1. Introduction
Obesity is a global health concern that has reached epidemic proportions [
1]. Obesity is caused by body fat accumulation in adipose tissue due to abnormalities in energy metabolism. It is associated with a number of metabolic diseases such as diabetes, cardiovascular disease, and low chronic inflammation [
2,
3]. Although pharmaceutical treatment of obesity costs approximately
$ 2 trillion per year [
4], the population of obese individuals is rapidly growing worldwide, indicating that prevention or intervention of obesity may be difficult due to the complex multisystem pathophysiology [
5]. Clinical guidelines from several countries recommend pharmacological agents such as orlistat for obese patients [
6,
7]. Orlistat is a Food and Drug Administration (FDA)-approved medicine for the treatment of obesity in the United States. It is an inhibitor of intestinal lipase that decreases dietary fat absorption [
8].
Although orlistat treatment helps to reduce weight [
9,
10], it is now generally believed that physical activity and a healthy diet may prevent weight gain and obesity by maintaining energy balance and efficiently burning excessive energy [
11]. Increasing consumption of fruits, vegetables, and edible plants and decreasing consumption of high-calorie foods leads to a decrease in weight gain and stabilization of weight in participants [
12]. The bioactive compounds present in these food categories that are implicated in obesity prevention include resveratrol, curcumin, conjugated linoleic acid, and omega-3 fatty acids [
13,
14,
15,
16].
Recent studies have indicated that seaweeds and their derivatives may also have potential therapeutic implications for obesity [
17,
18]. In particular,
Gelidium elegans (GENS), previously known as
Gelidium amansii, has been shown to have nutraceutical activities such as anti-adipogenesis and anti-obesity effects [
19,
20,
21]. However, the mechanism by which GENS is able to achieve its anti-obesity effects remains to be clearly defined. We have previously demonstrated that GENS has the potential to alter adipocyte phenotypes to beige-like adipocytes in vitro [
22]. This suggests that GENS may act as an energy expenditure enhancer by stimulating the PR domain-containing16 (PRDM16)/peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α)/uncoupling protein-1 (UCP-1) pathway in vivo. We therefore aimed to determine the molecular mechanism of GENS and the efficacy of the combination therapy of GENS and orlistat on weight gain, blood biochemistry and gene expression changes in high-fat diet (HFD)-induced obese mice.
2. Materials and Methods
2.1. Materials
GENS extract was obtained from NEWTREE Inc. (Seongnam, Kyeonggi, Korea). The composition of GENS extract is described in
Table 1. The following antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA): CCAAT/enhancer binding protein alpha (C/EBPα, SC-61), diacylglycerol O-acyltransferase-1 (DGAT-1, SC-32861), fatty acid synthase (FAS, SC-20140), peroxisome proliferator activated receptor gamma (PPARγ, SC-7273), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, SC-25778), sterol regulatory element binding protein-1 (SREBP-1, SC-366), and uncoupling proteins-1 (UCP-1, SC-6529). PR domain-containing16 (PRDM16, ab106410) were obtained