Copper to Zinc Ratio as Disease Biomarker in Neonates with Early-Onset Congenital Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Trace Elements
2.4. Ceruloplasmin
2.5. Interleukin 6 and C-reactive Protein
2.6. Statistical Analysis
3. Results
3.1. Cu and Zn Status at Day of Birth in Control and Infected Newborns
3.2. Cu and CP in Relation to Gestational Age and Infection
3.3. Associations of Zn with Gestational Age, Birth Weight, and Infection
3.4. Cu/Zn Ratio
4. Discussion
4.1. Early-Onset Congenital Infections as Disruptors of the Trace Element Homeostasis
4.2. The Cu/Zn Ratio as a Potential Biomarker of Early-Onset Congenital Infections
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stern, B.R. Essentiality and toxicity in copper health risk assessment: Overview, update and regulatory considerations. J. Toxicol. Environ. Health Part A 2010, 73, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Benedetti, G.; Albarede, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- De Romana, D.L.; Olivares, M.; Uauy, R.; Araya, M. Risks and benefits of copper in light of new insights of copper homeostasis. J. Trace Elem. Med. Biol. 2011, 25, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Hornig, D.H. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br. J. Nutr. 2007, 98 (Suppl. S1), 29–35. [Google Scholar] [CrossRef] [PubMed]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F.; Miller, L.V.; Hambidge, K.M. Zinc deficiency in infants and children: A review of its complex and synergistic interactions. Paediatr. Int. Child Health 2014, 34, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Milanino, R.; Marrella, M.; Gasperini, R.; Pasqualicchio, M.; Velo, G. Copper and zinc body levels in inflammation: An overview of the data obtained from animal and human studies. Agents Actions 1993, 39, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Besecker, B.Y.; Exline, M.C.; Hollyfield, J.; Phillips, G.; Disilvestro, R.A.; Wewers, M.D.; Knoell, D.L. A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am. J. Clin. Nutr. 2011, 93, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, F.; Gashut, R.A.; Talwar, D.; Duncan, A.; Beulshausen, J.F.; McMillan, D.C.; Kinsella, J. Assessment of plasma and red cell trace element concentrations, disease severity, and outcome in patients with critical illness. J. Crit. Care 2014, 29, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Rech, M.; To, L.; Tovbin, A.; Smoot, T.; Mlynarek, M. Heavy metal in the intensive care unit: A review of current literature on trace element supplementation in critically ill patients. Nutr. Clin. Pract. 2014, 29, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.A.; Padbury, J.F. Neonatal sepsis: An old problem with new insights. Virulence 2014, 5, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, E.; Gest, A. Neonatal sepsis: An old issue needing new answers. Lancet Infect. Dis. 2015, 15, 503–505. [Google Scholar] [CrossRef]
