Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Recruitment
2.2. Double-Blind Study
2.2.1. Blinding and Initial Assessment of Participants
2.2.2. Supplementation: Days 1–20
2.2.3. Final Assessment: Day 22
2.3. Open-Label Study
2.4. Differential Sugar (Lactulose/Mannitol) Absorption Test
2.4.1. The Test Principle
2.4.2. Sugar Ingestion and Urine Collection
2.4.3. Sugar Derivatization
2.4.4. Gas Chromatography
2.5. Stool Zonulin Assay
2.6. Reference Limits
2.7. Statistical Analysis
2.8. Bioethical Approval
3. Results
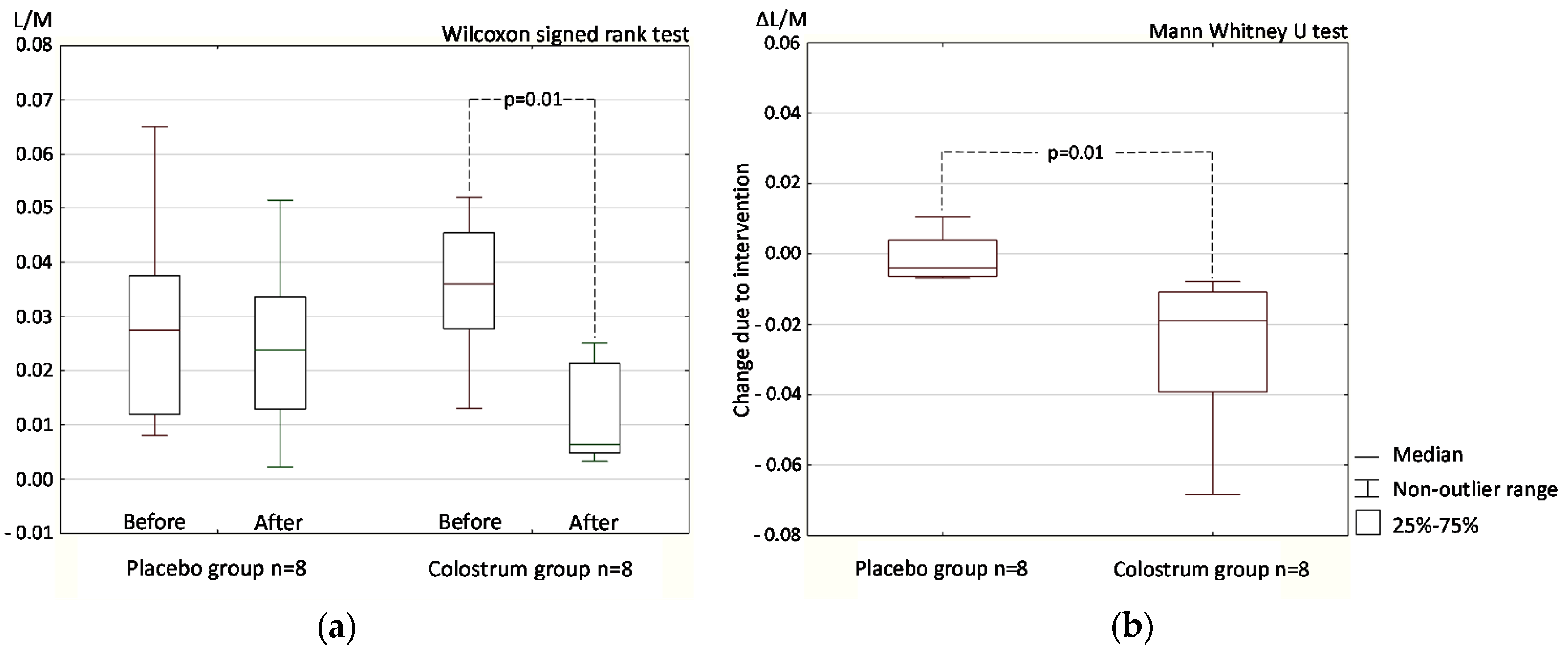
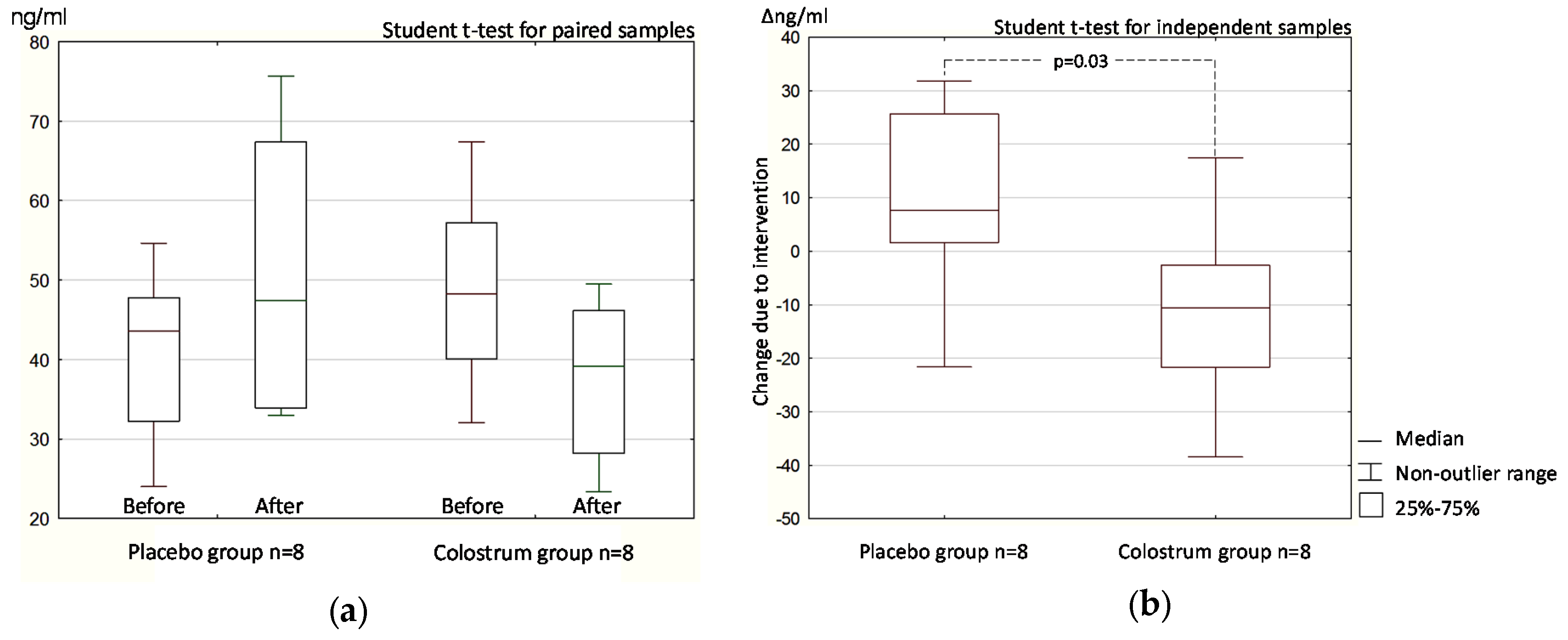
3.1. Double-Blind Phase
3.1.1. Intestinal Permeability (Differential Sugar Absorption Test)
3.1.2. Stool Zonulin Concentration
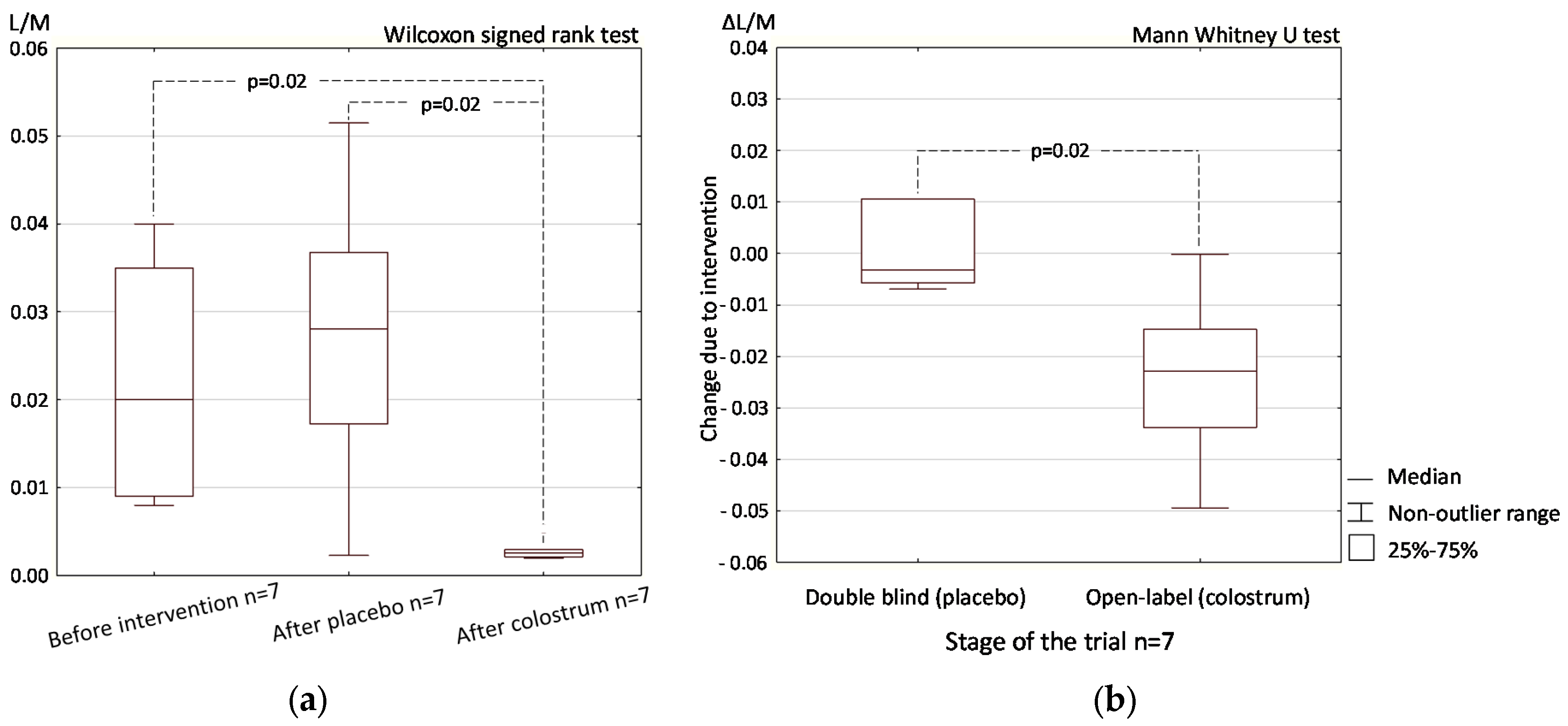
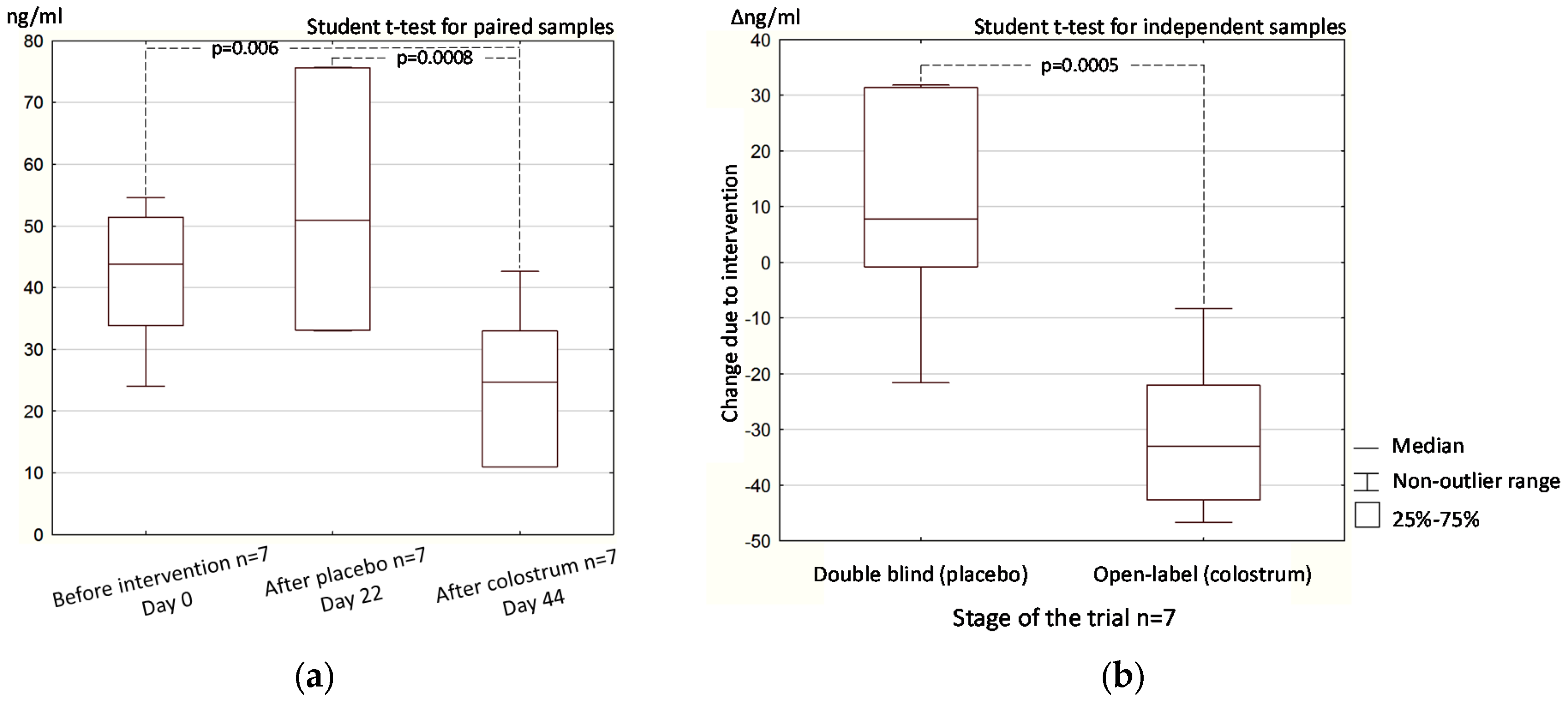
3.2. Open-Label (Crossover) Phase
3.2.1. Intestinal Permeability
3.2.2. Stool Zonulin Concentration
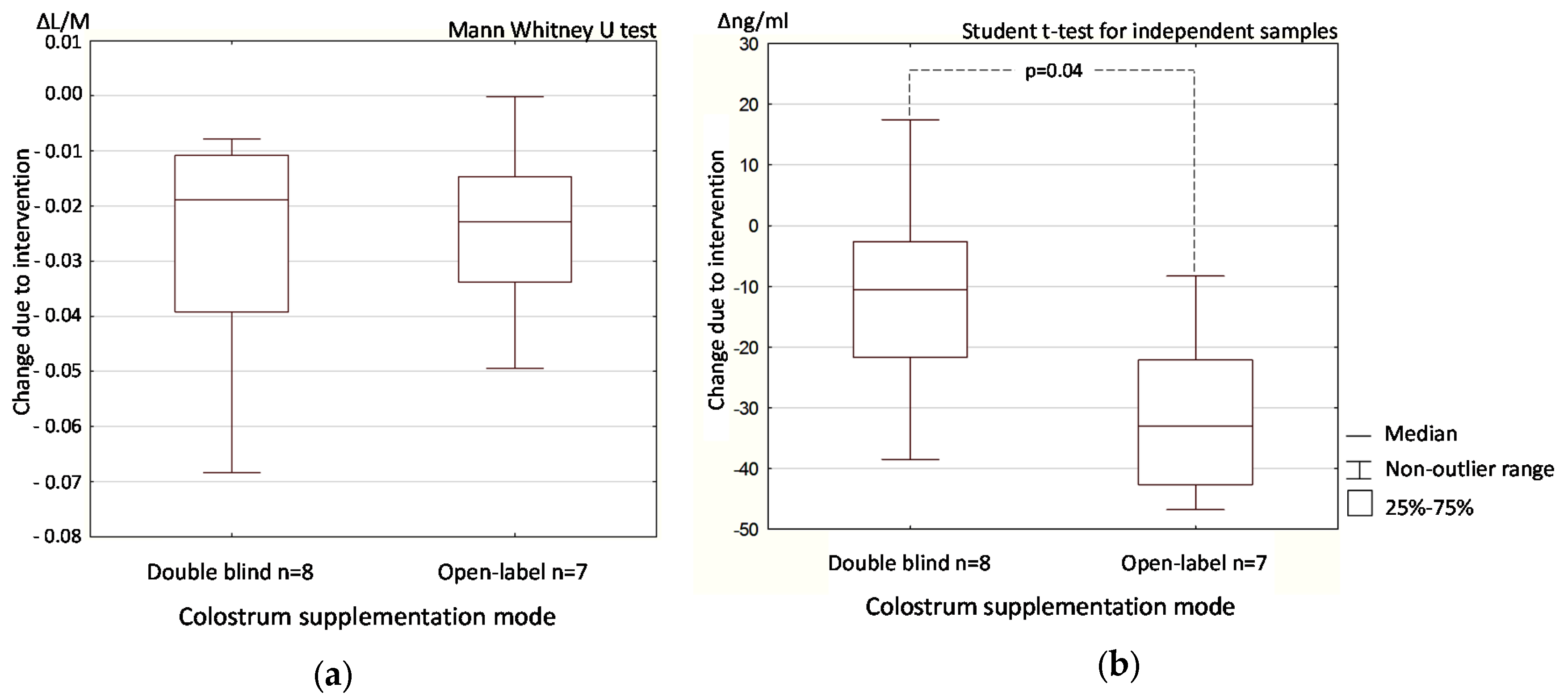
3.3. Colostrum Supplementation Effect in Double-Blind and Open-Label Phases
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bamias, G.; Nyce, M.R.; Sarah, A.; Rue, D.L.; Cominelli, F. New concepts in the pathophysiology of inflammatory bowel disease. Ann. Intern. Med. 2005, 143, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Shea-Donohue, T.; Urban, J.F. Neuroimmune Modulation of Gut Function. Handb. Exp. Pharmacol. 2016. [Google Scholar] [CrossRef]
