Analysis of the Anti-Cancer Effects of Cincau Extract (Premna oblongifolia Merr) and Other Types of Non-Digestible Fibre Using Faecal Fermentation Supernatants and Caco-2 Cells as a Model of the Human Colon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Green Cincau Extracts
2.2. In Vitro Fermentation of Dietary Fibre
2.3. Cell Culture
2.4. SCFA Analysis
2.5. MTT Proliferation Assay
2.6. Alkaline Phosphatase (AP) Activity Assay
2.7. Caspase 3–7 and Lactate Dehydrogenase (LDH) Assay
2.8. Statistical Analysis
3. Results
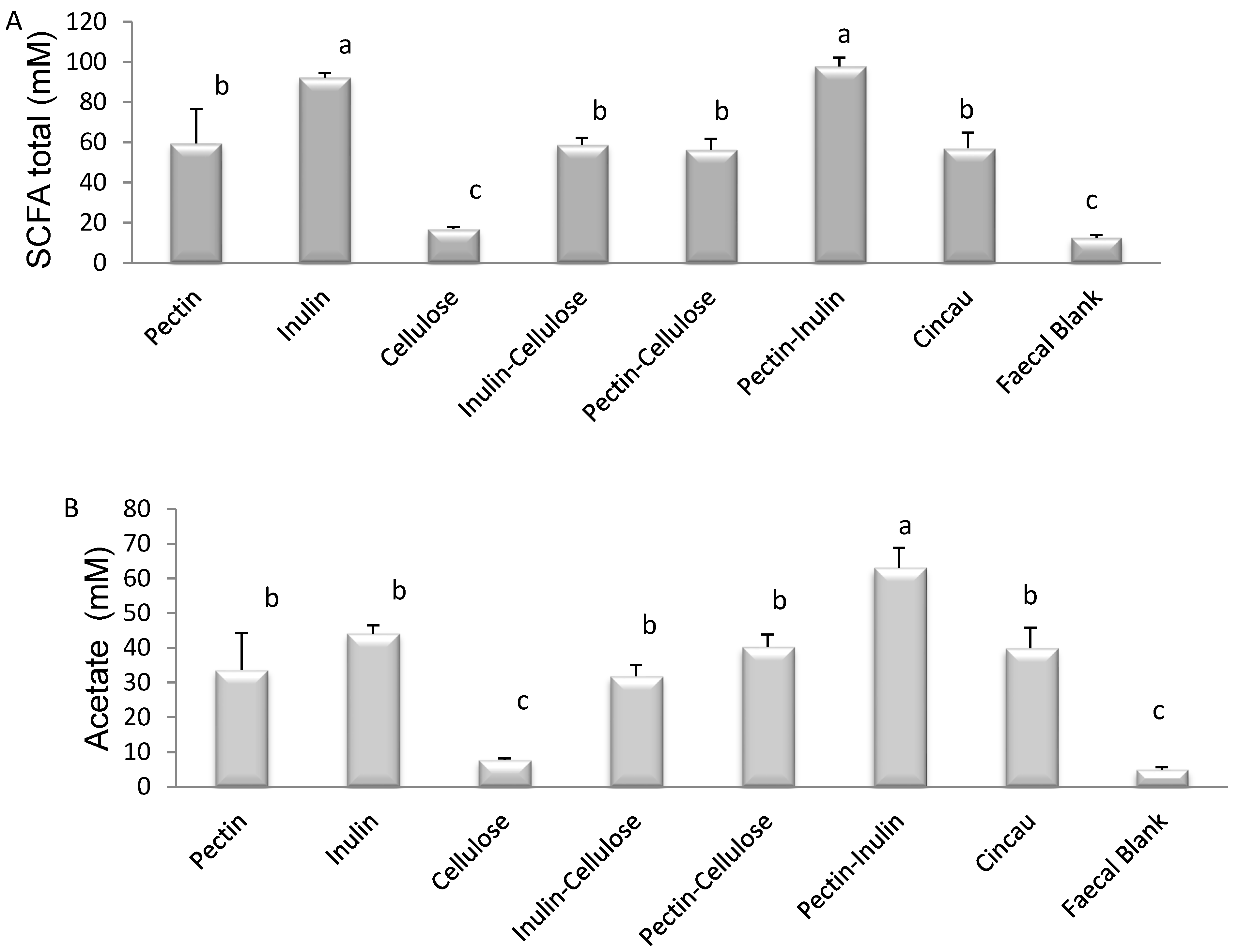
3.1. SCFA Content of Dietary Fibre Fermentation Supernatant
3.2. Effect of Dietary Fibre FS on Caco-2 Cell Viability
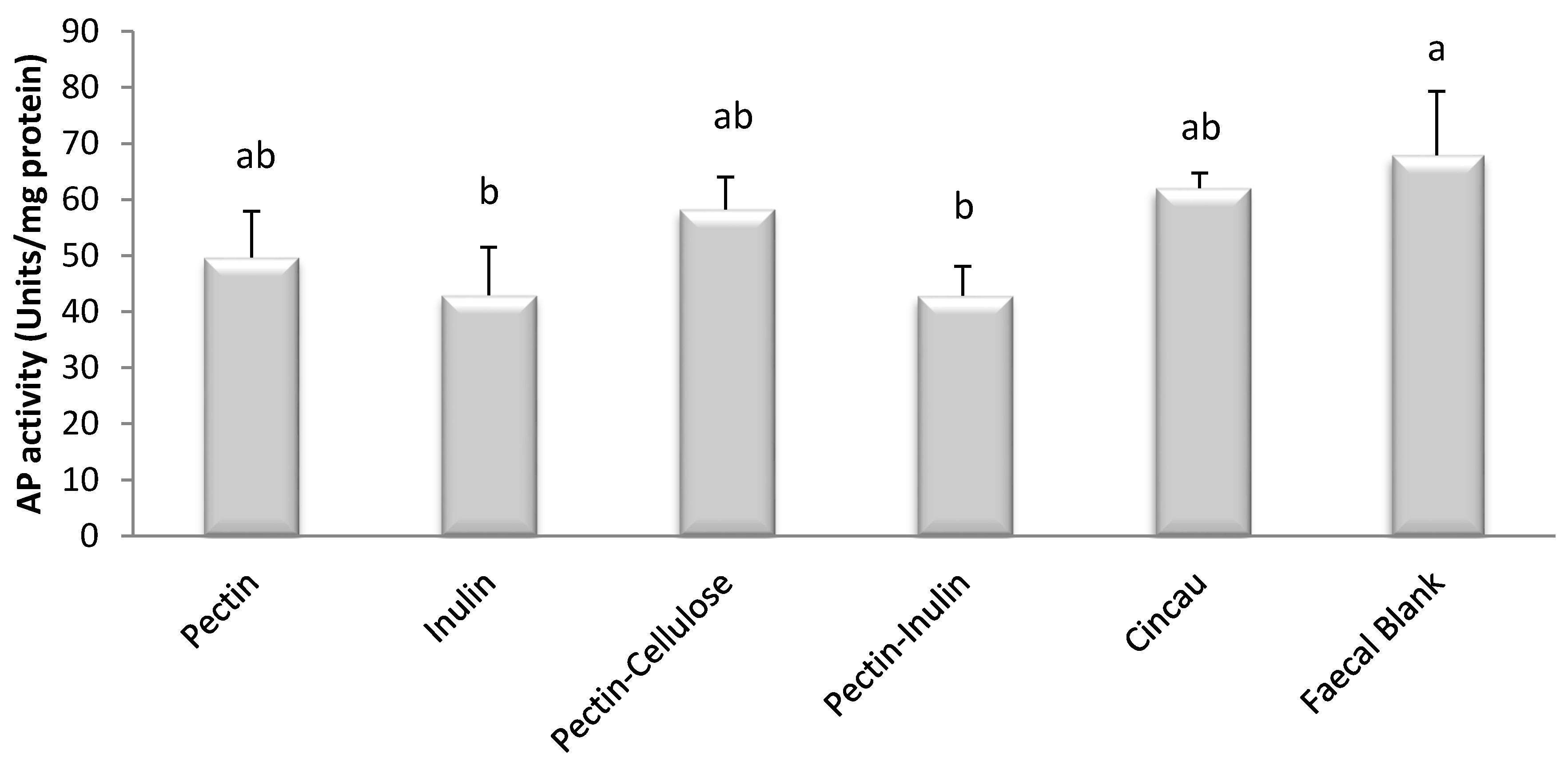
3.3. Effect of Dietary Fibre FS on Cell Differentiation
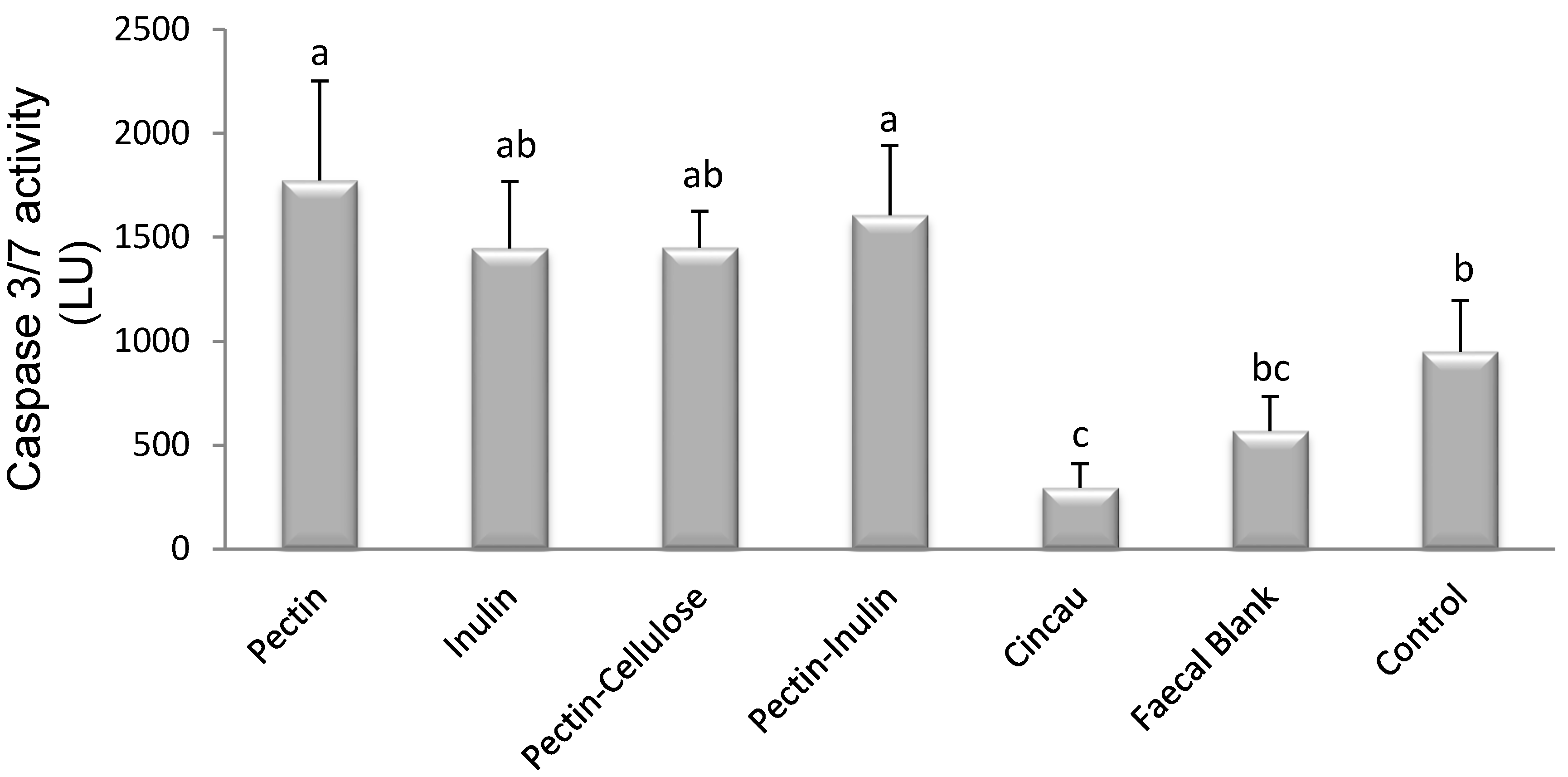
3.4. Effect of Dietary Fibre FS on Caspase 3/7 Activity
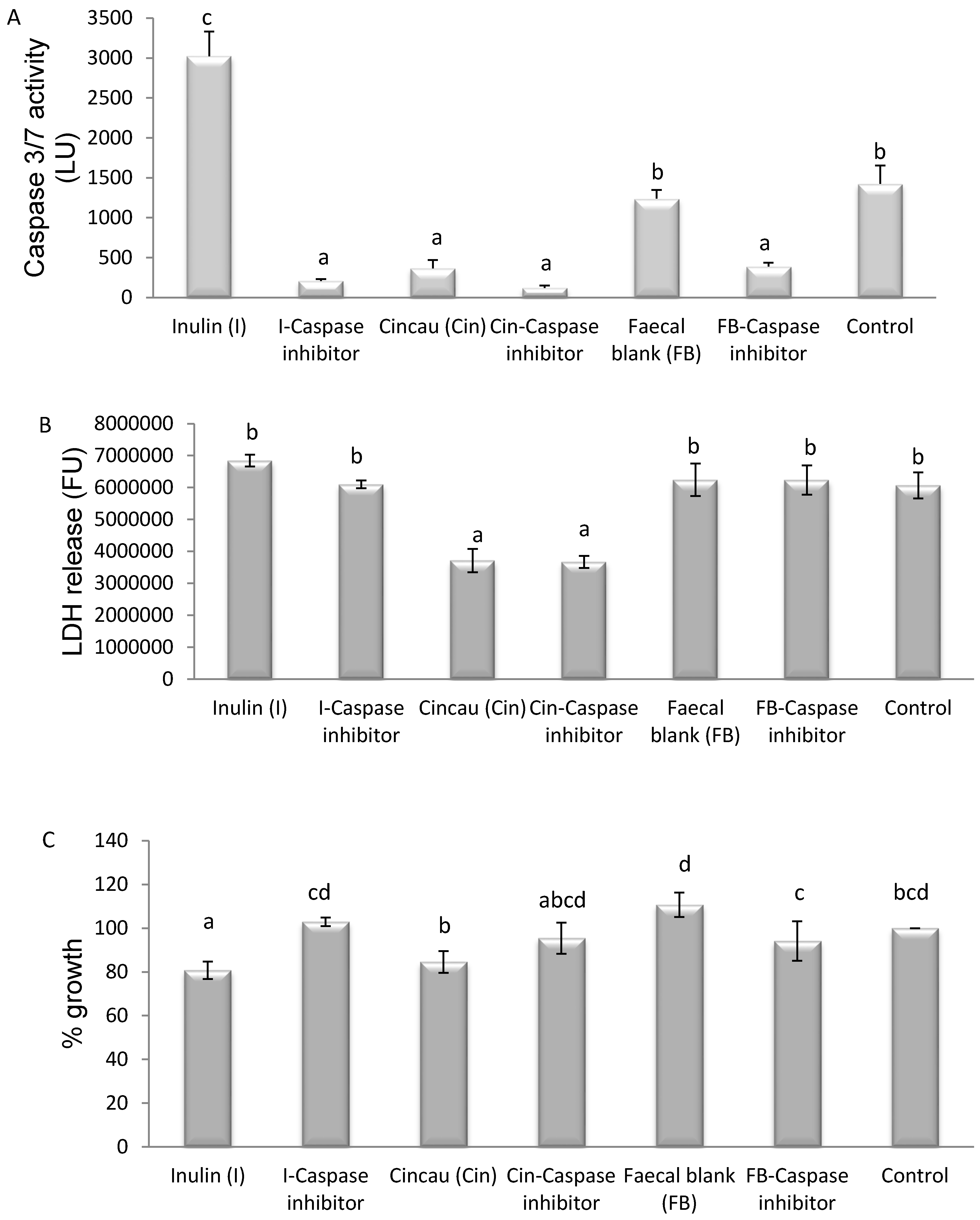
3.5. Mechanism of Cell Death Induced by FSs Containing SCFAs
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Center, M.M.; Jemal, A.; Ward, E. International trends in colorectal cancer incidence rates. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Encarnacao, J.C.; Abrantes, A.M.; Pires, A.S.; Botelho, M.F. Revisit dietary fiber on colorectal cancer: Butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015, 34, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Scharlau, D.; Borowicki, A.; Habermann, N.; Hofmann, T.; Klenow, S.; Miene, C.; Munjal, U.; Stein, K.; Glei, M. Mechanisms of primary cancer prevention by butyrate and other products formed during gut flora-mediated fermentation of dietary fibre. Mutat. Res. 2009, 682, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, R.H.; Young, G.P.; Bhathal, P.S. Effects of short chain fatty acids on a new human colon carcinoma cell line (lim1215). Gut 1986, 27, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Medina, V.; Edmonds, B.; Young, G.P.; James, R.; Appleton, S.; Zalewski, P.D. Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin a (inhibitors of histone deacetylase): Dependence on protein synthesis and synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer Res. 1997, 57, 3697–3707. [Google Scholar] [PubMed]
- Juskiewicz, J.; Zdunczyk, Z. Effects of cellulose, carboxymethylcellulose and inulin fed to rats as single supplements or in combinations on their caecal parameters. Comp. Biochem. Phys. A 2004, 139, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Pompei, A.; Cordisco, L.; Raimondi, S.; Amaretti, A.; Pagnoni, U.M.; Matteuzzi, D.; Rossi, M. In vitro comparison of the prebiotic effects of two inulin-type fructans. Anaerobe 2008, 14, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, U.; Nyman, M.; Ahrne, S.; Sullivan, E.O.; Fitzgerald, G. Bifidobacterium lactis bb-12 and lactobacillus salivarius ucc500 modify carboxylic acid formation in the hindgut of rats given pectin, inulin, and lactitol. J. Nutr. 2006, 136, 2175–2180. [Google Scholar] [PubMed]
- McIntyre, A.; Young, G.P.; Taranto, T.; Gibson, P.R.; Ward, P.B. Different fibers have different regional effects on luminal contents of rat colon. Gastroenterology 1991, 101, 1274–1281. [Google Scholar] [CrossRef]
- Pluske, J.R.; Durmic, Z.; Pethick, D.W.; Mullan, B.P.; Hampson, D.J. Confirmation of the role of rapidly fermentable carbohydrates in the expression of swine dysentery in pigs after experimental infection. J. Nutr. 1998, 128, 1737–1744. [Google Scholar] [PubMed]
- Henningsson, A.M.; Bjorck, I.M.; Nyman, E.M. Combinations of indigestible carbohydrates affect short-chain fatty acid formation in the hindgut of rats. J. Nutr. 2002, 132, 3098–3104. [Google Scholar] [PubMed]