- Hedegaard, S.S.; Wisborg, K.; Hvas, A.M. Diagnostic utility of biomarkers for neonatal sepsis-a systematic review. Infect. Dis. 2015, 47, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; Ferrara, T.; Maffucci, R.; Milite, P.; Del Buono, D.; Santoro, P.; Grimaldi, L.C. Neonatal sepsis: A difficult diagnostic challenge. Clin. Biochem. 2011, 44, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Bajcetic, M.; Spasic, S.; Spasojevic, I. Redox therapy in neonatal sepsis: Reasons, targets, strategy, and agents. Shock 2014, 42, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Alshaikh, B.; Yusuf, K.; Sauve, R. Neurodevelopmental outcomes of very low birth weight infants with neonatal sepsis: Systematic review and meta-analysis. J. Perinatol. 2013, 33, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Karahan, S.C.; Deger, O.; Orem, A.; Ucar, F.; Erem, C.; Alver, A.; Onder, E. The effects of impaired trace element status on polymorphonuclear leukocyte activation in the development of vascular complications in type 2 diabetes mellitus. Clin. Chem. Lab. Med. 2001, 39, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Karakas, Z.; Demirel, N.; Tarakcioglu, M.; Mete, N. Serum zinc and copper levels in southeastern turkish children with giardiasis or amebiasis. Biol. Trace Elem. Res. 2001, 84, 11–18. [Google Scholar] [CrossRef]
- Oyama, T.; Matsuno, K.; Kawamoto, T.; Mitsudomi, T.; Shirakusa, T.; Kodama, Y. Efficiency of serum copper/zinc ratio for differential diagnosis of patients with and without lung cancer. Biol. Trace Elem. Res. 1994, 42, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Donma, M.M.; Donma, O.; Tas, M.A. Hair zinc and copper concentrations and zinc: Copper ratios in pediatric malignancies and healthy children from southeastern turkey. Biol. Trace Elem. Res. 1993, 36, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Luterotti, S.; Kordic, T.V.; Letoja, I.Z.; Dodig, S. Contribution to diagnostics/prognostics of tuberculosis in children. Ii. Indicative value of metal ions and biochemical parameters in serum. Acta Pharm. 2015, 65, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Wiehe, L.; Cremer, M.; Wisniewska, M.; Becker, N.P.; Rijntjes, E.; Martitz, J.; Hybsier, S.; Renko, K.; Buhrer, C.; Schomburg, L. Selenium status in neonates with connatal infection. Br. J. Nutr. 2016, 116, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Young Infants Clinical Signs Study Group. Clinical signs that predict severe illness in children under age 2 months: A multicentre study. Lancet 2008, 371, 135–142. [Google Scholar]
- Goldstein, B.; Giroir, B.; Randolph, A.; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Dollner, H.; Vatten, L.; Austgulen, R. Early diagnostic markers for neonatal sepsis: Comparing c-reactive protein, interleukin-6, soluble tumour necrosis factor receptors and soluble adhesion molecules. J. Clin. Epidemiol. 2001, 54, 1251–1257. [Google Scholar] [CrossRef]
- Apgar, V. A proposal for a new method of evaluation of the newborn infant. Curr. Res. Anesth. Analg. 1953, 32, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Stosnach, H. Environmental trace-element analysis using a benchtop total reflection X-ray fluorescence spectrometer. Anal. Sci. 2005, 21, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Lombeck, I.; Fuchs, A. Zinc and copper in infants fed breast-milk or different formula. Eur. J. Pediatr. 1994, 153, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, E.; Liu, J.; Lou, X.; Jia, L.; Li, X.; Zhang, L. Gestational age-specific reference intervals for blood copper, zinc, calcium, magnesium, iron, lead, and cadmium during normal pregnancy. Clin. Biochem. 2013, 46, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Salmenpera, L.; Perheentupa, J.; Pakarinen, P.; Siimes, M.A. Cu nutrition in infants during prolonged exclusive breast-feeding: Low intake but rising serum concentrations of cu and ceruloplasmin. Am. J. Clin. Nutr. 1986, 43, 251–257. [Google Scholar] [PubMed]