- Kang, Y.B.; Cai, Y.; Zhang, H. Gut microbiota and allergy/asthma: From pathogenesis to new therapeutic strategies. Allergol. Immunopath. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bamias, G.; Pizarro, T.T.; Cominelli, F. Pathway-based approaches to the treatment of inflammatory bowel disease. Transl. Res. 2016, 167, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.S.; Sham, H.P.; Zarepour, M.; Vallance, B.A. Innate host responses to enteric bacterial pathogens: A balancing act between resistance and tolerance. Cell Microbiol. 2012, 14, 475–484. [Google Scholar]
- Fujimura, K.E.; Slusher, N.A.; Cabana, M.D.; Lynch, S.V. Role of the gut microbiota in defining human health. Expert Rev. Anti-Infect. 2010, 8, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, O. Leaking gut in type 1 diabetes. Curr. Opin. Gastroenterol. 2008, 24, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Gate-keeper function of the intestinal epithelium. Benef. Microbes. 2013, 4, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, C.; Fasano, A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers 2016, 4, e1251384. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Physiological, Pathological, and Therapeutic Implications of Zonulin-Mediated Intestinal Barrier Modulation. Living Life on the Edge of the Wall. Am. J. Pathol. 2008, 173, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 113, 4435–4440. [Google Scholar] [PubMed]
- Bjarnason, I.; Takeuchi, K. Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. J. Gastroenterol. 2009, 44, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Frauwallner, A. Exercise, intestinal barrier dysfunction and probiotic supplementation. Med. Sport Sci. 2012, 59, 47–56. [Google Scholar] [PubMed]