- Muir, J.G.; Yeow, E.G.; Keogh, J.; Pizzey, C.; Bird, A.R.; Sharpe, K.; O’Dea, K.; Macrae, F.A. Combining wheat bran with resistant starch has more beneficial effects on fecal indexes than does wheat bran alone. Am. J. Clin. Nutr. 2004, 79, 1020–1028. [Google Scholar] [PubMed]
- Khan, K.M.; Edwards, C.A. In vitro fermentation characteristics of a mixture of raftilose and guar gum by human faecal bacteria. Eur. J. Nutr. 2005, 44, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Pool-Zobel, B.L. Inulin-type fructans and reduction in colon cancer risk: Review of experimental and human data. Br. J. Nutr. 2005, 93, S73–S90. [Google Scholar] [CrossRef] [PubMed]
- Pool-Zobel, B.L.; Sauer, J. Overview of experimental data on reduction of colorectal cancer risk by inulin-type fructans. J. Nutr. 2007, 137, 2580s–2584s. [Google Scholar] [PubMed]
- Qamar, T.R.; Syed, F.; Nasir, M.; Rehman, H.; Zahid, M.N.; Liu, R.H.; Iqbal, S. Novel combination of prebiotics galacto-oligosaccharides and inulin-inhibited aberrant crypt foci formation and biomarkers of colon cancer in wistar rats. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Yang, L.C.; Chen, H.L. Effects of konjac glucomannan, inulin and cellulose on acute colonic responses to genotoxic azoxymethane. Food Chem. 2014, 155, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Vieiralves, N.F.; Gomes, M.I.; Salvadori, D.M.; Rodrigues, M.A.; Barbisan, L.F. Effects of lycopene, synbiotic and their association on early biomarkers of rat colon carcinogenesis. Food Chem. Toxicol. 2010, 48, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Chen, H.L. Effects of konjac glucomannan on putative risk factors for colon carcinogenesis in rats fed a high-fat diet. J. Agric. Food Chem. 2011, 59, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Bertkova, I.; Hijova, E.; Chmelarova, A.; Mojzisova, G.; Petrasova, D.; Strojny, L.; Bomba, A.; Zitnan, R. The