allergic rhinoconjunctivitis and atopic eczema symptom prevalence: An ecological analysis of the International Study of Asthma and Allergies in Childhood (ISAAC) data. ISAAC Phase One Study Group. Eur. Respir. J. 2001, 17, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Bakkeheim, E.; Mowinckel, P.; Carlsen, K.H.; Burney, P.; Carlsen, K.C. Altered oxidative state in schoolchildren with asthma and allergic rhinitis. Pediatr. Allergy Immunol. 2011, 22, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Chhabra, S.K.; Masood, A.; Raj, H.G. Increased oxidative stress and altered levels of antioxidants in asthma. J. Allergy Clin. Immunol. 2003, 111, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Iikura, K.; Katsunuma, T.; Saika, S.; Saito, S.; Ichinohe, S.; Ida, H.; Saito, H.; Matsumoto, K. Peripheral blood mononuclear cells from patients with bronchial asthma show impaired innate immune responses to rhinovirus in vitro. Int. Arch. Allergy Immunol. 2011, 155 (Suppl. 1), 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lemanske, R.F., Jr.; Dick, E.C.; Swenson, C.A.; Vrtis, R.F.; Busse, W.W. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J. Clin. Investig. 1989, 83, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.; Greenwood, D.; Kirk, S.; Cade, J. Lifestyle factors affecting fruit and vegetable consumption in the UK Women’s Cohort Study. Appetite 2001, 37, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Bopp, M.; Wilcox, S.; Laken, M.; Butler, K.; Carter, R.E.; McClorin, L.; Yancey, A. Factors associated with physical activity among African-American men and women. Am. J. Prev. Med. 2006, 30, 340–346. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Food Measured | Study Population | Age Group (Year) | Tool for Asthma Diagnosis | Dietary Assessment Methods | Variables Contrasted | Outcomes |
|---|---|---|---|---|---|---|---|
| Cook et al. [26], 1997 | F & V | 2650 | 8–11 | Questionnaire | FFQ a | >1 time/day vs. never | ↑ fresh fruit, salad, green vegetables consumption: ↑ FEV1, ↔ wheeze |
| La vecchia et al. [42], 1998 | Vegetables | 46,693 | ≥15 | Questionnaire | FFQ | Highest (>7 serving/week) vs. lowest (<7 serving/week) tertiles | ↑ vegetable consumption: ↓ bronchial asthma |
| Forastiere et al. [43], 2000 | Fruits | 4104 | 6–7 | ISAAC questionnaire | Questionnaire on dietary habits, citrus fruit consumption | 5–7 times/week vs. <1 time/week | ↑ fruit: ↓ any wheeze, ↓ shortness of breath with wheeze |
| Priftanji et al. [62], 2002 | F & V | 2653 | 20–44 | Questionnaire, | Questionnaire on dietary habits | At least once a week | ↑ taking fruit and vegetables: ↓ possible allergic asthma |
| Gilliland et al. [50], 2003 | F & V | 2566 | 11–19 | Pulmonary function testing | FFQ | ≤lowest vs. highest intake decile | ↓ intakes of all fruit juices: ↓ FEV1 and FVC among boys, ↓ intake of vegetable: ↓ FVC in girls, ↔ other respiratory symptoms |
| Woods et al. [31], 2003 | F & V | 1601 | 20–44 | ECRHS questionnaire | FFQ | 1–2 piece of apples, pears and berries/day and 2–4 servings leafy green vegetables and tomatoes/day | ↑ consumption of apples and pears: ↓ current asthma vegetable intake: ↔ |
| Awasthi et al. [54], 2004 | F & V | 3000 | 6–7 and 13–14 | ISAAC questionnaire | Validated questionnaire | F: ≥3 times/day V: ≥1 time/week | ↑ intakes of vegetables and fruits: ↓ wheeze |