from Abcam (Cambridge, MA, USA). Phospho-adenosine monophosphate-activated protein kinase (p-AMPK, CS-2603s), AMPK (CS-2603s), phospho-acetyl-CoA carboxylase (p-ACC, CS-3661s), and ACC (CS-3662s) were purchased from Cell Signaling Technology (Bedford, MA, USA). Orlistat and metformin were purchased from Cayman Chemical Company (Ann Arbor, MI, USA). Glucose and methylcellulose were purchased from Sigma (Sigma, St. Louis, MO, USA). All chemicals and reagents used were of analytical and obtained from commercial sources.
2.2. Animal Husbandry and Maintenance
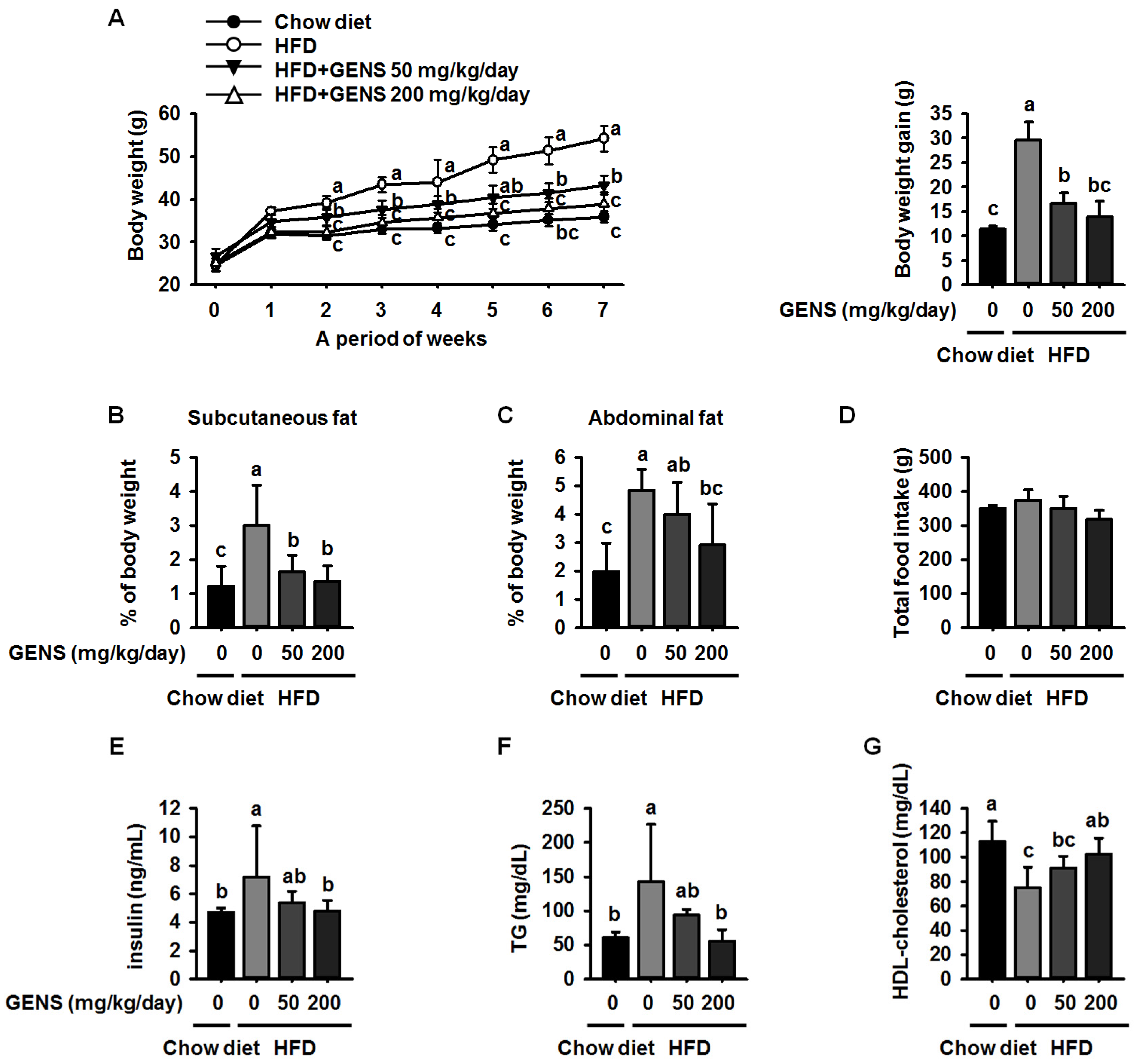
Male ICR mice (5 weeks old) were purchased form Orient Bio Co. (Gapyeong, Kyeonggi, Korea) and maintained in the animal facility at CHA University, Seongnam, Kyeonggi, Korea. The project was approved by the Institutional Animal Care and Use committee of CHA University (IACUC Approval Number 150071). Male mice were individually housed for 1 week under a 12-h light/dark cycle in temperature (20–24 °C) and humidity (44.5%–51.8%). After a 1-week adaptation period, mice were randomly divided into six groups (n = 6 per group). Mice were fed for 7 weeks with either 60% HFD (Central Lab Animal Inc., Seoul, Korea) or NIH-07 rodent chow diet (Zeigler Brothers, Gardners, PA, USA). GENS (50, 200 mg/kg/day) and/or orlistat (20 mg/kg/day) or an equal volume of vehicle (1.0% methylcellulose, HFD and chow diet group) were orally administered to the mice by gavage every day for 7 weeks. During the experiment period, and survival rates were investigated daily. The body weight, food intake, and water consumption were recorded weekly.
2.3. Concentration of Blood Glucose
The blood glucose concentration was measured from mice tail vein after 12 h (dark-period) of fasting every week, using a glucose analyzer (GlucoDr, Allmedicus, Kyeonggi, Korea).
2.4. Intraperitoneal Glucose Tolerance Test (IPGTT)
Mice were fasted for 12 h (dark-period) before IPGTT experiments, and glucose (1.0 g/kg body weight) was administered by intraperitoneal injection), as previously described [
24]. Blood samples were collected from tail vein at 30, 60, and 90 min to assess in vivo glucose clearance. Blood glucose levels were determined immediately using a glucometer (G-Doctor, Allmedicus, Anyang, Korea).
2.5. Biochemical Analysis
Mice were sacrificed by CO2 asphyxiation and cervical dislocation. Blood collected by direct cardiac puncture in an ethylenediaminetetraacetic acid (EDTA)-coated tube aseptically. Blood was allowed to clot for 1 h at room temperature and then plasma was isolated by centrifuging the blood at 13,000× g for 15 min at 4 °C to collect plasma. The plasma samples were collected and stored at −80 °C. Plasma triglycerides (TG) and high-density lipoprotein (HDL)-cholesterol levels were measured by enzymatically commercial kits (Roche, Mannheim, Germany), Plasma insulin levels were measured by enzymatically commercial kit (Wako Pure Chemical. Ltd., Osaka, Japan). The hepatic TG content was determined using a commercially available TG quantification kit (Cayman Chemical Company, Ann Arbor, MI, USA).
2.6. Organ Weight
Mice were euthanized using CO2 and cervical dislocation. Heart, lung, kidney, spleen, liver, abdominal fat, and subcutaneous fat were removed and weighed carefully.
