Effects of Higher Dietary Protein and Fiber Intakes at Breakfast on Postprandial Glucose, Insulin, and 24-h Interstitial Glucose in Overweight Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Breakfast Characteristics
2.4. Dietary Intake
2.5. Body Composition
2.6. 24-h Glucose (CGM)
2.7. Biochemical Analyses and Calculation
2.8. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Breakfast Treatments Effect on Outcome Measures
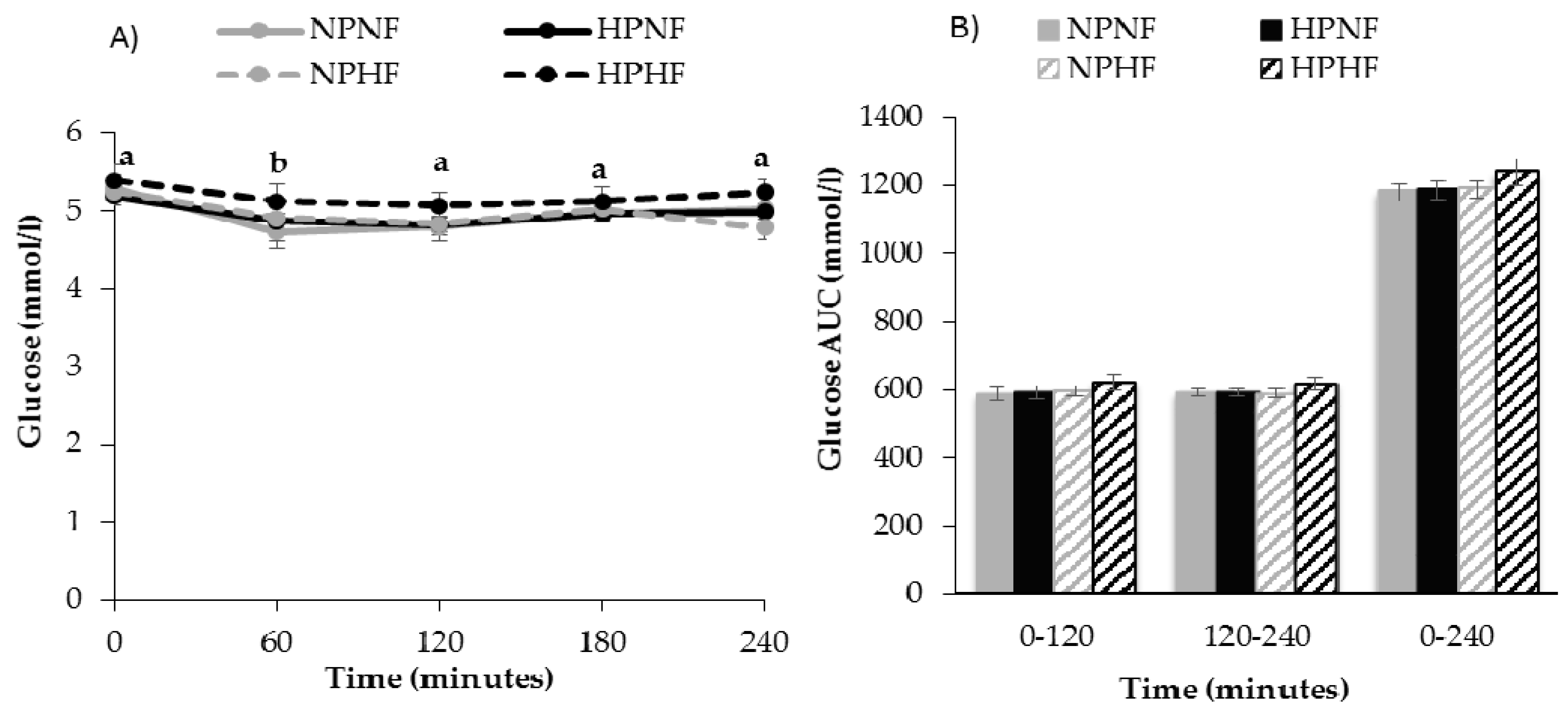
3.2.1. The Effect of Breakfast Treatment on Fasting Glucose and Insulin Variables
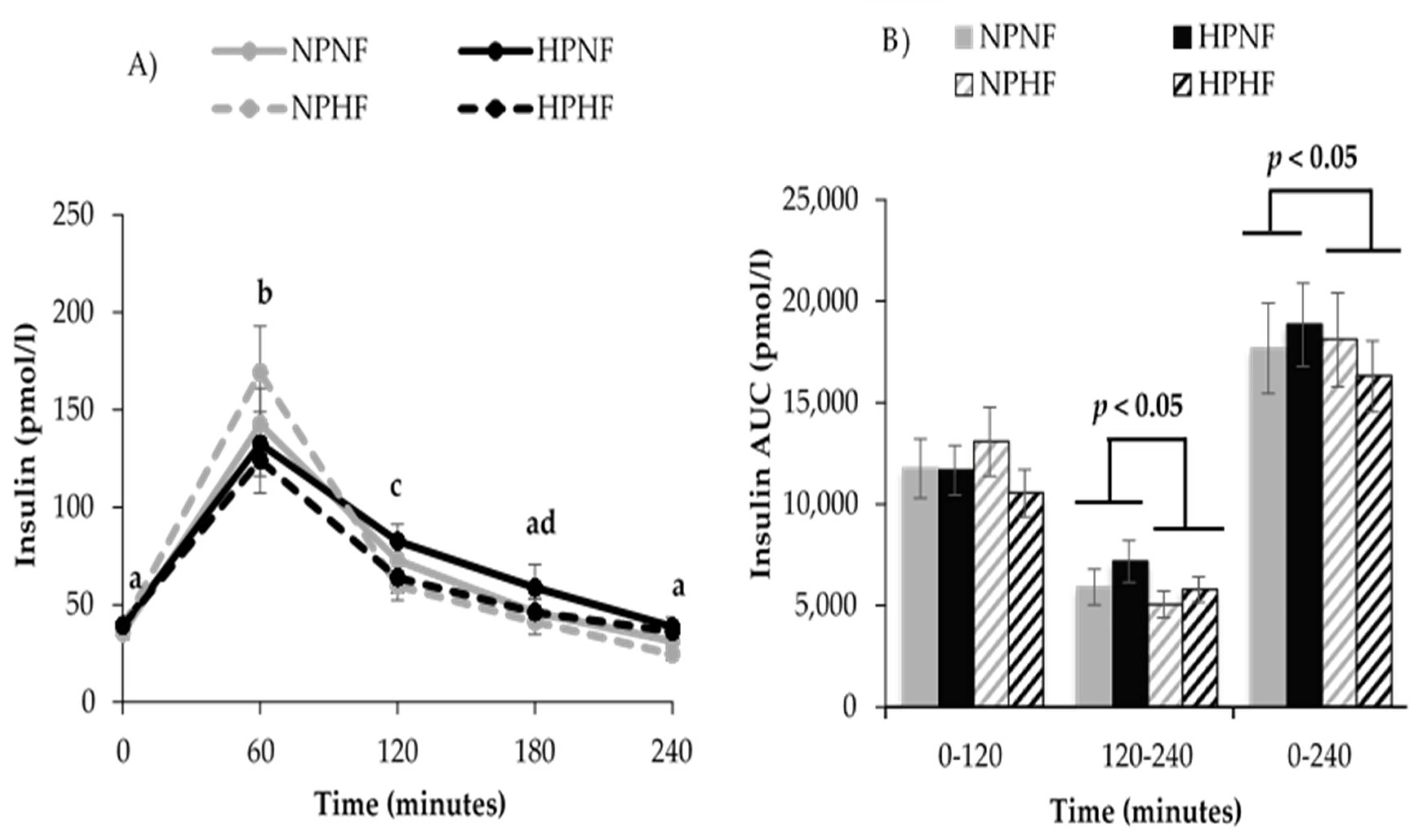
3.2.2. Time Course of Postprandial Glucose and Insulin Responses (AUC)
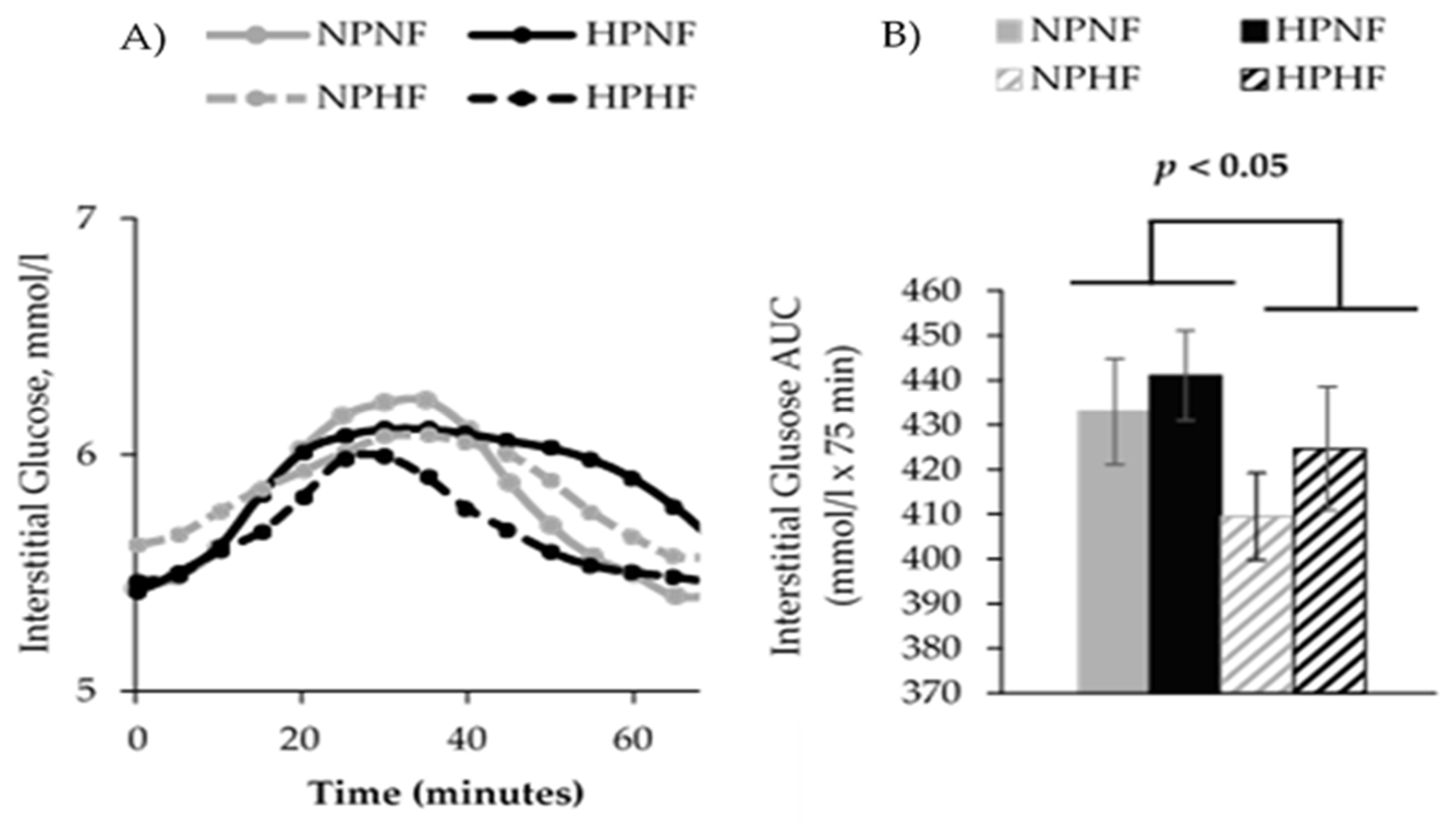
3.3. 24-h Interstitial Glucose Variables
3.4. Daily Energy and Macronutrients Intake
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Geiss, L.S.; Pan, L.; Cadwell, B.; Gregg, E.W.; Benjamin, S.M.; Engelgau, M.M. Changes in Incidence of Diabetes in U.S. Adults, 1997–2003. Am. J. Prev. Med. 2006, 30, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Cowie, C.C.; Rust, K.F.; Byrd-Holt, D.D.; Eberhardt, M.S.; Flegal, K.M.; Engelgau, M.M.; Saydah, S.H.; Williams, D.E.; Geiss, L.S.; Gregg, E.W. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002. Diabetes Care 2006, 29, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Kahn, R.; Robertson, R.M.; Rizza, R.A. Preventing Cardiovascular Disease and Diabetes: A Call to Action From the American Diabetes Association and the American Heart Association. Circulation 2006, 113, 2943–2946. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Pfeiffer, A.F. Foods for the prevention of diabetes: How do they work? Diabetes Metab. Res. Rev. 2012, 28, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Martinez-Gonzalez, M.A.; Bullo, M.; Ros, E. The role of diet in the prevention of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2011, 21, B32–B48. [Google Scholar] [CrossRef] [PubMed]
- Wyness, L. Understanding the role of diet in type 2 diabetes prevention. Br. J. Community Nurs. 2009, 14, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.L.; Dunbar, S.A.; Jaacks, L.M.; Karmally, W.; Mayer-Davis, E.J.; Wylie-Rosett, J.; Yancy, W.S., Jr. Macronutrients, food groups, and eating patterns in the management of diabetes: A systematic review of the literature, 2010. Diabetes Care 2012, 35, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Tosti, V.; Redman, L.M.; Fontana, L. Calorie restriction in humans: An update. Ageing Res. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Omodei, D.; Fontana, L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011, 585, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.L.; Burke, V.; Morton, A.R.; Beilin, L.J.; Puddey, I.B. Independent and additive effects of energy restriction and exercise on glucose and insulin concentrations in sedentary overweight men. Am. J. Clin. Nutr. 2004, 80, 308–316. [Google Scholar] [PubMed]
- Heymsfield, S.B.; Harp, J.B.; Reitman, M.L.; Beetsch, J.W.; Schoeller, D.A.; Erondu, N.; Pietrobelli, A. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am. J. Clin. Nutr. 2007, 85, 346–354. [Google Scholar] [PubMed]
- Blom, W.A.; Lluch, A.; Stafleu, A.; Vinoy, S.; Holst, J.J.; Schaafsma, G. Effect of a high-protein breakfast on the postprandial ghrelin response. Am. J. Clin. Nutr. 2006, 83, 211–220. [Google Scholar] [PubMed]
- Erdmann, J.; Topsch, R.; Lippl, F.; Gussmann, P.; Schusdziarra, V. Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J. Clin. Endocr. Metab. 2004, 89, 3048–3054. [Google Scholar] [CrossRef] [PubMed]
- Sheard, N.F.; Clark, N.G.; Brand-Miller, J.C.; Franz, M.J.; Pi-Sunyer, F.X.; Mayer-Davis, E.; Kulkarni, K.; Geil, P. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: A statement by the american diabetes association. Diabetes Care 2004, 27, 2266–2271. [Google Scholar] [CrossRef] [PubMed]
- Franc, S.; Dardari, D.; Peschard, C.; Riveline, J.-P.; Biedzinski, M.; Boucherie, B.; Petit, C.; Requeda, E.; Mistretta, F.; Varroud-Vial, M.; Charpentier, G. Can Postprandial Blood Glucose Excursion Be Predicted in Type 2 Diabetes? Diabetes Care 2010, 33, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- AlEssa, H.B.; Bhupathiraju, S.N.; Malik, V.S.; Wedick, N.M.; Campos, H.; Rosner, B.; Willett, W.C.; Hu, F.B. Carbohydrate quality and quantity and risk of type 2 diabetes in US women. Am. J. Clin. Nutr. 2015, 102, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Gross, L.S.; Li, L.; Ford, E.S.; Liu, S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: An ecologic assessment. Am. J. Clin. Nutr. 2004, 79, 774–779. [Google Scholar] [PubMed]