effect of probiotic microorganisms and bioactive compounds on chemically induced carcinogenesis in rats. Neoplasma 2010, 57, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V.; Chou, D.; Simi, B.; Ku, H.; Reddy, B.S. Prevention of colonic aberrant crypt foci and modulation of large bowel microbial activity by dietary coffee fiber, inulin and pectin. Carcinogenesis 1998, 19, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Veeriah, S.; Will, F.; Dietrich, H.; Pool-Zobel, B.L.; Schrenk, D. Histone-deacetylase inhibition and butyrate formation: Fecal slurry incubations with apple pectin and apple juice extracts. Nutrition 2008, 24, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Nurdin, S.U.; Zuidar, S.A.; Suharyono. Dried extract from green cincau leaves as potential fibre sources for food enrichment. Afr. Crop. Sci. J. 2005, 7, 655–658. [Google Scholar]
- Nurdin, S.U.; Hwang, J.K.; Hung, P. Effect of green cincau leaf (Premna oblongifolia merr.) water extracts on cytokines production in whole cell culture of mouse splenocytes. Indo Food Nutr. Prog. 2003, 10, 70–74. [Google Scholar]
- Nurdin, S.U. Evaluation of laxative effect and fermentability of gel forming component of green cincau leaves (Premna oblongifolia merr.). Teknologi dan Industri Pangan 2007, 18, 10–16. [Google Scholar]
- Belobrajdic, D.P.; Hino, S.; Kondo, T.; Jobling, S.A.; Morell, M.K.; Topping, D.L.; Morita, T.; Bird, A.R. High wholegrain barley beta-glucan lowers food intake but does not alter small intestinal macronutrient digestibility in ileorectostomised rats. Int. J. Food Sci. Nutr. 2016, 67, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, Method 994.13, Supplement Total Dietary Fibre (Determined as Neutral Sugar Residues, Uronic Acid Residues and Klason Lignin); AOAC: Arlington, VA, USA, 1995. [Google Scholar]