2.7. Tissue Samples Preparation for Oil Red O
Hepatic tissues were fixed in 4% paraformaldehyde, embedded in using tissue freezing medium, optimum cutting temperature (OCT) (Cell Path Ltd., Newtown, UK). To detect fat deposition in the liver, frozen sections of 6 μm for liver were rinsed with distilled water, stained with 0.18% Oil red O (Sigma, St. Louis, MO, USA) with 60% 2-propanol (Sigma, St. Louis, MO, USA) for 20 min at 37 °C, and then rinsed with distilled water and examined under light microscopy with Nikon Eclipse E600 (Nikon, Tokyo, Japan).
2.8. Western Blot Analysis
For protein extraction, frozen tissues were homogenized in specific lysis buffer (PRO-PREP; iNtRON Biotechnology Inc., Seoul, Korea) containing protease with phosphatase inhibitor cocktail 2 and 3 (Sigma, St. Louis, MO, USA). The lysates were clarified by centrifugation at 12,000×
g for 20 min at 4 °C. The protein concentration of clarified supernatants was determined by the Bradford assay (Bio Legend, San Diego, CA, USA). Protein samples (30 μg) were separated by sodium dodecyl sulfate polyacrylamide gel electrophores (SDS-PAGE), and transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA), as previously described [
25]. The membranes were probed with each antibodies and visualized using an enhanced chemiluminescence substrate. The signals were detected with LAS image software (Fuji, New York, NY, USA).
2.9. Quantitative Reverse Transcription Polymerase Chain Reaction (Quantitative RT-PCR) Analysis