- Hu, F.B. Are refined carbohydrates worse than saturated fat? Am. J. Clin. Nutr. 2010, 91, 1541–1542. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Intake of Refined Carbohydrates and Whole Grain Foods in Relation to Risk of Type 2 Diabetes Mellitus and Coronary Heart Disease. J. Am. Coll. Nutr. 2002, 21, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and Lifestyle Recommendations Revision 2006: A Scientific Statement From the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.; de Bruijne, L.M.; Stolk, R.P.; Peeters, P.H.M.; Bots, M.L.; Grobbee, D.E.; van der Schouw, Y.T. High Dietary Glycemic Load and Glycemic Index Increase Risk of Cardiovascular Disease Among Middle-Aged Women: A Population-Based Follow-Up Study. J. Am. Coll. Cardiol. 2007, 50, 14–21. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.W. Evidence-Based Approach to Fiber Supplements and Clinically Meaningful Health Benefits, Part 1: What to Look for and How to Recommend an Effective Fiber Therapy. Nutr. Today 2015, 50, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Chutkan, R.; Fahey, G.; Wright, W.L.; McRorie, J. Viscous versus nonviscous soluble fiber supplements: Mechanisms and evidence for fiber-specific health benefits. J. Am. Acad. Nurse Pract. 2012, 24, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Leeds, A.R.; Gassull, M.A.; Haisman, P.; Dilawari, J.; Goff, D.V.; Metz, G.L.; Alberti, K.G. Dietary fibres, fibre analogues, and glucose tolerance: Importance of viscosity. Br. Med. J. 1978, 1, 1392–1394. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Vuksan, V.; Vidgen, E.; Parker, T.; Faulkner, D.; Mehling, C.C.; Garsetti, M.; Testolin, G.; Cunnane, S.C.; et al. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: Serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am. J. Clin. Nutr. 2002, 75, 834–839. [Google Scholar] [PubMed]
- Dall’Alba, V.; Silva, F.M.; Antonio, J.P.; Steemburgo, T.; Royer, C.P.; Almeida, J.C.; Gross, J.L.; Azevedo, M.J. Improvement of the metabolic syndrome profile by soluble fibre–guar gum–in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2013, 110, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.G.; Derosa, G.; Bove, M.; Imola, F.; Borghi, C.; Gaddi, A. Psyllium improves dyslipidaemia, hyperglycaemia and hypertension, while guar gum reduces body weight more rapidly in patients affected by metabolic syndrome following an AHA Step 2 diet. Mediterr. J. Nutr. Metab. 2010, 3, 47–54. [Google Scholar] [CrossRef]