- Shenkin, A. Trace elements and inflammatory response: Implications for nutritional support. Nutrition 1995, 11, 100–105. [Google Scholar] [PubMed]
- Koo, W.W.K.; Succop, P.; Hambidge, K.M. Sequential concentrations of copper and ceruloplasmin in serum from preterm infants with rickets and fractures. Clin. Chem. 1991, 37, 556–559. [Google Scholar] [PubMed]
- Li, Z.H.; Wang, Y.; Wang, J.; Tang, Z.W.; Pounds, J.G.; Lin, Y.H. Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal. Chem. 2010, 82, 7008–7014. [Google Scholar] [CrossRef] [PubMed]
- Mohan, G.; Kulshreshtha, S.; Dayal, R.; Singh, M.; Sharma, P. Effect of therapy on serum zinc and copper in primary complex of children. Biol. Trace Elem. Res. 2007, 118, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Canatan, H.; Bakan, I.; Akbulut, M.; Halifeoglu, I.; Cikim, G.; Baydas, G.; Kilic, N. Relationship among levels of leptin and zinc, copper, and zinc/copper ratio in plasma of patients with essential hypertension and healthy normotensive subjects. Biol. Trace Elem. Res. 2004, 100, 117–123. [Google Scholar] [CrossRef]
- Faber, S.; Zinn, G.M.; Kern, J.C., 2nd; Kingston, H.M. The plasma zinc/serum copper ratio as a biomarker in children with autism spectrum disorders. Biomarkers 2009, 14, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Buntzel, J.; Glatzel, M.; Micke, O.; Mucke, R.; Schonekaes, K.; Bruns, F.; Frohlich, D. The copper-zinc-ratio as marker of tumor activity in head and neck cancer? Strahlenther. Onkol. 2005, 181, 122. [Google Scholar]
- Malavolta, M.; Giacconi, R.; Piacenza, F.; Santarelli, L.; Cipriano, C.; Costarelli, L.; Tesei, S.; Pierpaoli, S.; Basso, A.; Galeazzi, R.; et al. Plasma copper/zinc ratio: An inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population. Biogerontology 2010, 11, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.H.; Chen, P.C.; Yeh, M.S.; Hsiung, D.Y.; Wang, C.L. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin. Biochem. 2011, 44, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Cvijanovich, N.Z.; King, J.C.; Flori, H.R.; Gildengorin, G.; Vinks, A.A.; Wong, H.R. A safety and dose escalation study of intravenous zinc supplementation in pediatric critical illness. JPEN 2016, 40, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, M.K.; Choi, B.Y. The relationship between zinc status and inflammatory marker levels in rural Korean adults aged 40 and older. PLoS ONE 2015, 10, e0130016. [Google Scholar] [CrossRef] [PubMed]
- Cvijanovich, N.Z.; King, J.C.; Flori, H.R.; Gildengorin, G.; Wong, H.R. Zinc homeostasis in pediatric critical illness. Pediatr. Crit. Care Med. 2009, 10, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.; Talwar, D.; McMillan, D.C.; Stefanowicz, F.; O’Reilly, D.S. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am. J. Clin. Nutr. 2012, 95, 64–71. [Google Scholar] [CrossRef] [PubMed]

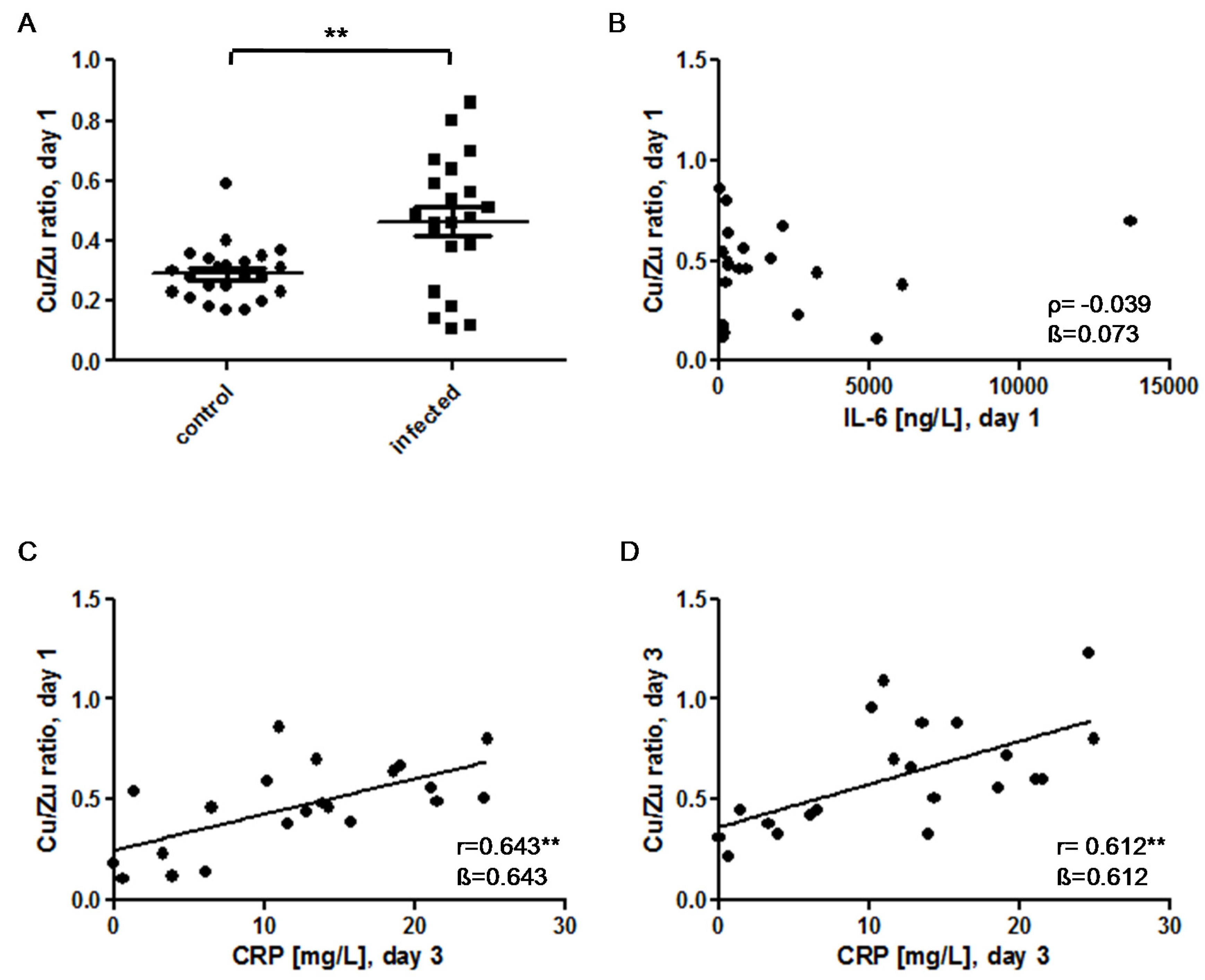
 ) indicates pregnancy length until term, and includes the preterm neonates (<37 weeks of gestation); ρ: Spearman’s rank correlation coefficient; r: Pearson correlation coefficient; β: standardised regression coefficient; **: p < 0.01.