| Wong et al. [63], 2004 | F & V | 10,902 | 10 | Questionnaire, skin-prick test | Questionnaire on F & V intakes | F: more than once daily vs. <once daily; V:more than once a week vs. <1 per week | ↑ intakes of fruit and vegetables: ↓ wheeze |
| Lewis et al. [27], 2005 | Fruits | 11,562 | 4–6 | Questionnaire | Questionnaire | ≥21 portions/week vs. 0 portions/week | Fruits: ↔ wheeze |
| Nja et al. [66], 2005 | F & V | 502 | 6–16 | Questionnaire, skin-prick test | Questionnaire | Daily intake vs. occasionally | ↑ intakes of fruit and vegetables: ↓ asthma |
| Tabak et al. [7], 2006 | Citrus and V | 598 | 8–13 | ISAAC questionnaire | FFQ | F: Highest (287 g/day vs. lowest (79 g/day) tertiles; V: Highest (140 g/day vs. lowest (53 g/day) tertiles | citrus fruits, vegetables: ↔ |
| Cardinale et al. [9], 2007 | F & V | 130 | 6–7 | Doctor-diagnosed asthma | FFQ | Always vs. never | ↑ intakes of fruit: ↓ asthma ↑ intakes of salads: ↓ FENO |
| Chatzi et al. [13], 2007 | F & V | 690 | 7–18 | ISAAC questionnaire, family history of allergic disease | FFQ | >1 time/day vs. <1 time/day | ↑ intake of grapes, oranges, apples and fresh tomatoes: ↓ wheezing |
| Garcia-Marcos et al. [39], 2007 | F & V | 20,106 | 6–7 | ISAAC questionnaire | Questionnaire | ≥3 times/week vs. never | ↑ intakes of fruit and vegetables: ↓ COA, ↓ CSA |
| Okoko et al. [1], 2007 | Fruits | 2640 | 5–10 | ISAAC questionnaire | FFQ | >1 serving/day vs. <1 serving/month | ↑ intakes of apples: ↓ ever-asthma ↑ intake of bananas and apples: ↓ ever wheeze and current wheeze |
| Tsai et al. [61], 2007 | F & V | 2218 | 11–12 | ATS questionnaire | FFQ | Daily intake vs. never | ↑ Fruit intakes: ↓ wheezing without cold, ↓ asthma, ↑ vegetable consumption: ↑ asthma |
| Barros et al. [67], 2008 | F & V | 174 | >16 | Doctor-diagnosed asthma and questionnaire | FFQ | F: <178.4 g/day vs. >304.97 g/day; V: <211.54 g/day vs. >426.63 g/day | ↑ consumption of fresh fruit: ↓ non-controlled asthma; vegetable intake: ↔; F&V: ↔ exhaled NO |
| Castro-Rodriguez et al. [40], 2008 | F & V | 1784 | 4.08 | Questionnaire | FFQ | >3 times/week vs. never | ↑ intake of vegetable: ↓ wheezing; fruits intake: ↔ |
| Garcia et al. [64], 2008 | Fruits | 3256 children and 3829 adolescents | 6–7 and 13–14 | ISAAC questionnaire | Questionnaire on dietary habits | ≥3 times/week vs. occasionally | ↑ fruit consumption: ↓ current asthma symptoms among the 13–14 year age-group |
| Takaoka et al. [57], 2008 | F & V | 153 females | Mean 21 | Doctor -diagnosed asthma, ISSAC/ECRHS questionnaire | FFQ | almost daily vs. never | ↑ intake of fruit: ↓ wheeze vegetables: ↔ |
| Nagel et al. [70], 2010 | F & V | 50,004 b | 8–12 | ISAAC questionnaire | FFQ | ≥3 times/week vs. never/occasionally | ↑ consumption of green vegetables: ↓ wheezers in non-affluent countries only; ↑ fruit intake: ↓ prevalence of current wheeze in affluent and non-affluent countries |
| Arvaniti et al. [34], 2011 | F & V | 700 | 10–12 | ISAAC questionnaire | FFQ | At least once/day | Fruits and vegetables: ↔ asthma |
| Lawson et al. [51], 2011 | F & V | 4726 | 11–15 | Doctor-diagnosed asthma | Validated questionnaire | High vs. low consumption | ↑ vegetable consumption: ↓ current asthma; fruit intake: ↔ |