- Paterson, B.M.; Lammers, K.M.; Arrieta, M.C.; Fasano, A.; Meddings, J.B. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: A proof of concept study. Aliment. Pharm. Therap. 2007, 26, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, S.; Ju, J.M.; Lamba, A.; Murray, J.A. The potential utility of tight junction regulation in celiac disease: Focus on larazotide acetate. Ther. Adv. Gastroenter. 2016, 9, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Zuhl, M.N.; Lanphere, K.R.; Kravitz, L.; Mermier, C.M.; Schneider, S.; Dokladny, K.; Moseley, P.L. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J. Appl. Physiol. 2014, 116, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, M.; Bogner, S.; Schippinger, G.; Steinbauer, K.; Fankhauser, F.; Hallstroem, S.; Schuetz, B.; Greilberger, J.F. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2012, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Wilms, E.; Gerritsen, J.; Smidt, H.; Besseling-van der Vaart, I.; Rijkers, G.T.; Garcia Fuentes, A.R.; Masclee, A.A.; Troost, F.J. Effects of Supplementation of the Synbiotic Ecologic® 825/FOS P6 on Intestinal Barrier Function in Healthy Humans: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0167775. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Macdonald, C.E.; Johnson, W.S. Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am. J. Clin. Nutr. 2000, 72, 5–14. [Google Scholar] [PubMed]