- Gunness, P.; Gidley, M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food Funct. 2010, 1, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.; Tosh, S.M.; Gibbs, A.L.; Brand-Miller, J.; Duncan, A.M.; Hart, V.; Lamarche, B.; Thomson, B.A.; Duss, R.; Wood, P.J. Physicochemical properties of oat beta-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomized clinical trial. Am. J. Clin. Nutr. 2010, 92, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Gibb, R.D.; McRorie, J.W.; Russell, D.A.; Hasselblad, V.; D’Alessio, D.A. Psyllium fiber improves glycemic control proportional to loss of glycemic control: A meta-analysis of data in euglycemic subjects, patients at risk of type 2 diabetes mellitus, and patients being treated for type 2 diabetes mellitus. Am. J. Clin. Nutr. 2015, 102, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Radavelli-Bagatini, S. Effects of psyllium on metabolic syndrome risk factors. Obes. Rev. 2012, 13, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.W. Evidence-Based Approach to Fiber Supplements and Clinically Meaningful Health Benefits, Part 2: What to Look for and How to Recommend an Effective Fiber Therapy. Nutr. Today 2015, 50, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Baum, J.I. Dietary Protein Impact on Glycemic Control during Weight Loss. J. Nutr. 2004, 134, 968S–973S. [Google Scholar] [PubMed]
- Salehi, A.; Gunnerud, U.; Muhammed, S.J.; Östman, E.; Holst, J.J.; Björck, I.; Rorsman, P. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on β-cells. Nutr. Metab. 2012, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q.; Gannon, M.C. Metabolic response of people with type 2 diabetes to a high protein diet. Nutr. Metab. (Lond.) 2004, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Norton, L.E.; Wilson, G.J.; Layman, D.K.; Moulton, C.J.; Garlick, P.J. Leucine content of dietary proteins is a determinant of postprandial skeletal muscle protein synthesis in adult rats. Nutr. Metab. 2012, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Gaziano, J.M.; Buring, J.E.; Lee, I.M. Egg Consumption and Risk of Type 2 Diabetes in Men and Women. Diabetes Care 2009, 32, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Mursu, J.; Tuomainen, T.