- Charoensiddhi, S.; Conlon, M.A.; Vuaran, M.S.; Franco, C.M.M.; Zhang, W. Impact of extraction processes on prebiotic potential of the brown seaweed ecklonia radiata by in vitro human gut bacteria fermentation. J. Funct. Foods 2016, 24, 221–230. [Google Scholar] [CrossRef]
- Zhou, Z.; Cao, X.; Zhou, J.Y.H. Effect of resistant starch structure on short-chain fatty acids production by human gut microbiota fermentation in vitro. Starch Stärke 2013, 65, 509–516. [Google Scholar] [CrossRef]
- Le Leu, R.K.; Brown, I.L.; Hu, Y.; Bird, A.R.; Jackson, M.; Esterman, A.; Young, G.P. A synbiotic combination of resistant starch and bifidobacterium lactis facilitates apoptotic deletion of carcinogen-damaged cells in rat colon. J. Nutr. 2005, 135, 996–1001. [Google Scholar] [PubMed]
- Young, F.M.; Phungtamdet, W.; Sanderson, B.J. Modification of mtt assay conditions to examine the cytotoxic effects of amitraz on the human lymphoblastoid cell line, wil2ns. Toxicol. In Vitro 2005, 19, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Beyer-Sehlmeyer, G.; Glei, M.; Hartmann, E.; Hughes, R.; Persin, C.; Bohm, V.; Rowland, I.; Schubert, R.; Jahreis, G.; Pool-Zobel, B.L. Butyrate is only one of several growth inhibitors produced during gut flora-mediated fermentation of dietary fibre sources. Br. J. Nutr. 2003, 90, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Erickson, R.H.; Gum, J.R.; Yoshioka, M.; Gum, E.; Kim, Y.S. Biosynthesis of alkaline-phosphatase during differentiation of the human colon cancer cell-line caco-2. Gastroenterology 1990, 98, 1199–1207. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Gerard, P.; Rabot, S.; Bruneau, A.; El Aidy, S.; Derrien, M.; Kleerebezem, M.; Zoetendal, E.G.; Smidt, H.; Verstraete, W.; et al. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environ. Microbiol. 