| Rosenlund et al. [17], 2011 | F & V | 2447 | 8 | Doctor-diagnosed asthma | FFQ | Quartile 4 (7.1 serving/day) vs. quartile 1 (1.8 serving/day) | Fruits and vegetables: ↔; apples/pears, carrots: ↓ asthma |
| Rosenkranz et al. [32], 2012 | F & V | 156,035 | ≥45 | Questionnaire, self-reported information | FFQ | Quintile 5 vs. 1 | ↑ fruit and vegetable intake: ↓ asthma in men |
| Agrawal et al. [55], 2013 | F & V | 156,316 | 20–49 | Questionnaire | FFQ | Daily intake vs. occasionally/never | ↑ fruit and vegetable intake: ↓ asthma |
| Ng et al. [69], 2013 | F & V | 2478 | ≥55 | Spirometry | SQFFQ | Once/day | Fruits and vegetables: ↔ respiratory function |
| Alphantonogeorgos et al. [35], 2014 | F & V | 1125 | 10–12 | ISAAC questionnaire | KIDMED FFQ | Once/day | ↑ intake of one fruit or fruit juice and vegetable: ↓ ever wheezing and current wheezing |
| Papadopoulou et al. [36], 2014 | F & V | 2023 | 9–10 | Doctor-diagnosed asthma | SQFFQ | Daily vs. never | Fruits, vegetables: ↔ asthma |
| Gomes de Luna Mde et al. [46], 2015 | F & V | 3015 | 13–14 | ISAAC questionnaire | Questionnaire | ≥3 times/week vs. < times/week | ↑ Fruit intake: ↓ asthma; vegetable intakes: ↔ |
| Author (Year) | Food Measured | Study Population | Age (Year) | Tool for Asthma Diagnosis | Dietary Assessment Methods | Variables Contrasted | Outcomes |
|---|---|---|---|---|---|---|---|
| Hijazi et al. [68], 2000 | F & V a | 114 cases with a history of asthma and wheeze in the last 12 months and 202 controls | 12 | ISAAC questionnaire and skin test | SQFFQ | >3 time/day vs. <2 | ↓ vegetables intake: ↑ asthma; fruit intake ↔ |
| Shaheen et al. [18], 2001 | F & V | 607 cases and 864 controls | 16–50 | Questionnaire | FFQ | ≥5 times/week vs. <once/month | ↓ Total fruit and total vegetable consumption: ↑ asthma |
| Patel et al. [23], 2006 | F & V | 515 cases and 515 controls | 45–75 | Physician diagnosed asthma | 7-day food diaries | Consumption above the median (F: 132.1 g, V: 96.9 g vs. no consumption) | ↑ intake of citrus fruit and total fruit intakes: ↓ asthma; vegetable intake ↔ |
| Pastorino et al. [44], 2006 | F & V | 528 | 13–14 | ISAAC questionnaire | Questionnaire about dietary habits | Weekly or daily vs. never consumption | ↑ intake of cooked vegetables: ↓ asthma; fruit intake ↔ |
| Romieu et al. [19], 2009 | F & V | 158 cases and 50 controls followed for 22 weeks | 6–14 | Physician diagnosed asthma | FFQ | F & V index = 0 vs. F & V index =4 | ↑ FVI: ↑ FEV1 and FVC; ↓ IL-8 in nasal lavage, ↓ FeNO level |
| Mendes et al. [45], 2011 | F & V | 104 cases with persistent asthma and 67 controls with intermittent asthma | 2–12 | Questionnaire | Dietary data collected during the last 30 days | Regular vs. occasional consumption | ↑ consumption of fruits: ↓ persistent asthma |
| Protudjer et al. [20], 2012 | F & V | 149 cases and 327 controls from a Cohort study | 8–10 and 11–14 | Skin-prick test ≥3 mm and asthma symptoms | FFQ | High vs. low score (>6 times/day vs. Almost never) | ↑ vegetable intake: ↓ allergic asthma, ↓ moderate/severe AHR; fruit intake: ↔ |
| Han et al. [49], 2015 | F & V | 351 cases and 327 controls | 6–14 | Physician-diagnosed asthma and ≥1 episode of wheeze in the previous year | Questionnaire | Quartile 4 vs. quartile 1 | ↑ consumption of vegetables: ↓ asthma, ↓ serum IL-17F |