Mouse liver RNA was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA was reverse transcribed with the Maxime PCR premix Kit (iNtRON Biotechnology, Seongnam, Korea). Quantitative RT-PCR using SYBR Green 2× master mix kit (m.biotech., Inc., Seoul, Korea) was run in hexaplicate on a CFX96 Touch real-Time PCR Detection System (Bio-Rad, Hercrules, CA, USA). The 18s was used for the relative quantization of the target genes based on the comparative ∆∆ threshold cycle (
Ct) method, as previously described [
26]. Primer sequences are shown in
Table 2.
2.10. Statistical Analysis
All statistical analyses were performed using the Statistical Package for Social Sciences version 12.0 (SPSS, Chicago, IL, USA). One-way analysis of variance (ANOVA) was used for comparisons among group. Significant differences between the mean values were assessed using Duncan’s test. All values are presented as the mean ± standard deviation (SD) values. The p-value in the multiple comparison results (e.g., a, b, c, and d) indicate significant differences among the groups, p < 0.05.
4. Discussion
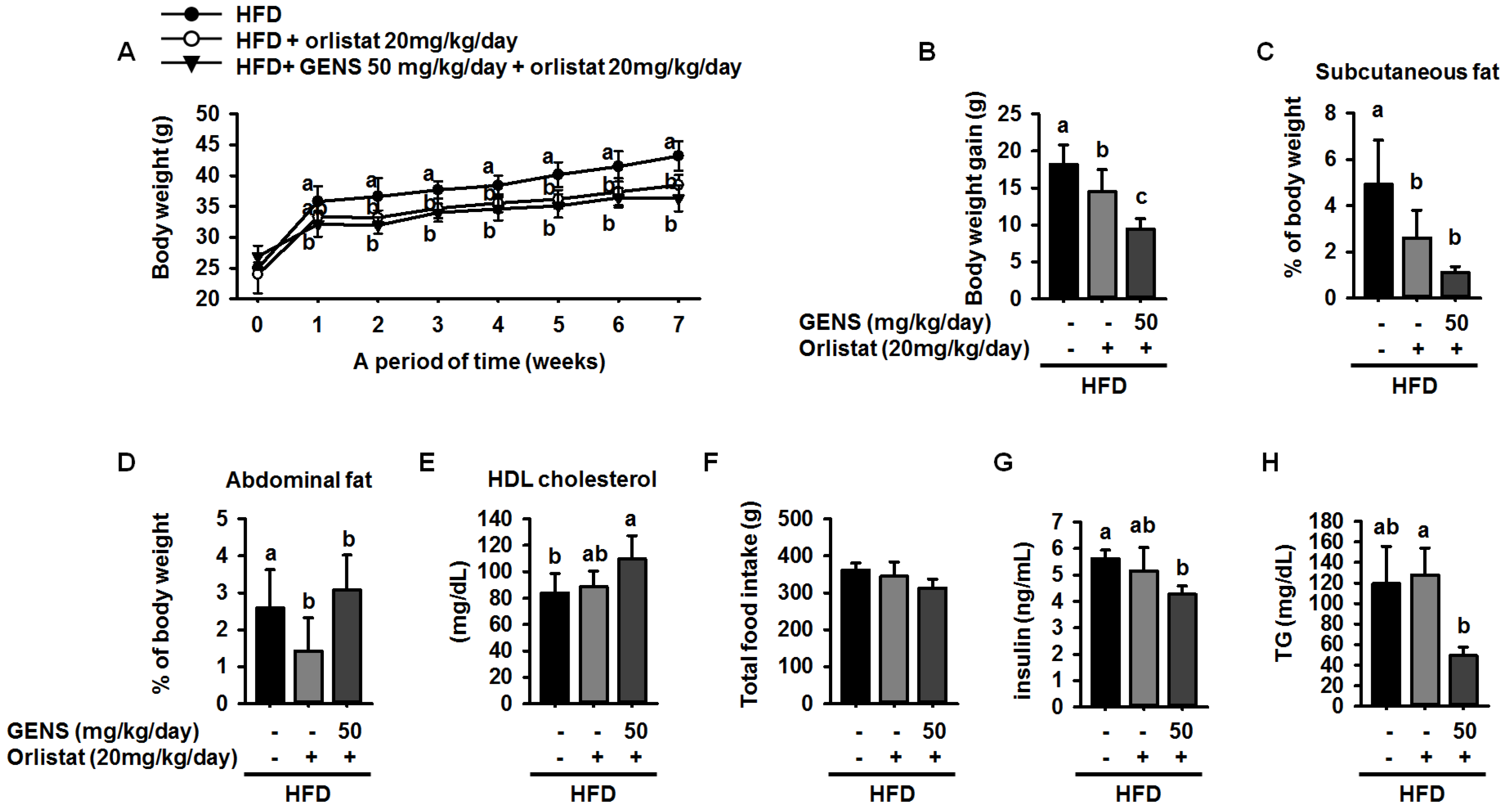
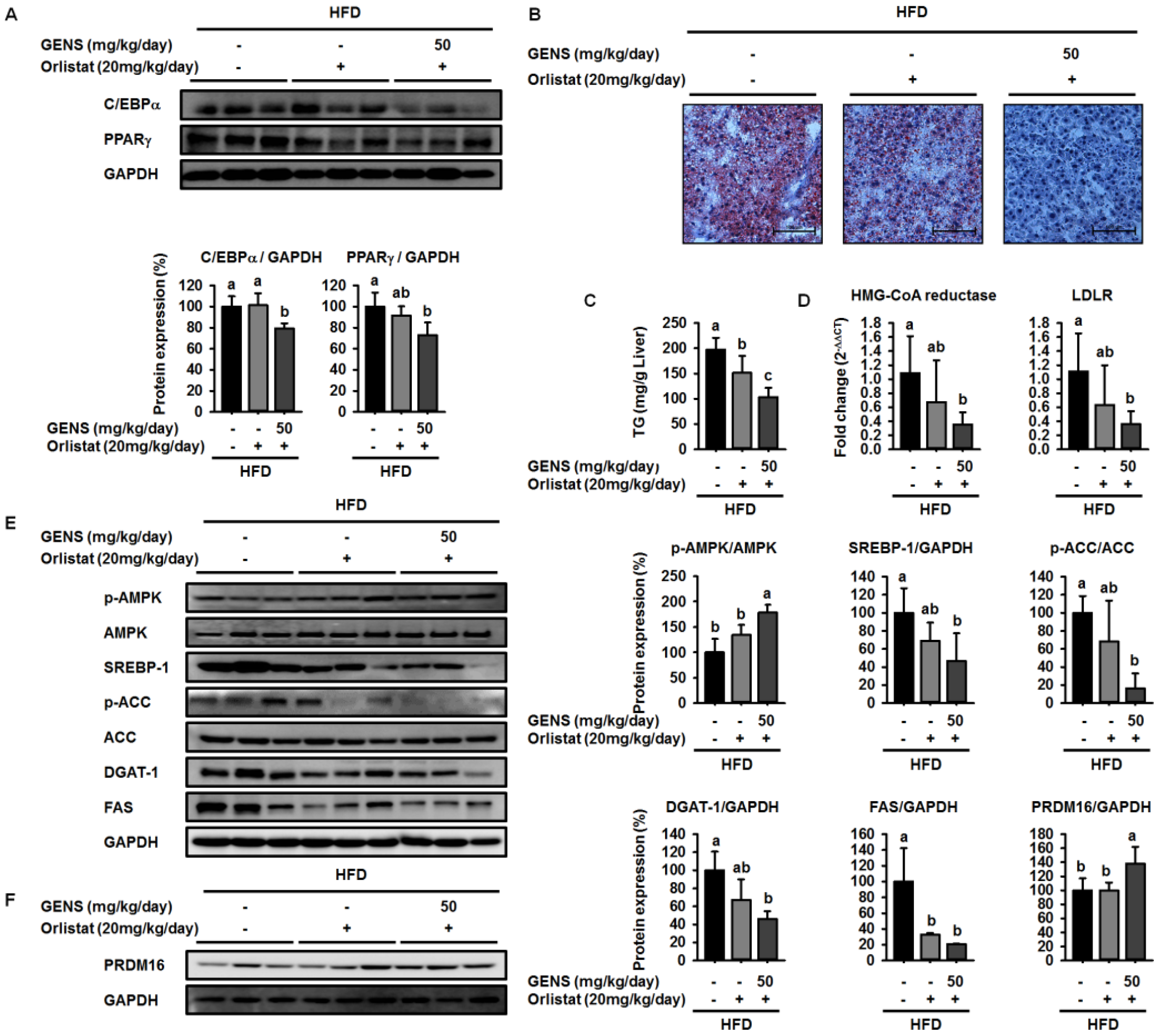
Here, we showed that GENS is sufficient to inhibit multiple characteristics of obesity, including weight gain, adipose tissue mass, hepatic lipogenesis, and hyperglycemia, in the HFD-induced obese mouse model. Notably, GENS altered energy metabolism by stimulating the expression of known thermogenesis regulator