2011, 13, 2667–2680. [Google Scholar] [CrossRef] [PubMed]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Lanneau, D.; de Thonel, A.; Maurel, S.; Didelot, C.; Garrido, C. Apoptosis versus cell differentiation: Role of heat shock proteins hsp90, hsp70 and hsp27. Prion 2007, 1, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.M.; Ko, T.C.; Evers, B.M. Caco-2 intestinal cell differentiation is associated with g(1) arrest and suppression of cdk2 and cdk4. Am. J. Physiol. 1998, 275, C1193–C1200. [Google Scholar] [PubMed]
- Orchel, A.; Dzierzewicz, Z.; Parfiniewicz, B.; Weglarz, L.; Wilczok, T. Butyrate-induced differentiation of colon cancer cells is pkc and jnk dependent. Dig. Dis. Sci. 2005, 50, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.; Beyer-Sehlmeyer, G.; Pool-Zobel, B.L. Mixtures of scfa, composed according to physiologically available concentrations in the gut lumen, modulate histone acetylation in human ht29 colon cancer cells. Br. J. Nutr. 2006, 96, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Hubatsch, I.; Ridderstrom, M.; Mannervik, B. Human glutathione transferase a4-4: An alpha class enzyme with high catalytic efficiency in the conjugation of 4-hydroxynonenal and other genotoxic products of lipid peroxidation. Biochem. J. 1998, 330 Pt 1, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Munjal, U.; Glei, M.; Pool-Zobel, B.L.; Scharlau, D. Fermentation products of inulin-type fructans reduce proliferation and induce apoptosis in human colon tumour cells of different stages of carcinogenesis. Br. J. Nutr. 2009, 102, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.; Rowland, I.R. Stimulation of apoptosis by two prebiotic chicory fructans in the rat colon. Carcinogenesis 2001, 22, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Anh, T.D.; Ahn, M.Y.; Kim, S.A.; Yoon, J.H.; Ahn, S.G. The histone deacetylase inhibitor, trichostatin a, induces g2/m phase arrest and apoptosis in yd-10b oral squamous carcinoma cells. Oncol. Rep. 2012, 27, 455–460. [Google Scholar] [PubMed]