-P.; Virtanen, H.E.K.; Voutilainen, S. Egg consumption and risk of incident type 2 diabetes in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2015, 101, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Blesso, C.N.; Andersen, C.J.; Barona, J.; Volek, J.S.; Fernandez, M.L. Whole egg consumption improves lipoprotein profiles and insulin sensitivity to a greater extent than yolk-free egg substitute in individuals with metabolic syndrome. Metabolism 2013, 62, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G.; Tagami, H. Interventions to lower the glycemic response to carbohydrate foods with a low-viscosity fiber (resistant maltodextrin): Meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2009, 89, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of Fat, Protein, and Glycemic Index on Postprandial Glucose Control in Type 1 Diabetes: Implications for Intensive Diabetes Management in the Continuous Glucose Monitoring Era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.H.; Jin, H.Y.; Lee, K.A.; Kang, S.M.; Kim, W.J.; Kim, M.G.; Park, J.H.; Chae, S.W.; Baek, H.S.; Park, T.S. The Correlation and Accuracy of Glucose Levels between Interstitial Fluid and Venous Plasma by Continuous Glucose Monitoring System. Korean Diabetes J. 2010, 34, 350–358. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture Agricultural Research Service. What We Eat in America, NHANES Service; United States Department of Agriculture Agricultural Research Service: Washington, DC, USA, 2011–2012. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables/ (accessed on 31 March 2017).
- Rains, T.M.; Leidy, H.J.; Sanoshy, K.D.; Lawless, A.L.; Maki, K.C. A randomized, controlled, crossover trial to assess the acute appetitive and metabolic effects of sausage and egg-based convenience breakfast meals in overweight premenopausal women. J. Nutr. 2015, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-M.; Heden, T.D.; Liu, Y.; Nyhoff, L.M.; Thyfault, J.P.; Leidy, H.J.; Kanaley, J.A. A High-Protein Breakfast Induces Greater Insulin and Glucose-Dependent Insulinotropic Peptide Responses to a Subsequent Lunch Meal in Individuals with Type 2 Diabetes. J. Nutr. 2015, 145, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.L.; Jenkins, D.J.A.; Wolever, T.M.S.; Rogovik, A.L.; Jovanovski, E.; Božikov, V.; Rahelić, D.; Vuksan, V. Comparable Postprandial Glucose Reductions with Viscous Fiber Blend Enriched Biscuits in Healthy Subjects and Patients with Diabetes Mellitus: Acute Randomized Controlled Clinical Trial. Croat. Med. J. 2008, 49, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S.; Campbell, J.E.; Geleva, D.; Anderson, G.H. High-Fiber Cereal Reduces Postprandial Insulin Responses in Hyperinsulinemic but not Normoinsulinemic Subjects. Diabetes Care 2004, 27, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; O’Connor, L.E.; Sands, L.P.; Slebodnik, M.B.; Campbell, W.W. Effects of dietary protein intake on body composition changes after weight loss in older adults: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Medtronic. A Practical Guide to Continuous Glucose Monitoring; Medtronic: North Ryde, Australia, 2011. [Google Scholar]
- Potteiger, J.A.; Jacobsen, D.J.; Donnelly, J.E. A comparison of methods for analyzing glucose and insulin areas under the curve following nine months of exercise in overweight adults. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Sayer, R.D.; Amankwaah, A.F.; Tamer, G.G.; Chen, N.; Wright, A.J.; Tregellas, J.R.; Cornier, M.-A.; Kareken, D.A.; Talavage, T.M.; McCrory, M.A.; et al. Effects of Dietary Protein and Fiber at Breakfast on Appetite, ad Libitum Energy Intake at Lunch, and Neural Responses to Visual Food Stimuli in Overweight Adults. Nutrients 2016, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.G.; Coffman, K.P.; Reid, R.L.; Krall, J.M.; Albrink, M.J. Effect of Test Meals of Varying Dietary Fiber Content on Plasma-Insulin and Glucose Response. Am. J. Clin. Nutr. 1981, 34, 328–334. [Google Scholar] [PubMed]
- Acheson, K.J.; Blondel-Lubrano, A.; Oguey-Araymon, S.; Beaumont, M.; Emady-Azar, S.; Ammon-Zufferey, C.; Monnard, I.; Pinaud, S.; Nielsen-Moennoz, C.; Bovetto, L. Protein choices targeting thermogenesis and metabolism. Am. J. Clin. Nutr. 2011, 93, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Bonnema, A.L.; Altschwager, D.K.; Thomas, W.; Slavin, J.L. The effects of the combination of egg and fiber on appetite, glycemic response and food intake in normal weight adults—A randomized, controlled, crossover trial. Int. J. Food Sci. Nutr. 2016, 67, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Makris, A.P.; Borradaile, K.E.; Oliver, T.L.; Cassim, N.G.; Rosenbaum, D.L.; Boden, G.H.; Homko, C.J.; Foster, G.D. The Individual and Combined Effects of Glycemic Index and Protein on Glycemic Response, Hunger, and Energy Intake. Obesity 2011, 19, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; de Luis, D.A.; Izaola, O.; La Calle, F.; del Olmo, L.; Fernandez, L.; Arranz, T.; Hernandez, J.M.G. Effect of soluble fiber intake in lipid and glucose leves in healthy subjects: A randomized clinical trial. Diabetes Res. Clin. Pract. 