- Champagne, C.P.; Raymond, Y.; Pouliot, Y.; Gauthier, S.F.; Lessard, M. Effect of bovine colostrum, cheese whey, and spray-dried porcine plasma on the in vitro growth of probiotic bacteria and Escherichia coli. Can. J. Microbiol. 2014, 60, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Floyd, D.N.; Macdonald, C.E.; Calnan, D.P.; Adenekan, R.O.; Johnson, W.; Goodlad, R.A.; Marchbank, T. Bovine colostrum is a health food supplement which prevents NSAID induced gut damage. Gut 1999, 44, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Cairangzhuoma, Y.M.; Muranishi, H.; Inagaki, M.; Uchida, K.; Yamashita, K.; Saito, S.; Yabe, T.; Kanamaru, Y. Skimmed, sterilized, and concentrated bovine late colostrum promotes both prevention and recovery from intestinal tissue damage in mice. J. Dairy Sci. 2013, 96, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Boldogh, I.; Aguilera-Aguirre, L.; Bacsi, A.; Choudhury, B.K.; Saavedra-Molina, A.; Kruzel, M. Colostrinin decreases hypersensitivity and allergic responses to common allergens. Int. Arch. Allergy Immunol. 2008, 146, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Pires, W.; Veneroso, C.E.; Wanner, S.P.; Pacheco, D.A.; Vaz, G.C.; Amorim, F.T.; Tonoli, C.; Soares, D.D.; Coimbra, C.C. Association Between Exercise-Induced Hyperthermia and Intestinal Permeability: A Systematic Review. Sports Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zuhl, M.; Schneider, S.; Lanphere, K.; Conn, C.; Dokladny, K.; Moseley, P. Exercise