molecules such as PRDM16 and UCP-1. Moreover, we demonstrated that combined treatment with GENS and orlistat attenuated the plasma TG content and body weight gain in HFD-fed mice. In obesity, excessive energy intake promotes an increase in the storage of lipids in adipose tissue [
39], thus resulting in an increase in adipose tissue mass through the formation of new adipocytes and an increase in the size of adipocytes [
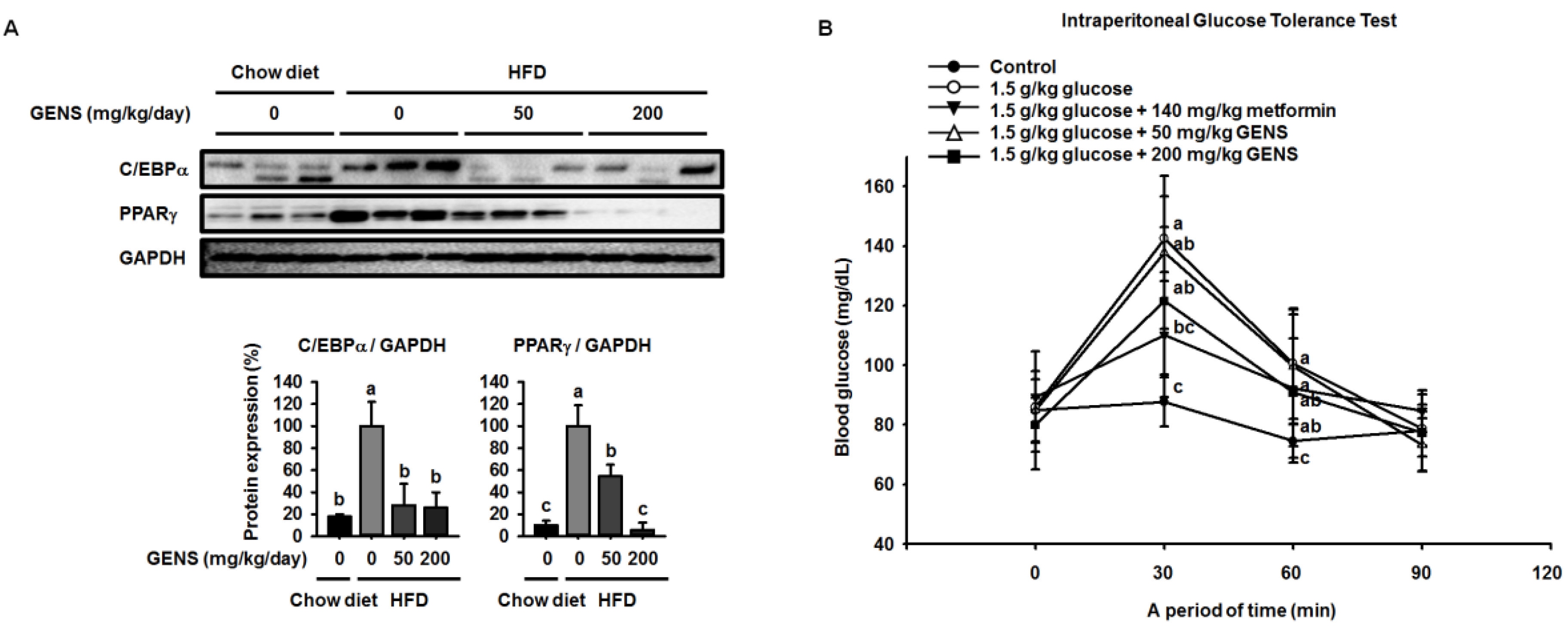
40]. These events involve cellular and molecular changes including morphological modifications of a number of critical adipogenic transcription factors, such as C/EBPα and PPARγ [
28]. HFD can activate the expression of C/EBPα and PPARγ in tissues and cause hyperglycemia resulting from diet-induced early insulin resistance through hematogenous spread [
41]. The elevated C/EBPα and PPARγ expression and increased hyperglycemia in obese mice was ameliorated by the intake of fruits, vegetables, and edible seaweed and/or its derivatives. This suggests the natural dietary substances can attenuate characteristics associated with obesity [
12,
46,
47,
48].
Our previous study, as well as recent studies, suggested that GENS may exert a protective effect on adipogenesis in adipocytes in vitro [
49] and in adipose tissue mass in vivo by regulating C/EBPα and PPARγ proteins [
19]. Consistent with previous results, our results showed that GENS decreased the expression of C/EBPα and PPARγ in white adipose tissue in HFD-fed mice.