2004, 65, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Karhunen, L.J.; Juvonen, K.R.; Flander, S.M.; Liukkonen, K.H.; Lahteenmaki, L.; Siloaho, M.; Laaksonen, D.E.; Herzig, K.H.; Uusitupa, M.I.; Poutanen, K.S. A Psyllium Fiber-Enriched Meal Strongly Attenuates Postprandial Gastrointestinal Peptide Release in Healthy Young Adults. J. Nutr. 2010, 140, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. The insulin resistance syndrome: Definition and dietary approaches to treatment. Annu. Rev. Nutr. 2005, 25, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Kn, F. Metabolic Regulation, 3rd ed.; Blackwell Publishing: Hoboken, NJ, USA, 2010. [Google Scholar]
- Jenkins, D.J.A.; Wolever, T.M.S.; Nineham, R.; Sarson, D.L.; Bloom, S.R.; Ahern, J.; Alberti, K.G.M.M.; Hockaday, T.D.R. Improved glucose tolerance four hours after taking guar with glucose. Diabetologia 1980, 19, 21–24. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, L.E.; Campbell, W.W. A novel fiber composite ingredient incorporated into a beverage and bar blunts postprandial serum glucose and insulin responses: A randomized controlled trial. Nutr. Res. 2016, 36, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Jovanovic, A.; Taylor, R. Utilizing the Second-Meal Effect in Type 2 Diabetes: Practical Use of a Soya-Yogurt Snack. Diabetes Care 2010, 33, 2552–2554. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.A.; Gardiner, J.; McBurney, M.I.; Anderson, S.; Weatherspoon, L.J.; Henry, D.N.; Hord, N.G. Effects of breakfast meal composition on second meal metabolic responses in adults with type 2 diabetes mellitus. Eur. J. Clin. Nutr. 2006, 60, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, A.; Gerrard, J.; Taylor, R. The Second-Meal Phenomenon in Type 2 Diabetes. Diabetes Care 2009, 32, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Östman, E.M.; Granfeldt, Y.; Björck, I.M.E. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 645–654. [Google Scholar] [PubMed]

| Dietary Variables | Breakfast Treatments | |||
|---|---|---|---|---|
| NPNF | HPNF | NPHF | HPHF | |
| * Energy (kcal) | 396 | 397 | 387 | 386 |
| Available Carbohydrate (g) | 51 | 50 | 51 | 48 |
| Sugar (g) | 18 | 22 | 11 | 14 |
| Total Fiber (g) | 2 | 2 | 8 | 8 |
| Soluble Fiber (g) | 0 | 1 | 6 | 7 |
| Insoluble Fiber (g) | 2 | 1 | 2 | 1 |
| Total Protein (g) | 12.5 | 25 | 12.5 | 25 |
| Total Fat (g) | 16 | 10 | 14 | 10 |
| Saturated Fat (g) | 4 | 3 | 4 | 3 |
| Monounsaturated Fat (g) | 6 | 3 | 6 | 3 |