) indicates pregnancy length until term, and includes the preterm neonates (<37 weeks of gestation); ρ: Spearman’s rank correlation coefficient; r: Pearson correlation coefficient; β: standardised regression coefficient; **: p < 0.01.
 ) indicates pregnancy length until term, and includes the preterm neonates (<37 weeks of gestation); ρ: Spearman’s rank correlation coefficient; r: Pearson correlation coefficient; β: standardised regression coefficient; **: p < 0.01.
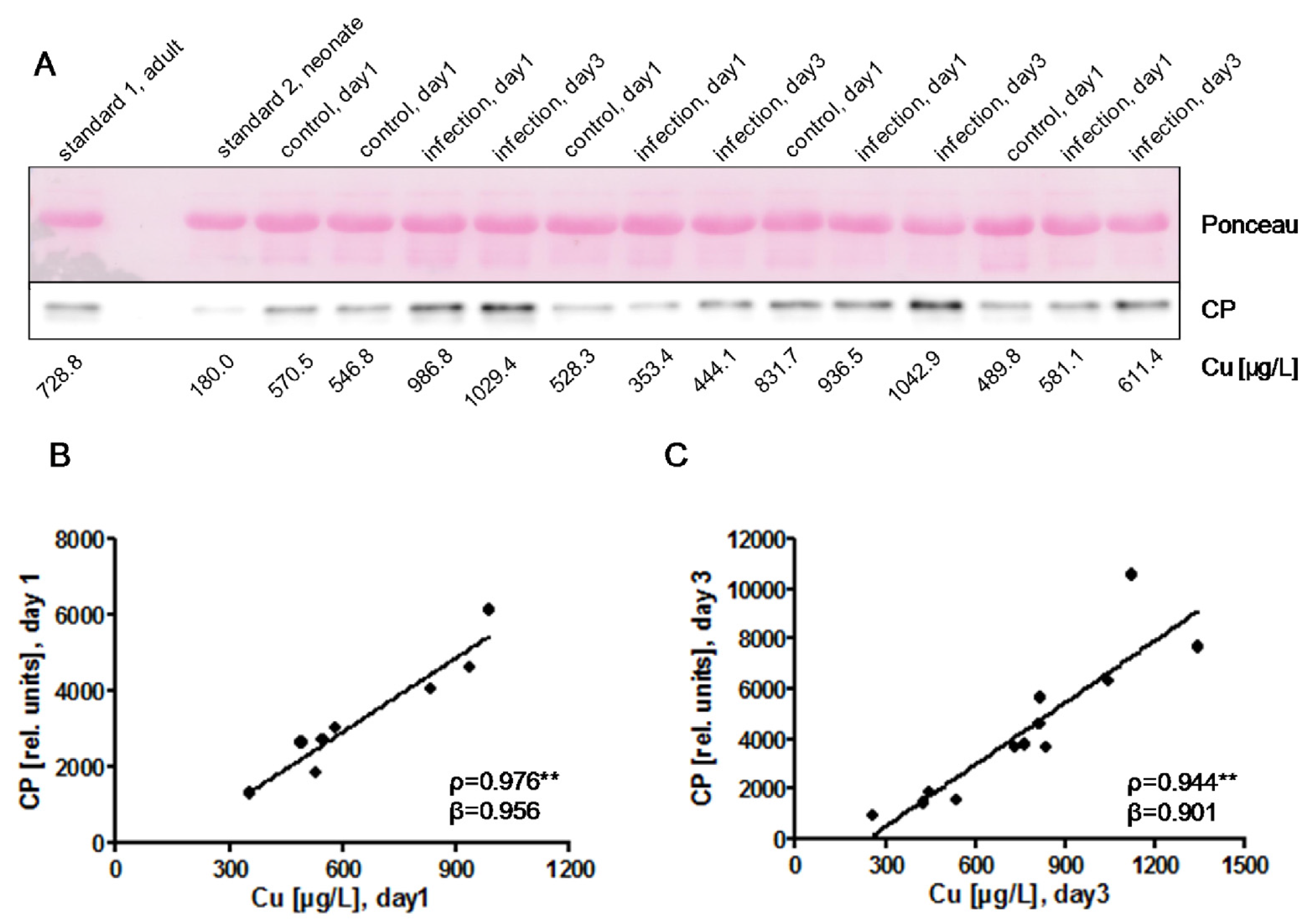
) indicates pregnancy length until term, and includes the preterm neonates (<37 weeks of gestation); ρ: Spearman’s rank correlation coefficient; r: Pearson correlation coefficient; β: standardised regression coefficient; **: p < 0.01.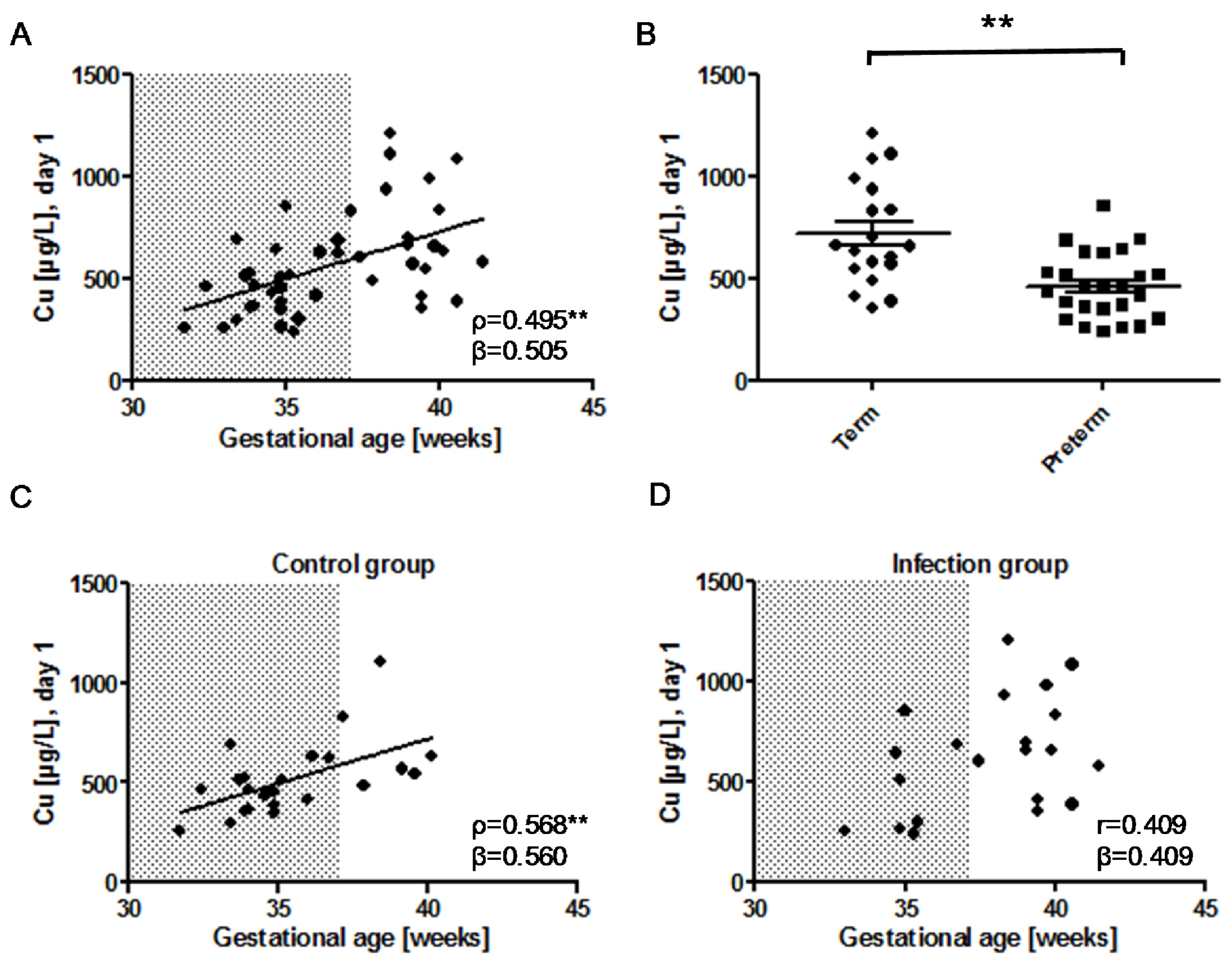
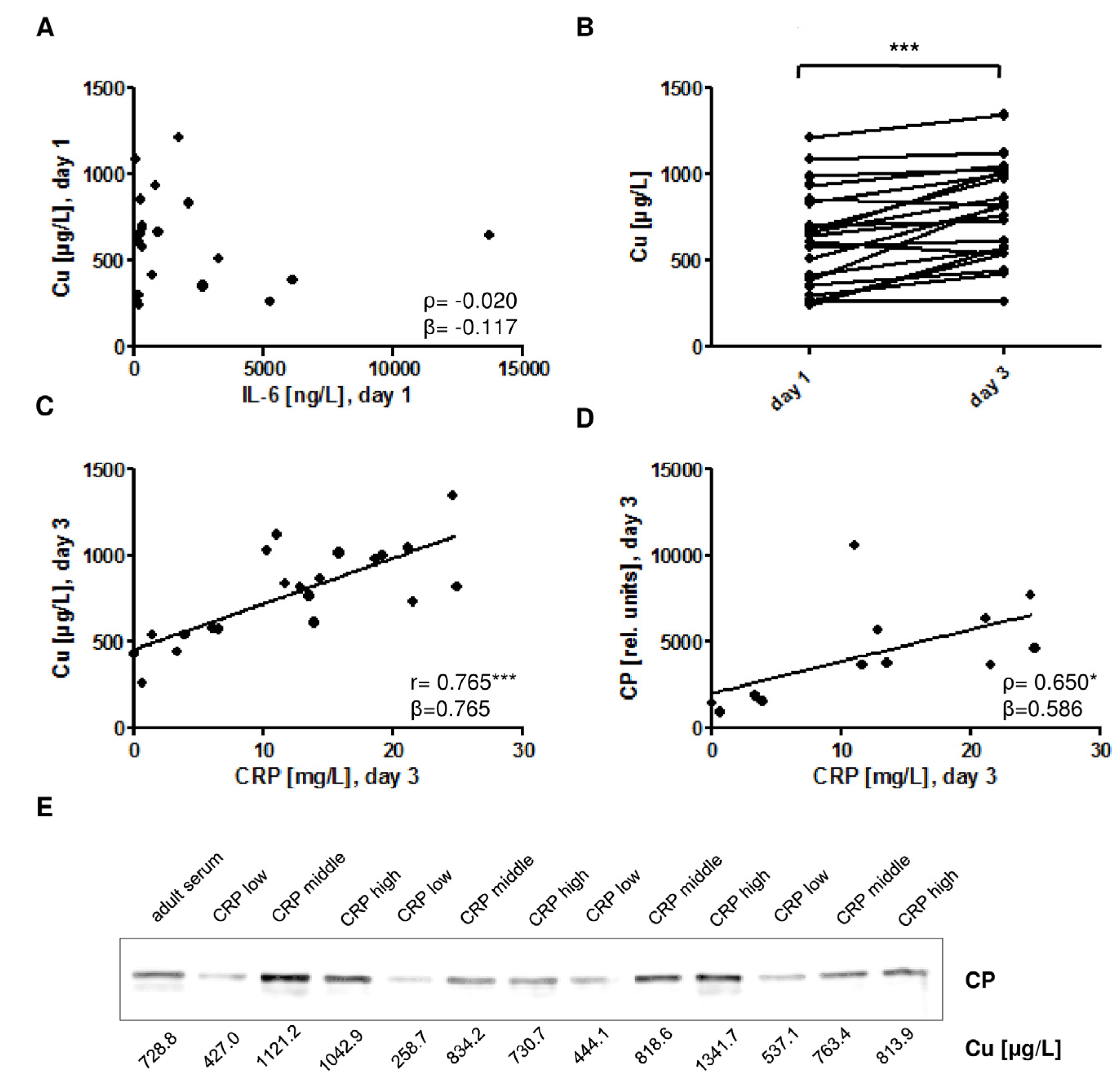
 ) indicates the preterm neonates (<37 weeks of gestation); ρ: Spearman’s rank correlation coefficient; r: Pearson correlation coefficient; β: standardised regression coefficient; *: p < 0.05; **: p < 0.01.