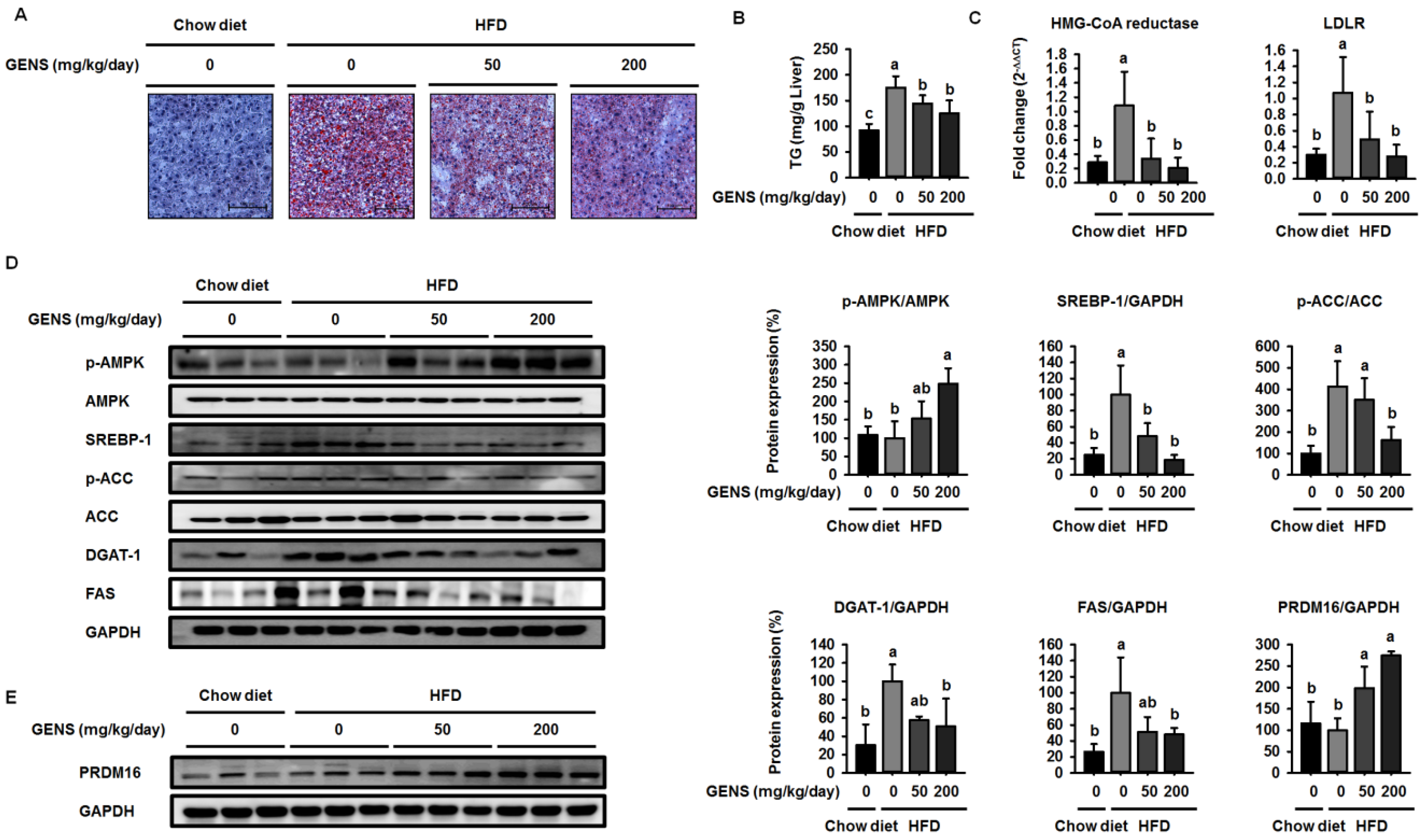
Diet-induced hepatic lipogenesis is associated with non-alcohol-induced fatty liver (NAFLD), a disease state that is a crucial factor in insulin resistance and early-stage diabetes [
50]. Prior studies have suggested that GENS has the potential to ameliorate hepatic lipid accumulation and plasma TG content in rats that have been fed a high fructose diet [
51].
However, these studies did not provide molecular mechanisms for how GENS regulates these processes. In this study, we demonstrated that GENS suppressed hepatic lipogenesis through modulation of the AMPK-SREBP-1 pathway in HFD-fed mice. In regulating energy metabolism, AMPK stimulates the interaction between PGC1α and PRDM16 resulting in increased mitochondrial biogenesis, thermogenesis, and energy expenditure [
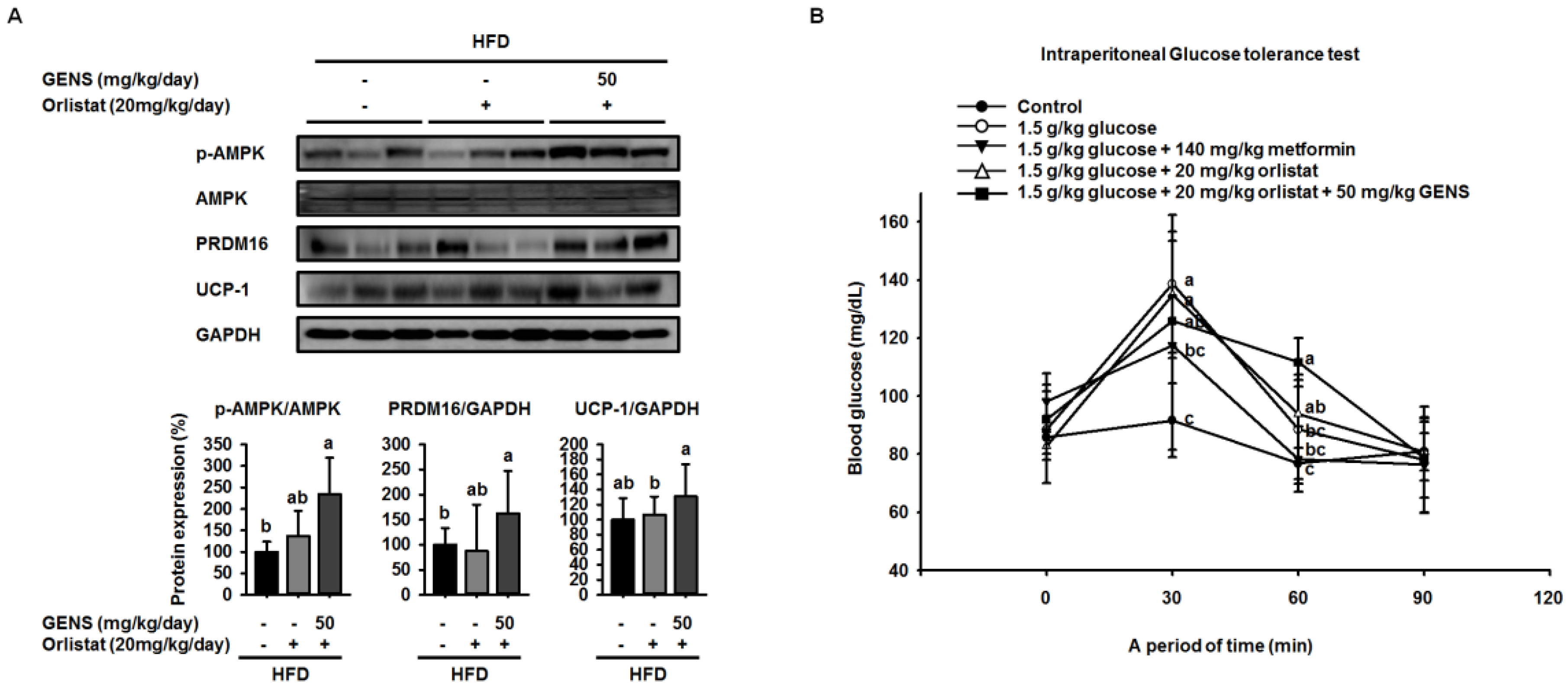
52]. Interestingly, we noticed that in HFD-fed mice treated with GENS, PRDM16 in the liver was significantly increased, indicating that GENS may elevate mitochondrial biogenesis or thermogenesis via activation of the thermogenic factor PRDM16 in these animals.
It has been previously demonstrated that HFD induces hepatic lipogenesis, leading to hyperglycemia [
53]. In this study, we revealed that hyperglycemia was induced in HFD-fed mice, whereas GENS significantly prevented HFD-mediated hyperglycemia. Although we could not address the detailed mechanism of the interaction between hepatic lipogenesis and hyperglycemia, we speculate that mitochondrial function such as biogenesis and thermogenesis might be involved in the link in these processes.
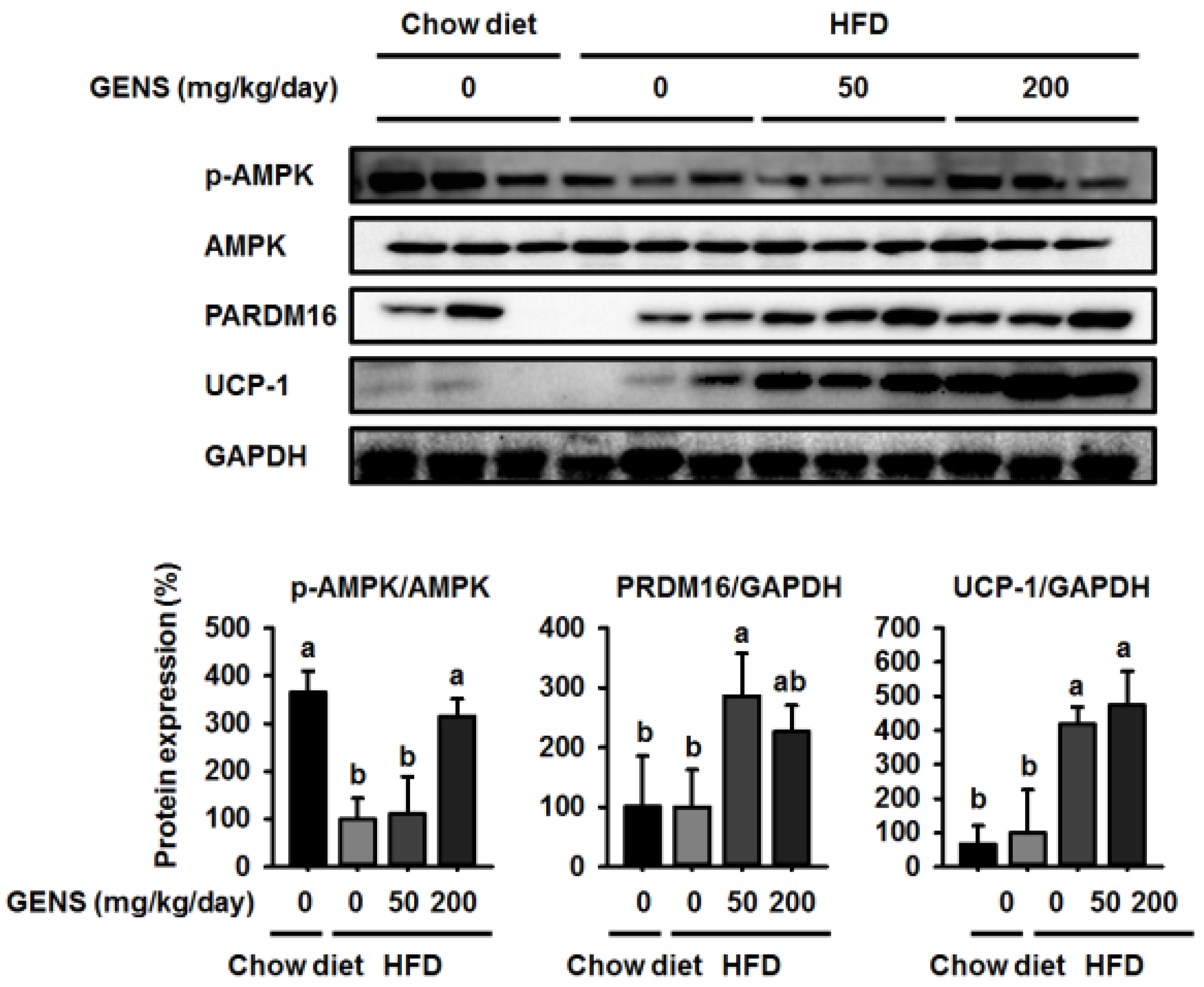
We hypothesized that if the anti-obesity effects of GENS were mainly due to the affect thermogenesis, GENS might alter the expression of thermogenesis-associated protein in brown adipose tissue. Thus, we analyzed the expression of thermogenic genes in brown adipose tissue, and our results revealed that levels of the most important thermogenesis-associated proteins AMPK, PRDM16, and UCP-1 were dramatically increased. Previous work has demonstrated that AMPK increases the expression of Sirt1 and enhances mitochondrial biogenesis through the regulation of PGC1α [