| Polyunsaturated Fat (g) | 3 | 1 | 2 | 1 |
| Trans Fat (g) | 0 | 0 | 0 | 0 |
| Cholesterol (mg) | 114 | 325 | 114 | 325 |
| Sodium (mg) | 767 | 723 | 765 | 720 |
| Variable | Mean ± SD |
|---|---|
| Age (years) | 26 ± 5 |
| Height (cm) | 175 ± 10 |
| Body Mass (kg) | 83.4 ± 10.2 |
| BMI (kg/m2) | 27.0 ± 1.3 |
| % Body Fat | 26.4 ± 9.5 |
| Serum glucose (mmol/L) | 5.2 ± 0.3 |
| Total cholesterol (mmol/L) | 4.3 ± 0.5 |
| Low-density lipoprotein cholesterol (mmol/L) | 2.6 ± 0.5 |
| High-density lipoprotein cholesterol (mmol/L) | 1.2 ± 0.2 |
| Triglycerides (mmol/L) | 1.1 ± 0.4 |
| Fasting Variables | Breakfast Treatments | ||||
|---|---|---|---|---|---|
| NPNF | HPNF | NPHF | HPHF | p | |
| Glucose (mmol/L) | 5.3 ± 0.2 | 5.3 ± 0.2 | 5.2 ±0.1 | 5.2 ± 0.2 | 0.924 |
| Insulin (pmol/L) | 36 ± 6 | 36 ± 6 | 42 ± 6 | 42 ± 6 | 0.695 |
| HOMA-IR | 1.39 ± 0.1 | 1.51 ± 0.2 | 1.55 ± 0.2 | 1.54 ± 0.2 | 0.713 |
| ISI | 33 ± 5.2 | 32 ± 4.7 | 30 ± 5.6 | 31 ± 4.3 | 0.803 |
| HOMA-β (%) | 79 ± 10.2 | 79 ± 9.5 | 115 ± 36.9 | 88 ± 18.2 | 0.740 |
| Glucose Variables | Breakfast Treatments | ||||
|---|---|---|---|---|---|
| NPNF | HPNF | NPHF | HPHF | p | |
| Peak (mmol/L) | 7.4 ± 0.2 | 7.1 ± 0.2 | 7.2 ± 0.1 | 7.2 ± 0.2 | 0.768 |
| Mean (mmol/L) | 5.5 ± 0.1 | 5.5 ± 0.1 | 5.5 ± 0.1 | 5.2 ± 0.1 | 0.255 |
| Variability (CV) | 0.99 ± 0.54 | 0.33 ± 0.23 | 0.30 ± 0.24 | 0.60 ± 0.38 | 0.534 |
| AUC (mmol/L × 1440 min) | 7982 ± 109 | 7977 ± 123 | 7755 ± 109 | 7860 ± 104 | 0.179 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amankwaah, A.F.; Sayer, R.D.; Wright, A.J.; Chen, N.; McCrory, M.A.; Campbell, W.W. Effects of Higher Dietary Protein and Fiber Intakes at Breakfast on Postprandial Glucose, Insulin, and 24-h Interstitial Glucose in Overweight Adults. Nutrients 2017, 9, 352. https://doi.org/10.3390/nu9040352
Amankwaah AF, Sayer RD, Wright AJ, Chen N, McCrory MA, Campbell WW. Effects of Higher Dietary Protein and Fiber Intakes at Breakfast on Postprandial Glucose, Insulin, and 24-h Interstitial Glucose in Overweight Adults. Nutrients. 2017; 9(4):352. https://doi.org/10.3390/nu9040352
Chicago/Turabian StyleAmankwaah, Akua F., R. Drew Sayer, Amy J. Wright, Ningning Chen, Megan A. McCrory, and Wayne W. Campbell. 2017. "Effects of Higher Dietary Protein and Fiber Intakes at Breakfast on Postprandial Glucose, Insulin, and 24-h Interstitial Glucose in Overweight Adults" Nutrients 9, no. 4: 352. https://doi.org/10.3390/nu9040352