regulation of intestinal tight junction proteins. Br. J. Sport Med. 2014, 48, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I.; MacPherson, A.; Hollander, D. Intestinal permeability: An overview. Gastroenterology 1995, 108, 1566–1581. [Google Scholar] [CrossRef]
- Playford, R.J.; MacDonald, C.E.; Calnan, D.P.; Floyd, D.N.; Podas, T.; Johnson, W.; Wicks, A.C.; Bashir, O.; Marchbank, T. Co-administration of the health food supplement, bovine colostrum, reduces the acute non-steroidal anti-inflammatory drug-induced increase in intestinal permeability. Clin. Sci. 2001, 100, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Marchbank, T.; Davison, G.; Oakes, J.R.; Ghatei, M.A.; Patterson, M.; Moyer, M.P.; Playford, R.J. The nutriceutical bovine colostrum truncates the increase in gut permeability caused by heavy exercise in athletes. Am. J. Physiol.-Gastr. Liver Physiol. 2011, 300, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Davison, G.; Marchbank, T.; March, D.S.; Thatcher, R.; Playford, R.J. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. Am. J. Clin. Nutr. 2016, 104, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Oda, H.; Wakabayashi, H.; Yamauchi, K.; Sato, T.; Xiao, J.Z.; Abe, F.; Iwatsuki, K. Isolation of a bifidogenic peptide from the pepsin hydrolysate of bovine lactoferrin. Appl. Environ. Microb. 2013, 79, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, V.; Benfeldt, E.; Valerius, N.H.; Paerregaard, A.; Michaelsen, K.F. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J. Pediatr. 2004, 145, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Robson-Ansley, P.; Howatson, G.; Tallent, J.; Mitcheson, K.; Walshe, I.; Toms, C.; DU Toit, G.; Smith, M.; Ansley, L. Prevalence of allergy and upper respiratory tract symptoms in runners of the London marathon. Med. Sci. Sports Exerc. 2012, 44, 999–1004. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean | SD |
|---|---|---|
| Body mass [kg] | 89.59 | 17.85 |
| Waist circumference [cm] | 80.32 | 27.35 |
| Hip circumference [cm] | 95.00 | 30.93 |
| Lean body mass (LBM) | 71.79 | 12.31 |
| Total body water (TBW) [%] | 51.71 | 8.89 |
| Peripheral body fat (PBF) [%] | 19.38 | 5.63 |
| Body mass index (BMI) | 27.47 | 4.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hałasa, M.; Maciejewska, D.; Baśkiewicz-Hałasa, M.; Machaliński, B.; Safranow, K.; Stachowska, E. Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes. Nutrients 2017, 9, 370. https://doi.org/10.3390/nu9040370
Hałasa M, Maciejewska D, Baśkiewicz-Hałasa M, Machaliński B, Safranow K, Stachowska E. Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes. Nutrients. 2017; 9(4):370. https://doi.org/10.3390/nu9040370
Chicago/Turabian StyleHałasa, Maciej, Dominika Maciejewska, Magdalena Baśkiewicz-Hałasa, Bogusław Machaliński, Krzysztof Safranow, and Ewa Stachowska. 2017. "Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes" Nutrients 9, no. 4: 370. https://doi.org/10.3390/nu9040370