- Hwang, J.J.; Kim, Y.S.; Kim, M.J.; Jang, S.J.; Lee, J.H.; Choi, J.; Ro, S.; Hyun, Y.L.; Lee, J.S.; Kim, C.S. A novel histone deacetylase inhibitor, cg0006, induces cell death through both extrinsic and intrinsic apoptotic pathways. Anti-Cancer Drug 2009, 20, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.M.; Donovan, M.; Cotter, T.G. Histone deacetylase activity regulates apaf-1 and caspase 3 expression in the developing mouse retina. Investig. Ophth. Vis. Sci. 2006, 47, 2765–2772. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.M.; Howarth, G.S.; Butler, R.N. Short-chain fatty acids induce apoptosis in colon cancer cells associated with changes to intracellular redox state and glucose metabolism. Chemotherapy 2012, 58, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Syu, K.Y.; Lin, J.K. Chemical composition of solanum nigrum linn extract and induction of autophagy by leaf water extract and its major flavonoids in au565 breast cancer cells. J. Agric. Food Chem. 2010, 58, 8699–8708. [Google Scholar] [CrossRef] [PubMed]
- Harun, F.B.; Jamalullail, S.M.S.S.; Yin, K.B.; Othman, Z.; Tilwari, A.; Balaram, P. Autophagic cell death is induced by acetone and ethyl acetate extracts from eupatorium odoratum in vitro: Effects on mcf-7 and vero cell lines. Sci. World J. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aryudhani, N. Mechanism of Antitumor Activity of Powdered Leaf Green Grass Jelly (Premna blongifolia merr.) in Mice Transplanted c3h Breast Tumor Cells. Master’s Thesis, Bogor Agricultural University, Bogor, Indonesia, 2011. [Google Scholar]