) indicates the preterm neonates (<37 weeks of gestation); ρ: Spearman’s rank correlation coefficient; r: Pearson correlation coefficient; β: standardised regression coefficient; *: p < 0.05; **: p < 0.01.
 ) indicates the preterm neonates (<37 weeks of gestation); ρ: Spearman’s rank correlation coefficient; r: Pearson correlation coefficient; β: standardised regression coefficient; *: p < 0.05; **: p < 0.01.
) indicates the preterm neonates (<37 weeks of gestation); ρ: Spearman’s rank correlation coefficient; r: Pearson correlation coefficient; β: standardised regression coefficient; *: p < 0.05; **: p < 0.01.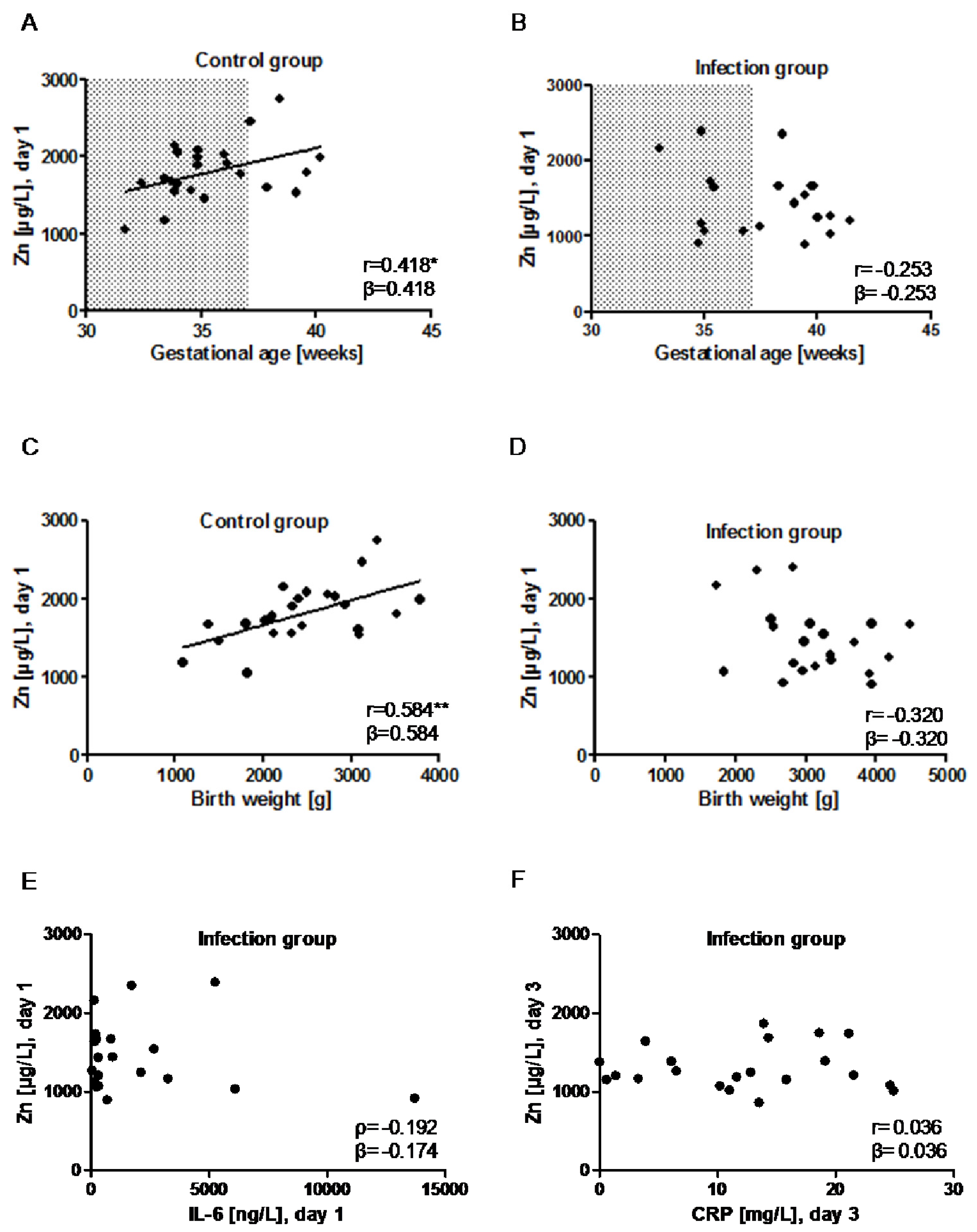
| Control Group | Infection Group | p-Value (Two-Sided) | |
|---|---|---|---|
| Participants | n = 23 | n = 21 | - |
| Gestation age [weeks] | 34.9 [33.9–37.1] | 38.4 [35.1–39.8] | 0.003 |
| Term infants | n = 6 (26%) | n = 13 (62%) | |
| Preterm infants (<37 gestation weeks) | n = 17 (74%) | n = 8 (38%) | |
| Birth Weight [g] | 2452.3 ± 694.1 | 3119.7 ± 733.7 | 0.003 |
| Vaginal birth | n = 7 (30%) | n = 10 (48%) | - |
| Twins | n = 7 (30%) | n = 1 (5%) | - |
| Apgar 1 min * | 9.0 [6.0–9.0] | 8.0 [4.5–9.0] | 0.505 |
| Apgar 5 min * | 9.0 [8.0–10.0] | 9.0 [7.5–10.0] | 0.951 |
| Cord arterial pH | 7.25 [7.2–7.29] | 7.23 [7.19–7.3] | 0.655 |
| pH on admission # | 7.33 [7.27–7.38] | 7.33 [7.26–7.37] | 0.673 |
| Base excess on admission ## | −2.15 [(−3.75)–(−0.90)] | −2.8 [(−5.32)–(−1.28)] | 0.283 |