| Author (Year) | Food Measured | Study Population | Age Group (Year) | Follow-Up (Year) | Tool for Asthma Diagnosis | Dietary Assessment Methods | Variables Contrasted | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Butland et al. [22], 1999 | Fresh fruit | 11,352 | 0–33 | 33 | Wheezing/whistling in the chest in the past doctor diagnosis | Validated questionnaire | >1 time/day vs. never | ↑ Fresh fruit, salads or raw vegetables consumption: ↓ the frequent wheezing |
| Knekt et al. [52], 2002 | Orange, apple, grapefruit, onion, white cabbage, berries, juices | 382 | 30–69 | 20 | Questionnaire | Dietary history | Quartile 4 vs. 1 | ↑ apple and orange intakes: ↓ asthma |
| Farchi et al. [41], 2003 | Cooked vegetables, salads, tomatoes, fresh fruit, citrus fruit, kiwi | 4104 | 6–7 | 1 | ISAAC questionnaire | FFQ | >4 times per week vs. never | ↑ Consumption of tomatoes, fruits and citrus fruit: ↓ shortness of breath |
| Romieu et al. [58], 2006 | F & V | 68,535 women | 40–65 | 3 | Questionnaire | FFQ | Quartile 4 vs. Quartile 1 (fruits: >336 vs. ≤145.3 g/day and vegetables: >90 vs. ≤39.3 g/day) | ↑ Consumption of tomatoes, carrots, and leafy vegetables: ↓ asthma |
| Fitzsimon et al. [65], 2007 | F & V | 631 mother-child pair | 3 | 3 | Doctor-diagnosed asthma | SQFFQ | Quartile 4 (8.9 serving/day) vs. quartile 1(2.3 serving/day) | ↑ Quartile of F & V intake in pregnancy: ↓ asthma in children |
| Willers et al. [21], 2007 | F & V | 1212 mother-child pair | At birth | 5 | ISAAC questionnaire | FFQ | >4 times/week vs. 0 ↔ 1 time/week | ↑ Maternal apple intake: ↓ ever wheeze, ↓ ever asthma and doctor-confirmed asthma vegetables: ↔ |
| Chatzi et al. [37], 2008 | F & V | 507 mothers and 468 children | 6.5 | 6.5 | Questionnaire on wheeze, whistling and skin-prick test | FFQ | Daily or weekly consumption vs. never | ↑ Consumption of vegetables: ↓ persistent wheeze, fruits: ↔ |
| Willers et al. [47], 2008 | F & V | 2832 mother-child pairs | 3 month–8 year | 8 | ISAAC questionnaire | Questionnaire about both mother’s and child’s diet | Daily vs. rare intake | ↑ Fruit intake: ↓ wheeze vegetables: ↔; F & V intake: ↔ |
| Bacopoulou et al. [33], 2009 | F & V | 2133 children | From birth | 18 | Doctor-diagnosed asthma and questionnaire about detailed information on asthma | Validated questionnaire | Daily intake vs. never | ↑ Fruit and vegetable intake: ↓ current asthma at 18 years |
| Miyake et al. [56], 2010 | F & V | 763 mother-child pair | 16–24 month | 2 | ISAAC questionnaire | DHQ | F: Quartile 4 (290.8 g/day) vs. 1 (49.6 g/day) V: Quartile 4 (288.4 g/day) vs. 1 (90.9 g/day) | F & V intake: ↔ wheeze |
| Uddenfeldt et al. [59], 2010 | Fruit | 8066 females and males | 16, 30–39, 60–69 | 13 | questionnaire | Questionnaire about frequency of current consumption | Daily intake vs. never | ↑ Fruit intake: ↓ asthma incidence |
| Nwaru et al. [53], 2011 | Food-based antioxidants | 2441 mother-child pair | 5 | 5 | ISAAC questionnaire | FFQ | Quantity of intake in diet | Food-based antioxidants: ↔ asthma |
| Willers et al. [48], 2011 | F & V | 4146 children | 2–3 and 7–8 | 8 | ISAAC questionnaire | Annual FFQ | Once weekly vs. long-term intake from age 2–8 years | ↑ Fruit intake: ↓ asthma symptoms; cooked ↑ vegetables intake: ↑ asthma |