54]. It is well known that PRDM16 plays a crucial role in controlling the expression of UCP-1 in brown adipocytes [
55]. Therefore, we postulated that GENS may stimulate thermogenesis or energy expenditure through the AMPK-PRDM16-UCP-1 pathway in HFD-fed mice.
Orlistat has been used to promote weight loss and plasma TG content normalization in NAFLD and obesity patients [
56,
57]. It has also been reported as a specific intestinal lipase that breaks down TG, thus resulting in a decrease in fat absorption [
58]. As orlistat treatment can promote the inhibition of dietary fat absorption and fatty acid synthesis, we utilized a different mechanism of activity than GENS to assess whether the combination of low-dose GENS and orlistat affected obesity characteristics in HFD-fed mice. We found that the combination was effective in weight-gain prevention in HFD-fed mice. More importantly, the combined treatment with low-dose GENS and orlistat significantly inhibited the expression of C/EBPα and PPARγ in white adipose tissue. It was reasonable to assume that the beneficial effect of GENS and orlistat on adipose tissue mass in HFD-fed mice was through the inhibition of adipogenesis since there was a significant difference in this variable between the HFD group, the orlistat group, and the low-dose GENS + orlistat group. As the highest level of reduction in hyperglycemia and plasma TG content was achieved with the combination of low-dose GENS and orlistat in HFD-fed mice, this combination seems ideal to reduce obesity features. In addition, we noticed that in these mice, the thermogenic genes AMPK, PRDM16, and UCP-1 were also synergistically activated by the combination treatment. This might indicate that an active UCP-1 protein is a heat generator that in mice contributes to loss of energy in the form of