| IL-6 [pg/mL], day 1 | 5.8 [4.2–17.0] | 498.5 [203.6–2523.0] | <0.001 |
| Clinical Characteristics | |
|---|---|
| Pneumonia/Respiratory Distress | n = 19 |
| Tachycardia/bradycardia | n = 6 |
| Fever/hypothermia | n = 2 |
| Irritability/lethargy | n = 3 |
| Coagulation disorder | n = 0 |
| IL-6 [ng/L], day 1 (median [IQR]) | 498.5 [203.6–2523.0] |
| CRP [mg/L], day 3, (mean ± SD) | 12.1 ± 7.8 |
| Antibiotic treatment [days] (median [IQR]) | 5.0 [3.0–5.0] |
| Significance | Odds Ratio | 95% CI | |
|---|---|---|---|
| Gestational age | 0.079 | 1.310 | 0.969–1.770 |
| Cu/Zn ratio | 0.034 | 164.224 | 1.457–18,506.693 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisniewska, M.; Cremer, M.; Wiehe, L.; Becker, N.-P.; Rijntjes, E.; Martitz, J.; Renko, K.; Bührer, C.; Schomburg, L. Copper to Zinc Ratio as Disease Biomarker in Neonates with Early-Onset Congenital Infections. Nutrients 2017, 9, 343. https://doi.org/10.3390/nu9040343
Wisniewska M, Cremer M, Wiehe L, Becker N-P, Rijntjes E, Martitz J, Renko K, Bührer C, Schomburg L. Copper to Zinc Ratio as Disease Biomarker in Neonates with Early-Onset Congenital Infections. Nutrients. 2017; 9(4):343. https://doi.org/10.3390/nu9040343
Chicago/Turabian StyleWisniewska, Monika, Malte Cremer, Lennart Wiehe, Niels-Peter Becker, Eddy Rijntjes, Janine Martitz, Kostja Renko, Christoph Bührer, and Lutz Schomburg. 2017. "Copper to Zinc Ratio as Disease Biomarker in Neonates with Early-Onset Congenital Infections" Nutrients 9, no. 4: 343. https://doi.org/10.3390/nu9040343