| Author (Year) | Study Population | Age Group (Year) | Notes | Intervention | Duration of Treatment | Tool for Asthma Diagnosis | Outcomes |
|---|---|---|---|---|---|---|---|
| Wood et al. [28], 2008 | 32 adults | Mean age of 52.1 | Participants were on the low antioxidant diet for 10 days before the study commenced. | Tomato extract (45 mg lycopene/day) vs. tomato juice (45 mg lycopene/day) vs. placebo | 3 × 7 day with a 10 days wash-out period between each treatment | Doctor-diagnosed asthma and having current (past 12 months) episodic respiratory symptoms | The LAO diet: ↓ %FEV1 a and %FVC, ↑ neutrophils increased both tomato juice and extract: ↓ airway neutrophil influx Neutrophils tomato extract: ↓ sputum neutrophil elastase activity |
| Baines et al. [29], 2009 | 10 adults diagnosed with stable asthma | Mean age of 63 | No control group | The LAO diet b | 14 days | Doctor-diagnosed asthma and respiratory symptoms | The LAO diet: ↑ genes involved in the inflammatory and immune responses including the innate immune receptors TLR2, IL1R2, CD93, ANTXR2, the innate immune signalling molecules IRAK2, 3, MAP3K8 and neutrophil proteases. |
| Fogarty et al. [24], 2009 | Intervention group n = 3233, Placebo group n = 3506 | 4–6 | The control group received usual diet. | A daily piece of fruit (generally including apples, oranges or pears) adding to their usual diet | 1 year | Questionnaire | ↔ |
| Wood et al. [30], 2012 | 137 adults | Mean age of 56 | Participants randomized to the low vs. high antioxidant diet (5 servings of vegetables and 2 servings of fruit daily) for 14 days before the study commenced. | High antioxidant diet group received placebo, while, low antioxidant diet group received tomato extract (45 mg lycopene/day). | 14 weeks | Doctor-diagnosed asthma and having current (past 12 months) episodic respiratory symptoms | The LAO diet: ↑ exacerbation, ↓ %FEV1 and %FVC |
| Lee et al. [60], 2013 | 192 | 10–12 | The control group received placebos | “fruit and vegetable” capsule c + Fish oil capsules+ Probiotic capsules vs. placebo | 16 weeks | Doctor-diagnosed asthma | The supplement group: ↑ FEV1, FVC and FEV1:FVC ratio, ↓ proportion of children using ICS |
| Garcia-Larsen et al. [25], 2014 | 32 | 6–10 | Participants were randomly allocated to one of four groups. The control group received usual diet. | Having an apple or a banana or an apple + banana in addition to their normal diet | 1 month | Respiratory tests | Groups 2 (adding banana) and 3 (adding banana + apple): ↓ levels of FENO |
| Calatayud-Saez et al. [38], 2016 | 104 children with childhood asthma criteria for at least 1 year | 1–5 | No control group | Dietary re-education by a nutritional education programme named “Learning to Eat from the Mediterranean” | 1 year | Doctor-diagnosed asthma | ↓ The use of ICS |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini, B.; Berthon, B.S.; Wark, P.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 341. https://doi.org/10.3390/nu9040341
Hosseini B, Berthon BS, Wark P, Wood LG. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients. 2017; 9(4):341. https://doi.org/10.3390/nu9040341
Chicago/Turabian StyleHosseini, Banafshe, Bronwyn S. Berthon, Peter Wark, and Lisa G. Wood. 2017. "Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis" Nutrients 9, no. 4: 341. https://doi.org/10.3390/nu9040341