- Cruz-Bravo, R.K.; Guevara-Gonzalez, R.G.; Ramos-Gomez, M.; Oomah, B.D.; Wiersma, P.; Campos-Vega, R.; Loarca-Pina, G. The fermented non-digestible fraction of common bean (Phaseolus vulgaris L.) triggers cell cycle arrest and apoptosis in human colon adenocarcinoma cells. Genes Nutr. 2014, 9, 359. [Google Scholar] [CrossRef] [PubMed]


| Dietary Component | Concentration |
|---|---|
| Moisture | 4.4 |
| Fat | 4.4 |
| Protein | 13.3 |
| Ash | 12.2 |
| Starch | 1.8 |
| Resistant starch | 0.5 |
| Soluble NSP | 5.8 |
| Insoluble NSP | 46.3 |
| Total non-starch polysaccharides (NSPs) | 52.1 |
| Dietary Fibre | Ratio (%) |
|---|---|
| Pectin | 100 |
| Inulin | 100 |
| Cellulose | 100 |
| Pectin + cellulose | 50:50 |
| Pectin + inulin | 50:50 |
| Inulin + cellulose | 50:50 |
| Cincau extract | 100 |
| Faecal blank (FB) | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurdin, S.U.; Le Leu, R.K.; Young, G.P.; Stangoulis, J.C.R.; Christophersen, C.T.; Abbott, C.A. Analysis of the Anti-Cancer Effects of Cincau Extract (Premna oblongifolia Merr) and Other Types of Non-Digestible Fibre Using Faecal Fermentation Supernatants and Caco-2 Cells as a Model of the Human Colon. Nutrients 2017, 9, 355. https://doi.org/10.3390/nu9040355
Nurdin SU, Le Leu RK, Young GP, Stangoulis JCR, Christophersen CT, Abbott CA. Analysis of the Anti-Cancer Effects of Cincau Extract (Premna oblongifolia Merr) and Other Types of Non-Digestible Fibre Using Faecal Fermentation Supernatants and Caco-2 Cells as a Model of the Human Colon. Nutrients. 2017; 9(4):355. https://doi.org/10.3390/nu9040355
Chicago/Turabian StyleNurdin, Samsu U., Richard K. Le Leu, Graeme P. Young, James C. R. Stangoulis, Claus T. Christophersen, and Catherine A. Abbott. 2017. "Analysis of the Anti-Cancer Effects of Cincau Extract (Premna oblongifolia Merr) and Other Types of Non-Digestible Fibre Using Faecal Fermentation Supernatants and Caco-2 Cells as a Model of the Human Colon" Nutrients 9, no. 4: 355. https://doi.org/10.3390/nu9040355