heat. The animal study was performed at 20–24 °C, whereas thermoneutrality for mice is 28–30 °C. Therefore, the UCP-1 protein would be expected to be active in the mice housed under the conditions used in the present study. As the GENS mice had less adipose tissue, both subcutaneous and abdominal, they also have less insulating material. This could mean that these mice had to generate more heat through UCP-1 activation. Obviously, this would contribute to less adipose tissue mass as observed, but the mechanism could be independent of any effect of GENS or orlistat. Under these circumstances, a reduced fat absorption would contribute to less available energy, and hence accentuate the mobilization of fatty acids from white adipose tissue for UCP-1 heat generation. Although the mechanisms of action of GENS and orlistat are distinct, we have provided partial evidence that GENS and orlistat can act synergistically in HFD-fed mice.
5. Conclusions
It is known that the incidence of overweight and obesity is responsible for increased abnormalities in multiple signaling pathways, including adipogenesis, lipolysis, and energy expenditure-associated pathways [
59,
60], and can cause hyperglycemia and NAFLD. In this study, we provided evidence to suggest that in HFD-fed mice, thermogenesis is activated in response to GENS consumption, possibly through the AMPK-PRDM16-UCP-1 pathway.
This results in the modulation of hepatic lipogenesis as reflected by altered AMPK-PRDM16-UCP-1 pathway and suppression of white adipose tissue mass expansion. Moreover, we postulated that combination of GENS and orlistat is associated with more significant weight loss than the orlistat alone in HFD-fed mice. In particular, our results demonstrated a synergistic effect between GENS and orlistat on hyperglycemia and hepatic lipogenesis in HFD-fed mice. Therefore, we suggest that combination therapy with GENS and orlistat may positively influence the outcomes related to obesity characteristics in a diet-induced obese population.